Sucrose Inclusion in Gestating and Lactating Diets of Sows Modifies the Feeding Behavior of Post-Weaning Pigs for Sweet Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Gestation and Lactation
2.2. Post-Weaning Measurements
2.3. Statistical Analysis
3. Results
3.1. Preference Thresholds
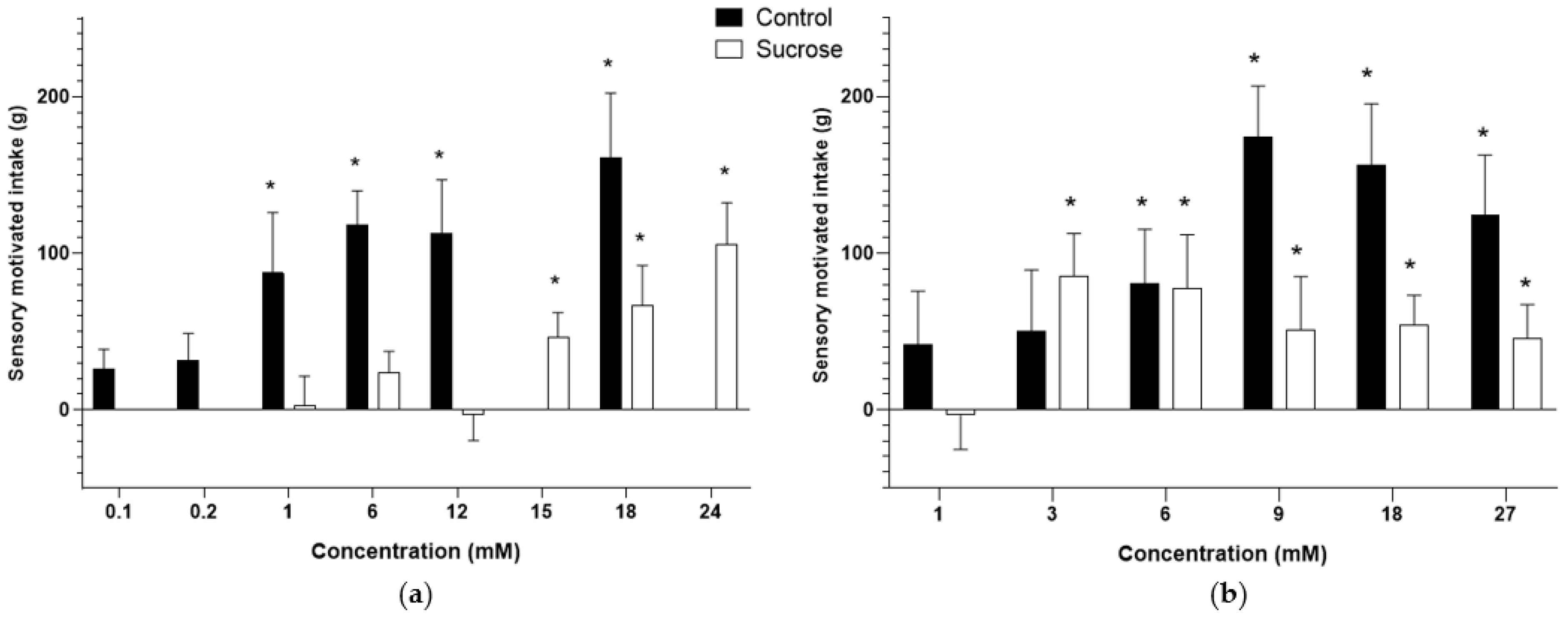
3.2. Sensory-Motivated Intake
3.3. Total Consumption
3.4. Consumption Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Counsilman, J.J.; Lim, L.M. The definition of weaning. Anim. Behav. 1985, 33, 1023–1024. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: Understanding the keys to health. Trends. Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef]
- Roura, E. Taste beyond taste. In Manipulating Pig Production XIII: Proceedings of the Thirteenth Biennial Conference of the Australasian Pig Science Association (APSA); van Barneveld, R., Ed.; Finsbury Green: Adelaide, Australia, 2011; pp. 106–117. ISBN 978-0-9806880-1-6. [Google Scholar]
- Roura, E.; Koopmans, S.; Lallès, J.; Le Huerou-Luron, I.; De Jager, N.; Schuurman, T.; Val-Laillet, D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 2016, 29, 60–90. [Google Scholar] [CrossRef]
- Guzmán-Pino, S.A.; Lazcano, C.; De Luca, V.; Figueroa, J.; Valenzuela, C.; Roura, E. Dietary Inclusion of Monosodium Glutamate in Gestating and Lactating Sows Modifies the Preference Thresholds and Sensory-Motivated Intake for Umami and Sweet Solutions in Post-Weaned Pigs. Animals 2019, 9, 336. [Google Scholar] [CrossRef]
- Janssen, S.; Depoortere, I. Nutrient sensing in the gut: New roads to therapeutics? Trends Endocrin. Met. 2013, 24, 92–100. [Google Scholar] [CrossRef]
- Moran, A.W.; Al-Rammahi, M.A.; Arora, D.K.; Batchelor, D.J.; Coulter, E.A.; Daly, K.; Ionescu, C.; Bravo, D.; Shirazi-Beechey, S.P. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br. J. Nutr. 2010, 104, 637–646. [Google Scholar] [CrossRef]
- Baldwin, B.A. Quantitative studies on taste preference in pigs. Proc. Nutr. Soc. 1976, 35, 69–73. [Google Scholar] [CrossRef]
- Baldwin, B.A. Postingestive factors influencing operant sugar intake in pigs. Physiol. Behav. 1996, 60, 283–286. [Google Scholar] [CrossRef]
- Dulac, C. The physiology of taste, vintage 2000. Cell 2000, 100, 607–610. [Google Scholar] [CrossRef]
- Guzmán-Pino, S.A.; Solà-Oriol, D.; Figueroa, J.; Dwyer, D.M.; Pérez, J.F. Effect of a long-term exposure to concentrated sucrose and maltodextrin solutions on the preference, appetence, feed intake and growth performance of post-weaned piglets. Physiol. Behav. 2015, 141, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.; Wanner, M.; Tinti, J.M.; Nofre, C. Gustatory responses of pigs to various natural and artificial compounds known to be sweet in man. Food Chem. 2000, 68, 375–385. [Google Scholar] [CrossRef]
- Roura, E.; Humphrey, B.; Tedó, G.; Ipharragerre, I. Unfolding the codes of short-term feed appetence in farm and companion animals. A comparative oronasal nutrient sensing biology review. Can. J. Anim. Sci. 2008, 88, 535–558. [Google Scholar] [CrossRef]
- Roura, E.; Tedó, G. Feed appetence in pigs: An oronasal sensing perspective. In Voluntary Feed Intake in Pigs; Torrallardona, D., Roura, E., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2009; pp. 105–140. ISBN 978-90-8686-096-8. [Google Scholar]
- Schaal, B.; Hummel, T.; Soussignan, R. Olfaction in the fetal and premature infant: Functional status and clinical implications. Clin. Perinatol. 2004, 31, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Oostindjer, M.; Bolhuis, J.E.; van den Brand, H.; Kemp, B. Prenatal flavor exposure affects flavor recognition and stress-related behavior of piglets. Chem. Senses 2009, 34, 775–787. [Google Scholar] [CrossRef]
- Langendijk, P.; Bolhuis, J.E.; Laurenssen, B.F. Effects of pre- and postnatal exposure to garlic and aniseed flavor on pre- and postweaning feed intake in pigs. Livest. Sci. 2007, 108, 284–287. [Google Scholar] [CrossRef]
- Oostindjer, M.; Bolhuis, J.E.; van den Brand, H.; Roura, E.; Kemp, B. Prenatal flavor exposure affects growth, health and behavior of newly weaned piglets. Physiol. Behav. 2010, 99, 579–586. [Google Scholar] [CrossRef]
- Oostindjer, M.; Kemp, B.; van den Brand, H.; Bolhuis, J.E. Facilitating ‘learning from mom how to eat like a pig’ to improve welfare of piglets around weaning. Appl. Anim. Behav. Sci. 2014, 160, 19–30. [Google Scholar] [CrossRef]
- Figueroa, J.; Solaà-Oriol, D.; Vinokurovas, L.; Manteca, X.; Peérez, J.F. Prenatal flavour exposure through maternal diets influences flavour preference in piglets before and after weaning. Anim. Feed Sci. Technol. 2013, 183, 160–167. [Google Scholar] [CrossRef]
- Figueroa, J.; Solà-Oriol, D.; Guzmán-Pino, S.A.; Chetrit, C.; Borda, E.; Pérez, J.F. The use of porcine digestible peptides and their continuity effect in nursery pigs. J. Anim. Sci. 2016, 94, 1531–1540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blavi, L.; Solà-Oriol, D.; Mallo, J.J.; Pérez, J.F. Anethol, cinnamaldehyde, and eugenol inclusion in feed affects postweaning performance and feeding behavior of piglets. J. Anim. Sci. 2016, 94, 5262–5271. [Google Scholar] [CrossRef] [PubMed]
- Val-Laillet, D.; Elmore, J.S.; Baines, D.; Naylor, P.; Naylor, R. Long-term exposure to sensory feed additives during the gestational and postnatal periods affects sows’ colostrum and milk sensory profiles, piglets’ growth, and feed intake. J. Anim. Sci. 2018, 96, 3233–3248. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- Dwyer, D.M. Licking and liking: The assessment of hedonic responses in rodents. Q. J. Exp. Psychol. 2012, 65, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Frías, D.; Tadich, T.; Franco-Rosselló, R.; Dwyer, D.M.; Figueroa, J. Consumption patterns: A proposed model for measurement of solution palatability in pigs. J. Anim. Sci. 2016, 94, 103–105. [Google Scholar] [CrossRef]
- Figueroa, J.; Frías, D.; Solà-Oriol, D.; Tadich, T.; Franco-Rosselló, R.; Núñez, V.; Dwyer, D.M. Palatability in pigs, the pleasure of consumption. J. Anim. Sci. 2019, 97, 2165–2174. [Google Scholar] [CrossRef]
- Blavi, L.; Solà-Oriol, D.; Llonch, P.; López-Vergé, S.; Martín-Orúe, S.M.; Pérez, J.F. Management and Feeding Strategies in Early Life to Increase Piglet Performance and Welfare around Weaning: A Review. Animals 2021, 11, 302. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflugers Arch–Eur. J. Physiol. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef]
- Fowden, A.L.; Forhead, A.J.; Silver, M.; MacDonald, A.A. Glucose, lactate and oxygen metabolism in the fetal pig during late gestation. Exp. Physiol. 1997, 82, 171–182. [Google Scholar] [CrossRef]
- Père, M.C. Maternal and fetal blood levels of glucose, lactate, fructose, and insulin in the conscious pig. J. Anim. Sci. 1995, 73, 2994–2999. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Bauman, D.E. Adaptations of glucose metabolism during pregnancy and lactation. J. Mammary Gland. Biol. Neoplasia 1997, 2, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Kensinger, R.S.; Harrell, R.J.; Bauman, D.E. Nutrient uptake and endocrine regulation of milk synthesis by mammary tissue of lactating sows. J. Anim. Sci. 1995, 73, 36–56. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, F.; Zhang, Y.; Lv, Y.; Heng, J.; Min, T.; Li, L.; Guan, W. Recent progress of porcine milk components and mammary gland function. J. Anim. Sci. Biotechnol. 2018, 9, 77. [Google Scholar] [CrossRef]
- Park, M.S.; Yang, Y.X.; Shinde, P.L.; Choi, J.Y.; Jo, J.K.; Kim, J.S.; Lohakare, J.D.; Yang, B.K.; Lee, J.K.; Kwon, I.K.; et al. Effects of dietary glucose inclusion on reproductive performance, milk compositions and blood profiles in lactating sows. J. Anim. Physiol. Anim. Nutr. 2010, 94, 677–684. [Google Scholar] [CrossRef]
- Nicolaïdis, S. Prenatal imprinting of postnatal specific appetites and feeding behavior. Metabolism 2008, 57 (Suppl. 2), S22–S26. [Google Scholar] [CrossRef]

| Percentage | Gestating | Lactating |
|---|---|---|
| Composition | ||
| Maize | 57.2 | 59.4 |
| Wheat | 25.0 | 8.0 |
| Soybean oil | - | 4.0 |
| Soybean meal | 13.4 | 24.2 |
| L-Lysine HCl | 0.2 | 0.6 |
| DL-Methionine | - | 0.2 |
| L-Threonine | - | 0.2 |
| L-Tryptophan | - | 0.05 |
| Mycotoxins inactivator | 0.1 | 0.1 |
| Mycotoxins absorbent | 0.1 | 0.2 |
| Artificial sweetener | 0.01 | - |
| Mineral–vitamin–phytase mix | 0.4 | 0.4 |
| Calcium carbonate | 1.2 | 1.0 |
| Sodium bicarbonate | 0.7 | - |
| Dicalcium phosphate | 0.7 | 0.7 |
| Salt | 0.5 | 0.5 |
| Copper sulphate | - | 0.05 |
| Ammonium chloride | 0.3 | - |
| Vegetable choline chloride | 0.2 | 0.1 |
| Chemical analysis | ||
| DM | 87.6 | 89.3 |
| CP | 15.4 | 18.7 |
| CF | 3.8 | 2.6 |
| EE | 2.5 | 5.3 |
| NFE | 60.0 | 56.3 |
| Ash | 5.9 | 6.4 |
| Solution (mM) | Consumption (g) | |||
|---|---|---|---|---|
| Control | Sucrose | SEM | p-Value | |
| Sucrose | ||||
| 1 | 415.9 | 267.2 | 21.60 | <0.001 |
| 6 | 426.0 | 342.7 | 23.73 | 0.021 |
| 12 | 481.0 | 369.7 | 15.74 | <0.001 |
| 18 | 481.3 | 297.4 | 22.15 | <0.001 |
| MSG | ||||
| 1 | 436.7 | 418.7 | 15.44 | 0.410 |
| 3 | 472.9 | 448.4 | 12.13 | 0.152 |
| 9 | 455.4 | 451.4 | 14.01 | 0.833 |
| 27 | 445.2 | 479.1 | 16.77 | 0.143 |
| Solution (mM) | Consumption Pattern | ||||
|---|---|---|---|---|---|
| Control | Sucrose | SEM | p-Value | ||
| Sucrose | |||||
| 1 | 5.1 | 3.8 | 0.35 | 0.014 | |
| 6 | 6.3 | 4.0 | 0.39 | <0.001 | |
| 12 | 6.2 | 5.2 | 0.35 | 0.065 | |
| 18 | 6.4 | 5.4 | 0.54 | 0.179 | |
| MSG | |||||
| 1 | 5.7 | 6.0 | 0.46 | 0.616 | |
| 3 | 7.0 | 8.5 | 0.59 | 0.073 | |
| 9 | 5.6 | 7.0 | 0.58 | 0.090 | |
| 27 | 7.0 | 7.7 | 0.75 | 0.504 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa, J.; Valenzuela, C.; Guzmán-Pino, S.A. Sucrose Inclusion in Gestating and Lactating Diets of Sows Modifies the Feeding Behavior of Post-Weaning Pigs for Sweet Solutions. Vet. Sci. 2022, 9, 233. https://doi.org/10.3390/vetsci9050233
Figueroa J, Valenzuela C, Guzmán-Pino SA. Sucrose Inclusion in Gestating and Lactating Diets of Sows Modifies the Feeding Behavior of Post-Weaning Pigs for Sweet Solutions. Veterinary Sciences. 2022; 9(5):233. https://doi.org/10.3390/vetsci9050233
Chicago/Turabian StyleFigueroa, Jaime, Carolina Valenzuela, and Sergio A. Guzmán-Pino. 2022. "Sucrose Inclusion in Gestating and Lactating Diets of Sows Modifies the Feeding Behavior of Post-Weaning Pigs for Sweet Solutions" Veterinary Sciences 9, no. 5: 233. https://doi.org/10.3390/vetsci9050233
APA StyleFigueroa, J., Valenzuela, C., & Guzmán-Pino, S. A. (2022). Sucrose Inclusion in Gestating and Lactating Diets of Sows Modifies the Feeding Behavior of Post-Weaning Pigs for Sweet Solutions. Veterinary Sciences, 9(5), 233. https://doi.org/10.3390/vetsci9050233







