1. Introduction
Escherichia coli (
E. coli) is a common member of the gut microbiome in birds. However, several strains—known as avian pathogenic
Escherichia coli (APEC)—are implicated in avian colibacillosis, which has been described as the most common bacterial disease in poultry, having a serious economic impact on poultry production [
1,
2,
3,
4]. APEC is responsible for different systemic or localized infections [
5] such as colisepticemia (characterized by the presence of fibrinous exudates in various organs) [
6], respiratory infections and air sacculitis [
7], swollen head syndrome [
1], peritonitis/salpingitis/salpingoperitonitis [
4,
8], yolk sack infections in day-old chicks and skin infections (cellulitis) [
6,
9].
E. coli can be a primary pathogen, causing clinical disease; occasionally, other predisposing factors must be present to help
E. coli express its pathogenic effect. Various infectious agents have been described to be complicated with
E. coli. Many viruses, especially those with an effect on the respiratory system such as paramyxovirus (Newcastle disease) [
10], coronavirus (infectious bronchitis) [
11,
12], metapneumovirus (avian rhinotracheitis or swollen head syndrome) [
13,
14], orthomyxovirus(influenza) [
15,
16] and laryngotracheitis virus(infectious laryngotracheitis) [
17] can trigger avian colibacillosis. Viruses that can act in a similar way to immunosuppressive factors such as
herpesviruses (Marek disease) or
circovirus (chicken anemia virus) can stimulate colibacillosis just as many other viruses can also stimulate
E. coli infections [
1,
18]. Bacteria such as
Mycoplasma gallisepticum [
1] or
Mycoplasma synoviae [
19], or parasites such as
Eimeria or
Ascaridia (or red mites) can also predispose to an
E. coli infection [
1,
20].
The scope of this study was to investigate the serogroup prevalence of avian
E. coli strains isolated from birds demonstrating colibacillosis lesions, characterizing the presence and frequency of different O-somatic antigens, in Greece. As other studies worldwide have already considered with the broiler production, our project was focused on flocks from layer production. Most of the studies related to broilers have revealed the prevalent role of O78, O2 and O1 serogroups as APEC strains [
1,
2,
7,
8]. Furthermore, a high diversity of O-serogroups have also been isolated from broilers suffering from colibacillosis and the presence of untypeable
E. coli strains has also been established [
1,
2,
7].
As it has been reported that the use of
E. coli vaccines induces homologous immunity to the vaccine strain used, data on the
E. coli serotype status of an area can support colibacillosis vaccination prevention schemes [
21,
22]. Furthermore, we evaluated the possibility that other infectious agents such as
Mycoplasma gallisepticum, Mycoplasma synoviae, the infectious bronchitis virus and the infectious laryngotracheitis virus correlated with the presence of colibacillosis and increased mortality in hens [
23].
2. Materials and Methods
2.1. Sampling
A total of 60 different farms and 140 flocks, including birds with colibacillosis lesions from diverse geographical areas in Greece were included in the present study. All samples were collected during the period 2016–2017. A mortality threshold of 0.1% per week was used to divide the flocks between ‘normal’ or ‘increased’ mortality (ISA management guide) [
24]. A total of 231
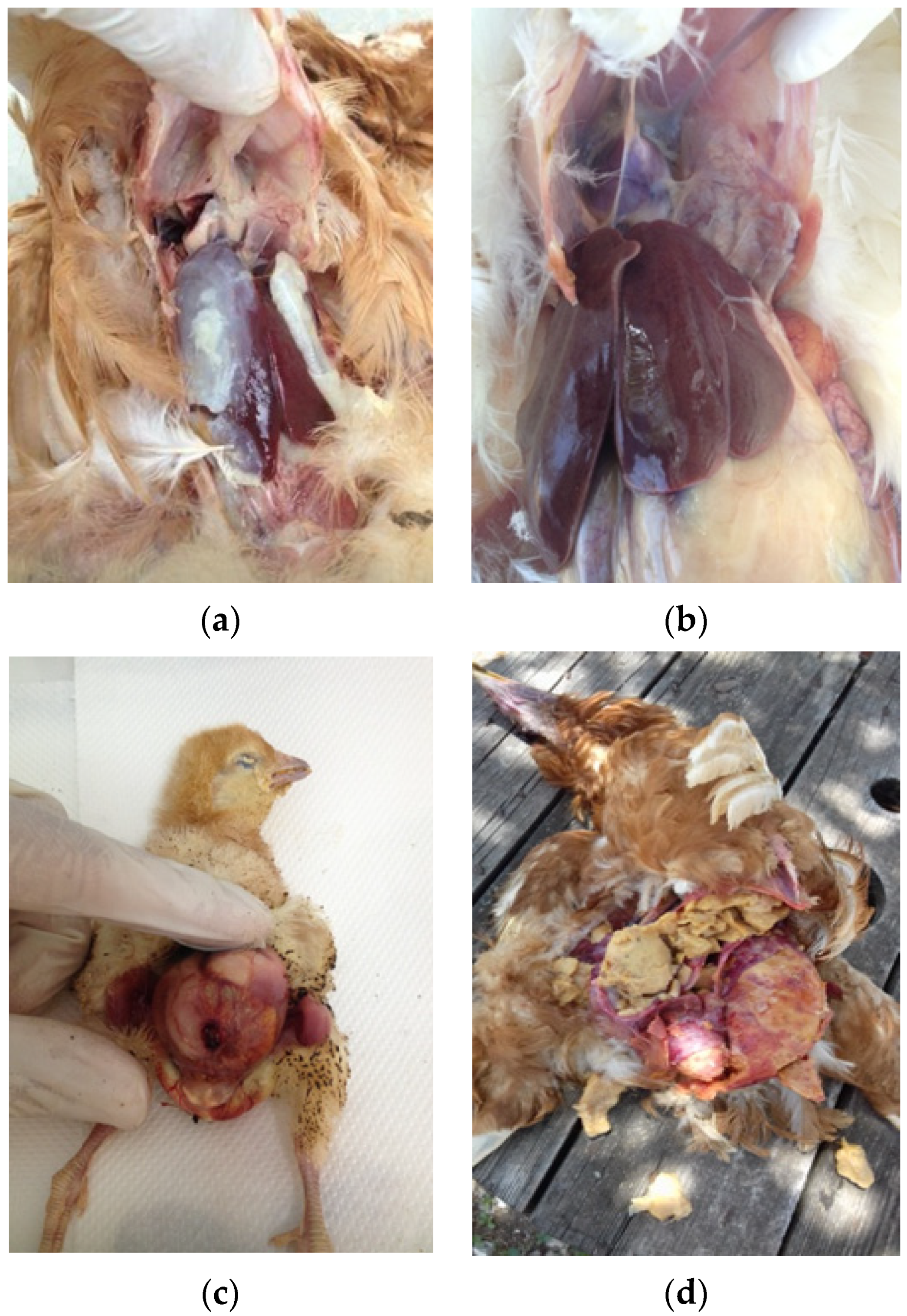
E. coli isolates were recovered from organ samples, including the liver, pericardium, air sacks, yolk sacks and peritoneum/ovaries, which were collected from rearing pullets, commercial layers and layer breeders. During each farm visit, we collected 3–5 dead birds per flock according to farm availability. All birds were necropsied and in case there was a demonstration of colibacillosis macroscopic lesions such as perihepatitis, pericarditis, air sacculitis, omphalitis and peritonitis (
Figure 1a–d), sampling was performed from the most apparent lesion.
2.2. Bacterial Isolation
Bacterial isolation was performed according to the following protocol. All samples were streaked on MacConkey agar (Bioprepare, Attica, Greece) and sheep blood agar (Bioprepare, Attica, Greece). Colonies that were phenotypically identified as Escherichia coli were further assessed for their biochemical properties by an analytical profile index (API) system. A total of 43 samples was evaluated by MICROGEN API GNA+B in a vet lab (Vet Analysis Lab, Athens, Greece) and, as a result, 24 samples were biochemically confirmed as E. coli and 19 needed further confirmation. Additionally, 207 samples, the 19 inconclusive and 188 new samples, were sent for an analysis to the Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna ‘Bruno Ubertini’, Italy. Biochemical confirmation was conducted using the same protocol (MICROGEN API GNABatch 41362, expanuary 2018) and, for all inconclusive samples, a further biochemical confirmation was conducted using API 20E (BioMerieux, Batch 1004845000, exp. 10 February 2017). In total, 231 samples were confirmed as E. coli.
2.3. Serogroup Characterization
A total of 207 samples biochemically identified as
E. coli in Italy were also serotyped by using a slow agglutination procedure in microtiter plates according to the Guinee agglutination method [
24]. This technique is based on the agglutination that occurs when an
E. coli culture is mixed with its homologous O antiserum [
25,
26].
The following agglutination protocol was used: a pure culture of E. coli isolates was obtained and it was used to prepare a bacterial suspension with a final concentration corresponding with a McFarland standard of 6.0. The suspension was treated at 100 °C for 1 h to obtain the final antigenic suspension. The heat treatment inactivated the K antigen. The bacterial suspension was then tested with a range of different antisera. Thirty different antisera that included the most common APEC serogroups (O1, O2, O5, O8, O9, O15, O18, O20, O22, O26, O45, O49, O55, O64, O78, O86, O88, O101, O103, O111, O113, O118, O128, O138, O139, O141, O147, O149, O153 and O157) were initially used for serotyping the E. coli strains. Each antiserum was diluted 1:40 and then inoculated in microtiter plates (U-bottom); a quantity of 100 µL of the antigenic suspension was then added in each well. The two suspensions were mixed and the plates were incubated at 37 °C for 18 h. After the incubation, the presence of agglutination was checked for positivity. Additionally, 24 samples that were confirmed in a laboratory (Vet Analysis Lab, Athens, Greece) as E. coli were sent for serotyping using agglutination testing to the Biovac laboratory (Biovac, Beaucouze, France).
2.4. Data Collection of Predisposing Factors
To assess the relation of other infectious agents with colibacillosis-increased mortality, the occurrence of other pathogens was investigated. Additionally, the following data were obtained: the results of serological testing through an ELISA, an applied vaccination program regarding these specific infectious agents and the presence of clinical symptoms indicating the disease. For the serological testing, 10 blood samples were collected from the flock at the same visit when the E. coli sampling took place. When possible, a second sampling after 3–4 weeks (pair samples) was performed. The flocks that were sampled for serological testing were randomly selected and organized according to the compliance of the farmer to allow for blood sampling. Blood samples from each flock were collected by awing branchial vein puncture. The samples were centrifuged at 4500 rpm for 10 min and the serum was collected.
2.5. Mycoplasma gallisepticum and Mycoplasma synoviae
None of the flocks sampled were vaccinated against either
Mycoplasma gallisepticum or
Mycoplasma synoviae. Therefore, the presence of positive antibody titers revealed an exposure to mycoplasma. The presence of 20% positive samples out of the 10 samples collected from each flock led to a characterization of an ‘infected’ flock (OIE terrestrial manual) [
27]. The serum was tested by the ELISA method (Zoetis/Proflok MG kit, lot 1,501,808 and Zoetis/Proflok MS kit, lot 1500907). When the samples were negative, a second sampling was performed within 3–6 weeks to check for seroconversion. Therefore, we managed to characterize the infection status of 61 different flocks.
2.6. Infectious Bronchitis
Concerning infectious bronchitis, all sampled flocks were vaccinated against both mass and variant strains during the rearing period. To characterize the flock IBV infection status, we considered the flock antibody titers in combination with the flock vaccination program and the presence of clinical symptoms that indicated an IBV infection.
In order to assess the antibody titers against IBV with the ELISA method (Zoetis/Proflok IBV kit, lot 132880), we collected blood samples from 51 flocks. For 37 flocks, we managed to perform a paired sampling at an interval of 3–5 weeks whereas for 14 flocks, a single sampling procedure was performed. For flocks that were pair sampled, seroconversion was checked. In cases where a single sampling was performed, the mean titer was compared according to the Zoetis IBV guidelines for infection. Very high titers that were not in accordance with the vaccination program used were considered to be suggestive of an infection.
2.7. Infectious Laryngotracheitis
Regarding infectious laryngotracheitis (ILT) testing, both vaccinated and unvaccinated flocks were included in our study. In order to perform a characterization of the flock infection status for ILT, we used the serological profile of the flocks performed with the ELISA method (Zoetis/Proflok ILT kit, lot 123146) in combination with the flock vaccination program and the presence of clinical symptoms that indicated an ILV infection. The presence of antibody titers in cases where no vaccination was applied combined with the presence of clinical symptoms/lesions indicated an infection. When a vaccination had been applied, a rise in the mean antibody titer along with the clinical appearance were taken into consideration. The procedure for collecting the blood samples was the same as for IBV. Therefore, we collected blood samples from 51 flocks. For 37 flocks, we used pair sampling whereas for 14 flocks, a single sampling procedure was followed.
2.8. Statistics
The percentages of each serotype in birds with high and low mortality and among the types of birds as well as the percentages of the serogrouped samples from each geographic area tested were compared using Med Calc software. The association between specific pathogens and increased mortality due to an E. coli infection was tested using a chi-squared test. For all the analyses, the statistical significance was set at p < 0.05.
4. Discussion
The presence of untypeable
E. coli isolates has been reported in previous studies [
28,
29,
30,
31,
32,
33,
34,
35,
36]. Approximately 180 O antigens are used today to characterize the strains in O-serogroups [
1]. Therefore, the number of strains that remain untypeable is related to the number of applied monospecific antisera. In the present study, a series of 30 antisera was used in two different labs. The use of a higher number of antisera might reduce the presence of untypeable strains. Nevertheless, in studies where all (181) antisera were applied for
E. coli characterization, several isolates still remained untyped [
37,
38,
39]. The reason for unsuccessful typing could be the presence of surface antigens such as the K antigen in the bacterial cell of
E. coli that inhibits the agglutination of the O antigen [
26]. Furthermore, it is not possible to serotype rough strains that can autoagglutinate and the presence of new serotypes that have not yet been identified remains a possibility [
1].
In our study,
E. coli of serogroups O78 and O2 showed that these were the most prevalent serogroups identified among the flocks with increased mortality in both pullets and layers, which was in agreement with previous studies [
29,
32,
33,
35,
36,
40,
41,
42,
43,
44,
45,
46,
47,
48], confirming their predominance in many parts of the world and in both broilers and layers with colisepticemia. Furthermore, a variety of
E. coli serogroups manifested between increased and normal mortality flocks, which has also been established in different research projects [
34,
41,
42,
49]. However, O78, O2 and O1 serogroups are not always the most prevalent serogroups of APEC strains in epidemiological studies. In contrast to our findings, various other serogroups have been reported to be predominant [
30,
39,
50,
51].
The presence of O111
E. coli strains in birds with colibacillosis has been previously demonstrated. Zanella et al. [
52] detected O111 isolates in layers with colibacillosis and polyserositis in Italy. Srinivasan et al. [
53] found that the O111, O166 and O64
E. coli serogroups were the most common ones in layers with egg peritonitis in India whereas another study in the U.S. also found O111 and O78 strains in layers with peritonitis [
54]. Furthermore, Giovanardi et al. [
55] managed to detect an
E. coli strain that was assigned to the O111 serogroup in a turkey suffering from colibacillosis and Khalifa et al. [
56] reported the isolation of O111
E. coli strains in one-week-old broiler chicks with omphalitis. Finally, Mora et al. [
57] revealed the presence of two emerging clonal serogroups of the O111 serogroup, emphasizing the increasing occurrence of this serogroup during recent years in Spain.
Other serogroups that were identified in our study have also been previously reported in studies of poultry suffering with colibacillosis such as O88 [
33,
46], O8 [
46,
50], O1 [
40,
43,
44,
47,
48], O18 [
31,
43,
47,
48], O45 [
46,
48], O103 [
31], O5 [
30,
42], O15 [
30,
35] and O147 [
42]. Several serogroups were exclusively present in the increased mortality flocks (O78, O111, O18, O1 and O103) or within the normal mortality group (O147, O15 and O5) where as several serogroups were detected in both groups of birds (O2, O88, O8 and O45). These findings are in agreement with several research projects where common O-serogroups were isolated both from birds with normal mortality and birds with clinical colibacillosis [
34,
37,
43]. Furthermore, Rodriguez-Siek et al. [
31] attempted to characterize and compare APEC and fecal strains, reporting that several isolates from each category were assigned to unshared serogroups; however, common serogroups were detected in both the APEC and fecal strains. To summarize our findings with relevant studies conducted at broiler farms, the high prevalence of the O78 and O2 groups was in accordance with similar references from research projects performed on broiler poultry. The presence of less common serogroups such as O111 [
56,
57] has also been reported in broilers similar to various other serogroups [
32,
34,
37,
42] or untypeable strains [
28,
32,
34].
The pathogenicity of
E. coli strains is attributed to different virulence factors. There is a huge diversity in the virulence factors of APEC strains. Virulence factors are divided into adhesins, iron acquisition systems, invasions, toxins and protectins [
58] and although it seems that there is no specific APEC genotype for all strains, it has been reported that certain virulence factor patterns are more likely to be detected in APEC strains compared with non-pathogenic
E. coli strains. As a result, many different serogroups might include pathogenic strains [
7,
40,
59] and strains that belong to the same serogroup could differ in pathogenicity [
60]. Several serogroups such as O78 and O2 have been found to include strains that more often contain certain virulence patterns andthus have a pathogenic action. This explains why those serogroups are more prevalent in studies investigating APEC serogroups. The presence of an increased number of virulence factors or of certain virulence factor patterns that are responsible for
E. coli pathogenicity in serogroups O78 and O2 has been reported in various research projects [
31,
40,
43,
61,
62]. Similar findings have been reported for pathogenic
E. coli strains that belong to the O111 serogroup. Yaguchi et al. [
34] revealed that O111 isolated strains were homogenous regarding their virulence gene pattern and consistent with the important virulence factors of APEC strains. It seems that the virulence factor characterization might contribute to the identification of APEC strains.
Finally, in the present study, we observed a different serogroup occurrence trait of two serogroups (O78, O2) between the two age-dependent groups of birds (rearing and laying period). The O78 serogroup was mainly found in young birds in comparison with the O2 serogroup that was detected only in the laying flocks. On the contrary, Dias da Silveira et al. [
30] observed no differences in the presence of
E. coli serogroups of isolates between day-old broiler chicks and adult broilers with colibacillosis. Similar findings of shared serogroups between different aged groups of birds were reported by Paudel et al. [
63].
Our attempt to find an association between Mycoplasma and colibacillosis revealed a high prevalence of both Mycoplasma gallisepticum and Mycoplasma synoviae infections in the layers. However, no statistically significant difference was recorded between Mycoplasma gallisepticum infections and increased mortality (p = 0.66) due to colibacillosis. Similar findings were observed for Mycoplasma synoviae infections (p = 0.78).
The data for
mycoplasma prevalence in poultry vary from country to country. Nevertheless,
Mycoplasma synoviae infections in layers seem to be high in many countries (Germany: 95%; U.S.: 84%; UK: 78%) [
64]. A Dutch survey reported that the surveillance program for
Mycoplasma synoviae infections in commercial layer flocks revealed 73% positive flocks [
65]. A Belgian study in commercial layers with
E. coli infections revealed a high level of bird flock infections from
Mycoplasma synoviae whereas none of the flocks were infected by
Mycoplasma gallisepticum [
41].
Our findings were in accordance with several other research projects that revealed no significant interaction between
Mycoplasma gallisepticum/synoviae infections and colibacillosis outbreaks [
41] or revealed that colibacillosis can occur in mycoplasma-free birds [
66]. On the other hand, several studies have established the predisposing role of
Mycoplasma [
19,
67,
68].
Mycoplasma gallisepticum or
synoviae infections may play a role in stimulating colibacillosis mortality. However, bird exposure to
mycoplasmas is not necessarily followed by a colibacillosis outbreak because in our study, both MG and MS showed a high prevalence in the normal mortality flocks. This trait couldbe explained by the fact that
mycoplasma can infect poultry and remain in a latent state, waiting for the appropriate combination of infectious agents or environmental factors to cause clinical disease [
27]. Furthermore, the role of MS in respiratory infections is not always pronounced.
Concerning the role of IBV and ILT, no statistically significant difference was observed between the flocks with normal and increased E. coli mortality. However, it seemed that the flocks that were possibly infected with IBV tended to have a higher risk of mortality related to an E. coli infection because the six flocks suspected for an IBV infection had increased E. coli mortality. Similar findings were reported for ILT where the occurrence of infection was higher in the increased E. coli mortality groups. This could be explained by the pathogenic action of IBV and ILT damaging the respiratory epithelium and thus facilitating the establishment of the E. coli infection.
In contrast to our findings, the relationship of IBV and
E. coli has been reported in various research projects [
11,
69,
70,
71] as well as the association between ILT infections and colibacillosis [
17,
72]. However, our data about IBV and
E. coli infections were in agreement with [
41], who reported no significant relationship between those two agents.