Comparison of Analytical Values after Changing to the International Standardized Method for Lactate Dehydrogenase and Alkaline Phosphatase Measurements in Mouse and Rat
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analysis of Serum Lactate Dehydrogenase and Alkaline Phosphatase Activity
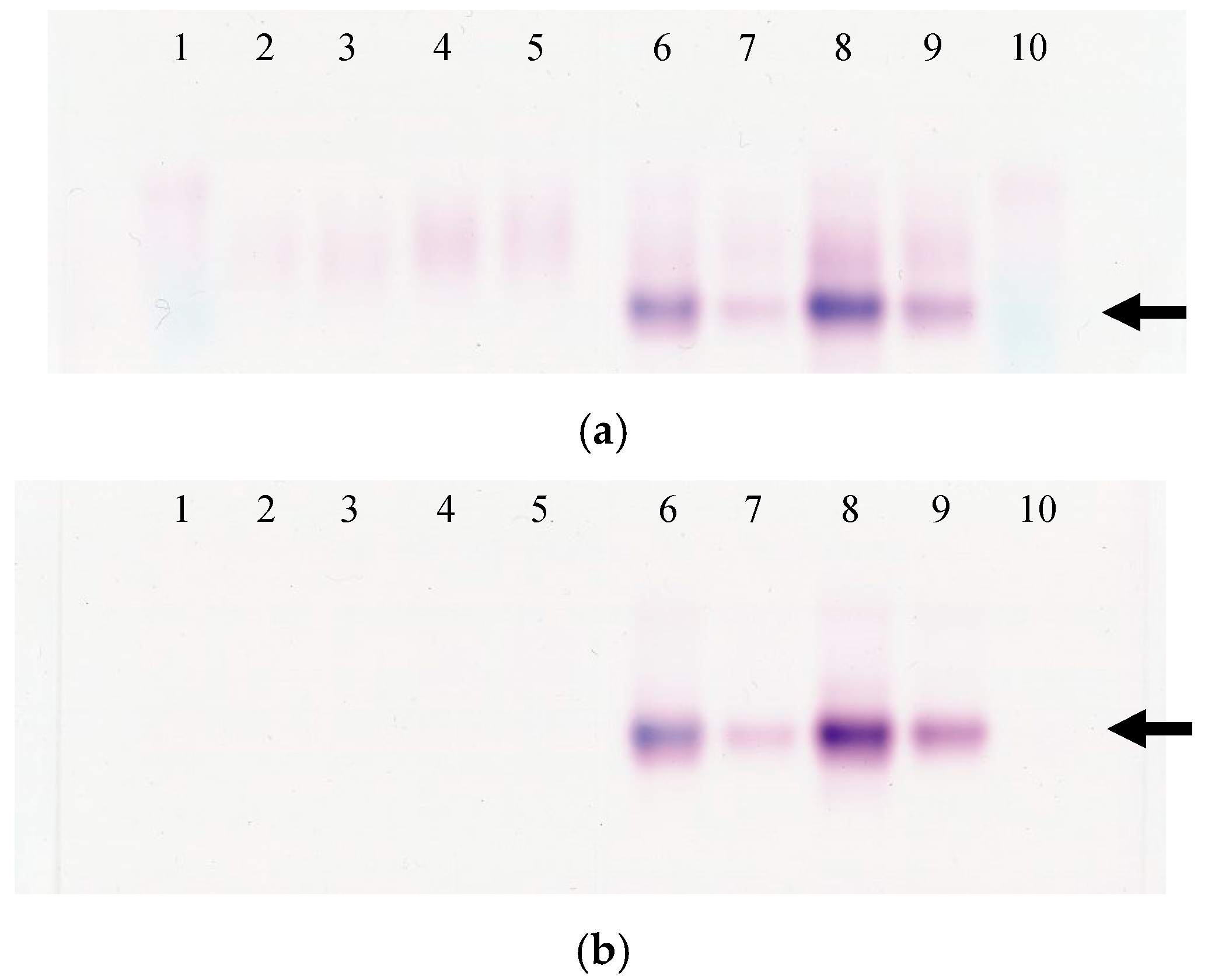
2.3. Analysis of Alkaline Phosphatase Isozyme
2.4. Statistical Analysis
3. Results
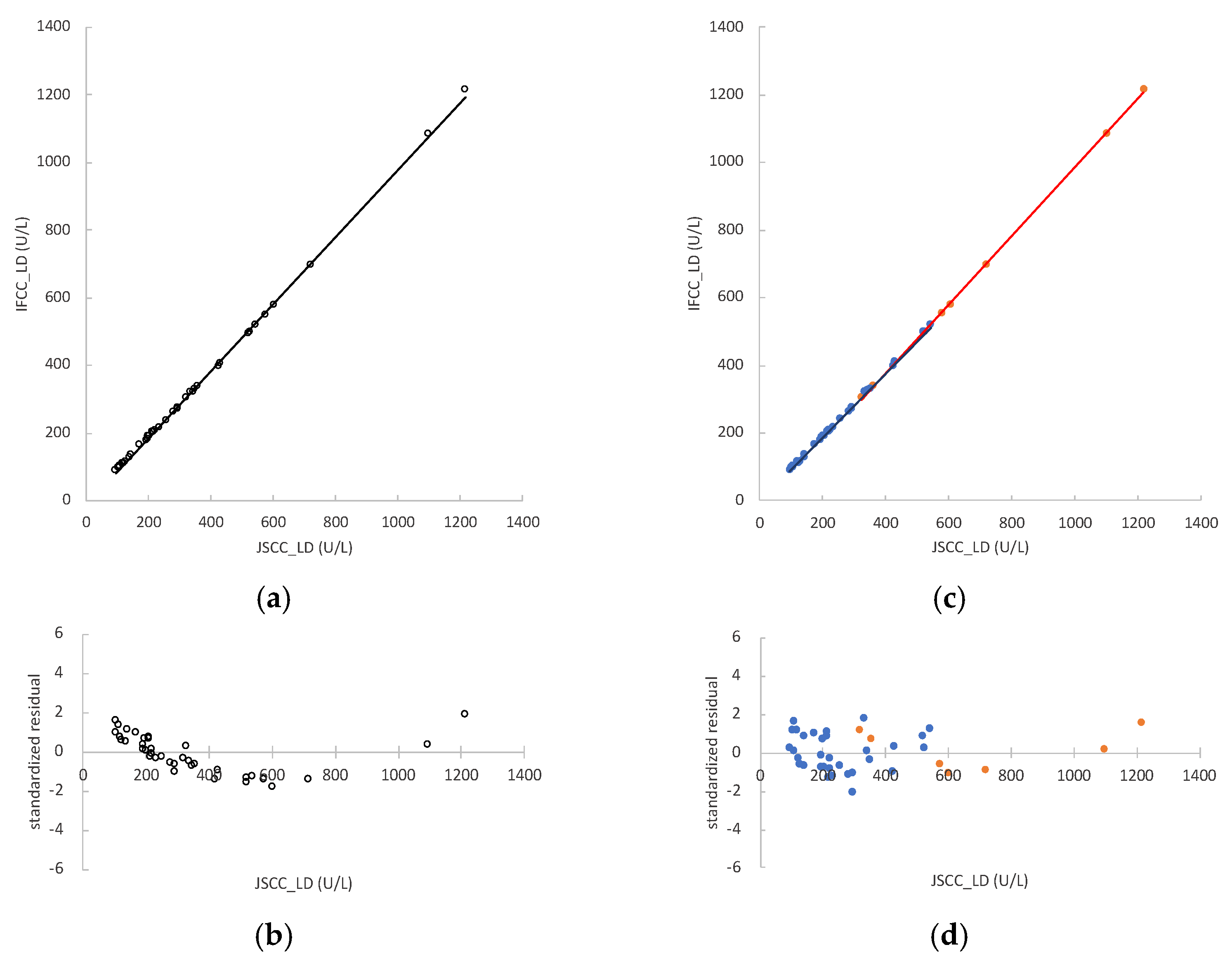
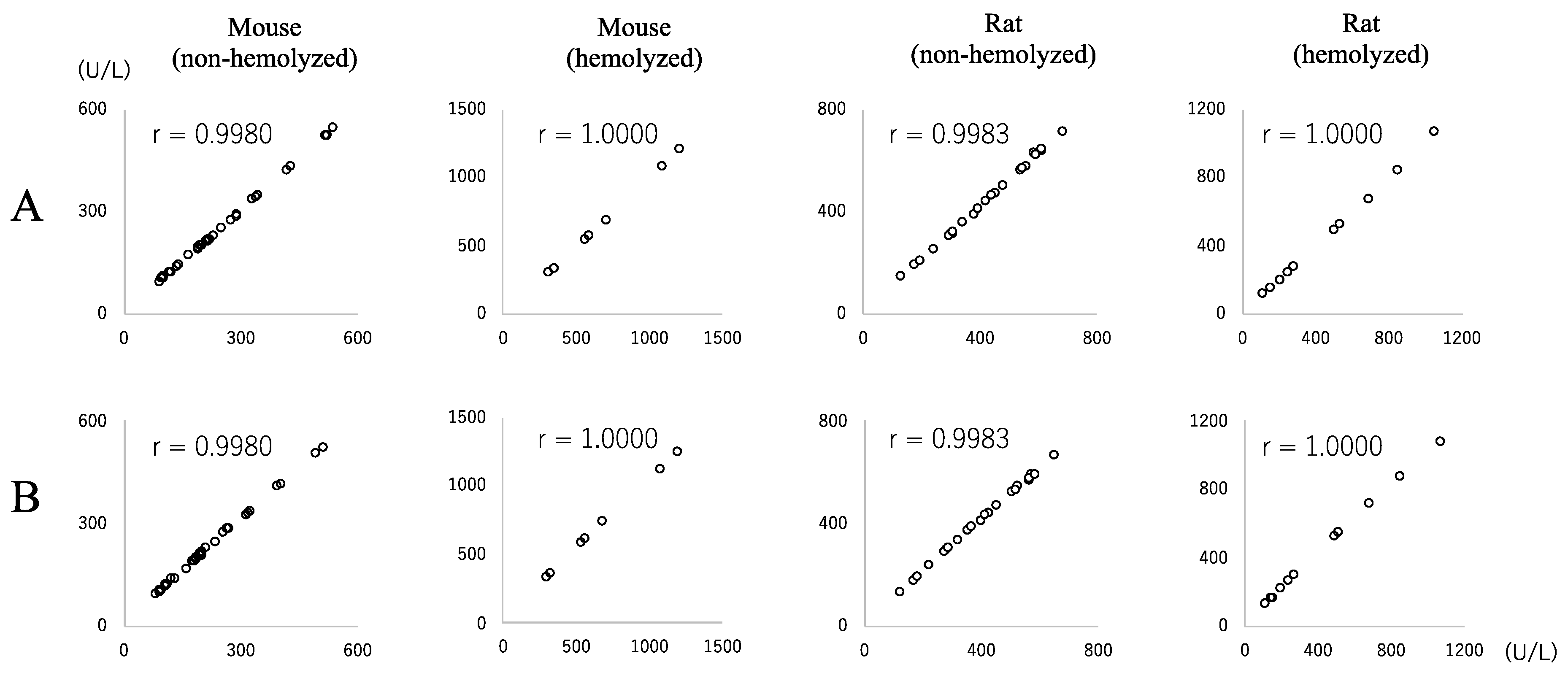
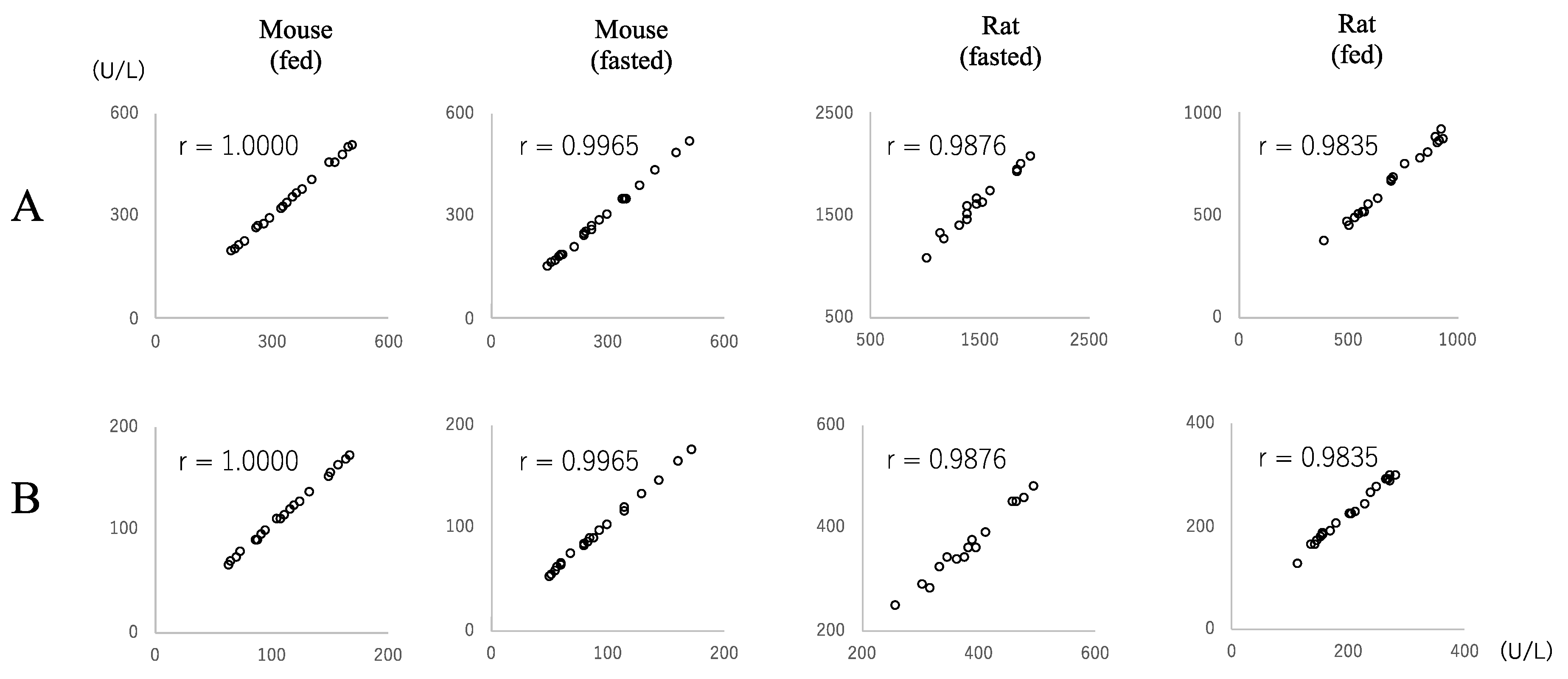
3.1. LD Activity in Mouse Samples and Regression Analyses between JSCC and IFCC Methods
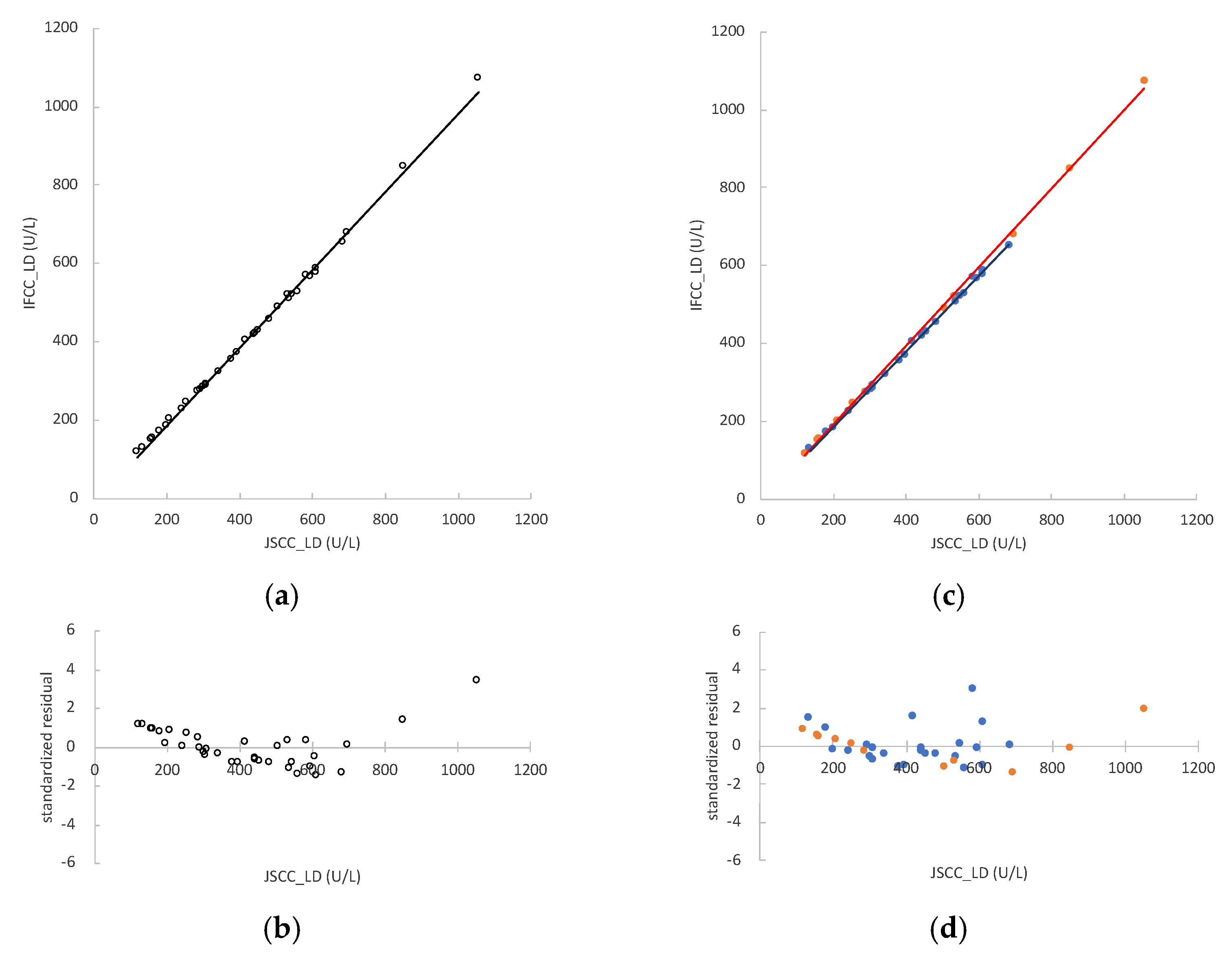
3.2. LD Activity in Rat Samples and Regression Analyses between JSCC and IFCC Methods
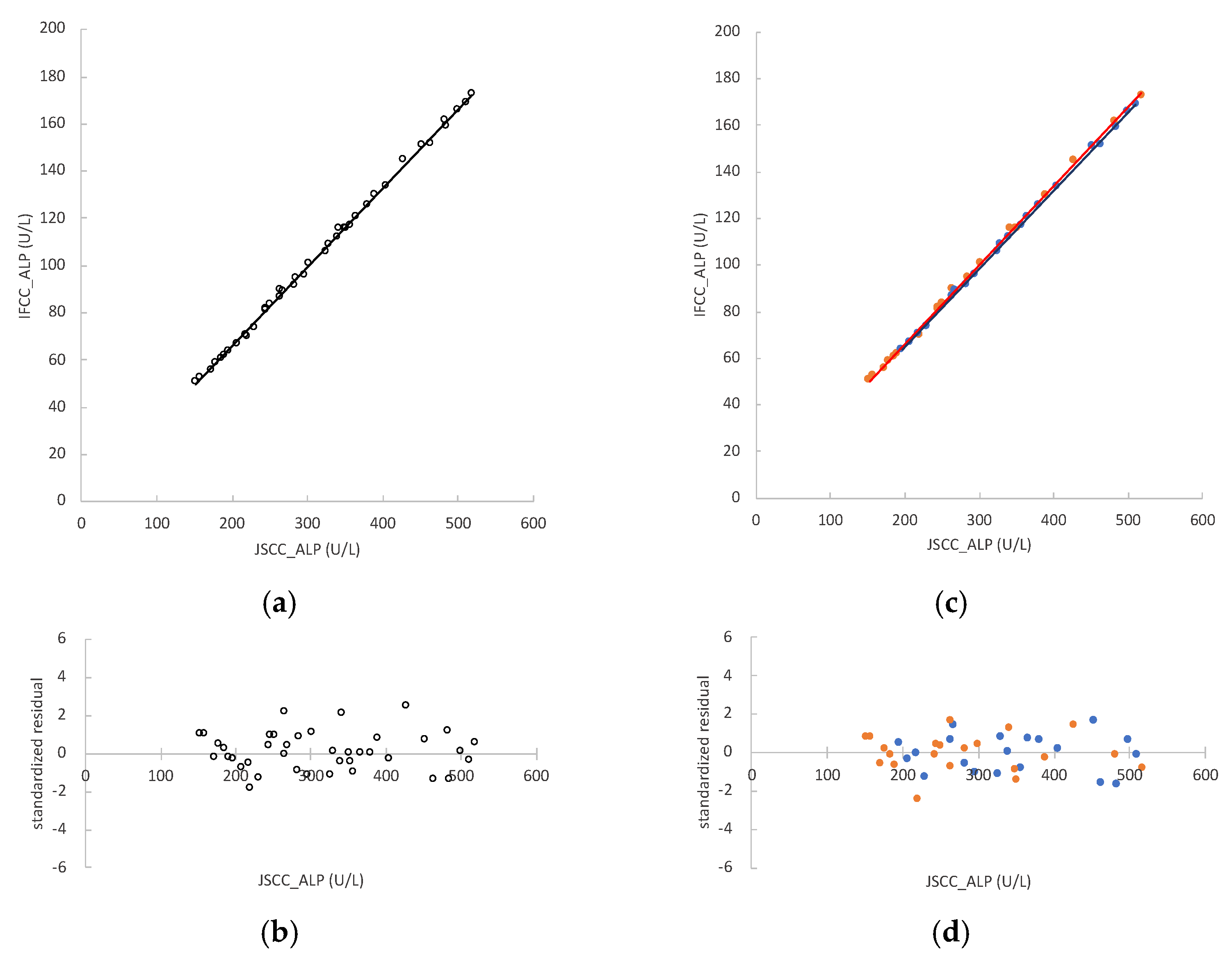
3.3. ALP Activity in Mouse Samples and Regression Analyses between the JSCC and IFCC Methods
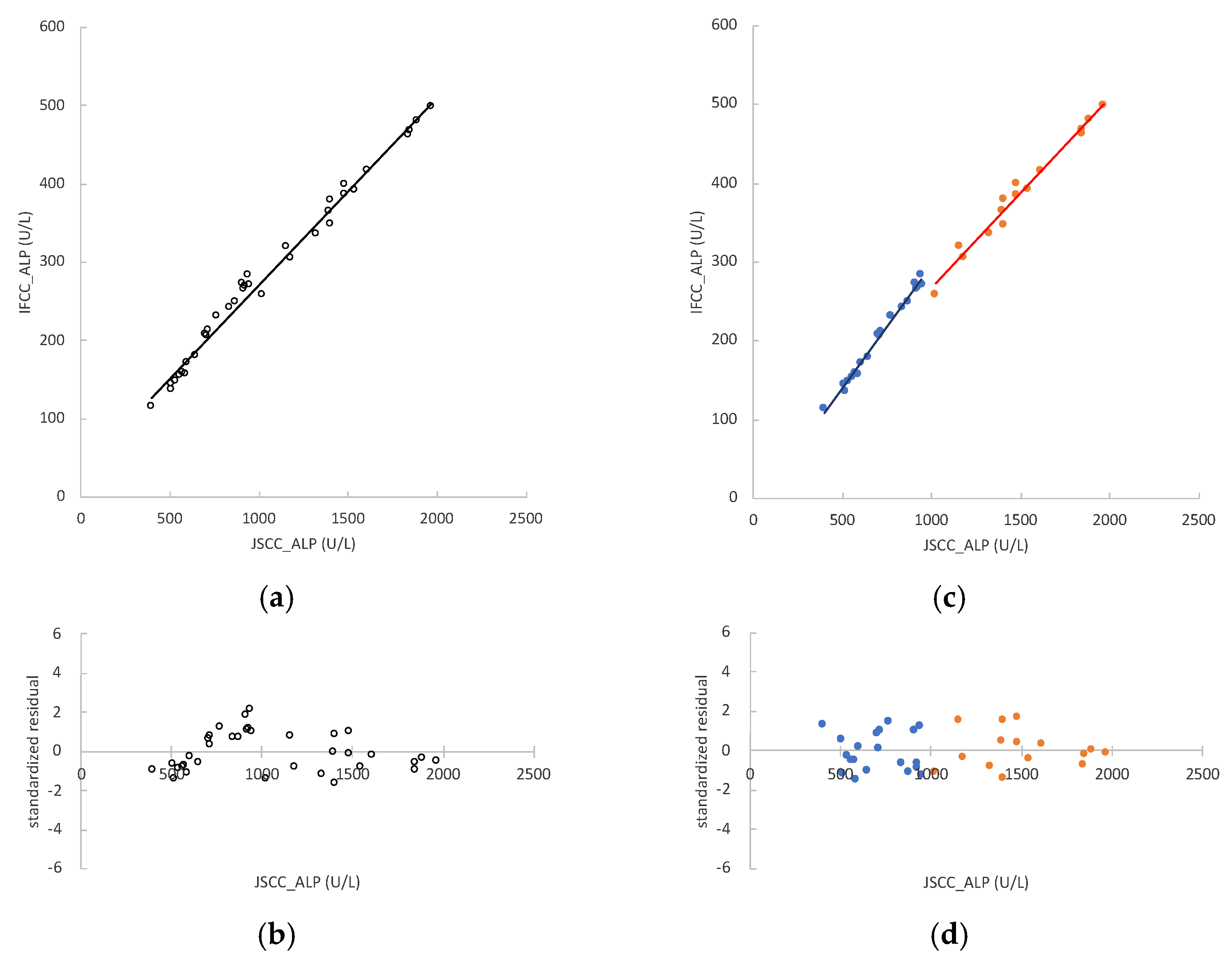
3.4. ALP Activity in Rat Samples and Regression Analyses between JSCC and IFCC Methods
3.5. Analysis of ALP Isozyme in Mouse and Rat Serum
3.6. Conversion Factor between JSCC and IFCC Methods for Measuring LD Values
3.7. Conversion Factor between JSCC and IFCC Methods for Measuring ALP Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, M.J.; Buehner, M.; Chandrasekhar, K.; Ford, G.C.; Hackert, M.L.; Liljas, A.; Rossmann, M.G.; Smiley, I.E.; Allison, W.S.; Everse, J.; et al. Structure function relationships in lactate dehydrogenase. Proc. Natl. Acad. Sci. USA 1973, 70, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Don, D.; Nathan, O.K. D-and L-lactic acid dehydrogenases in Lactobacillus plantarum. J. Biol. Chem. 1960, 235, 810–818. [Google Scholar] [CrossRef]
- Storey, K.B. Comparative enzymology—New insights from studies of an “old” enzyme, lactate dehydrogenase. Comp. Biochem. Physiol. Part B 2016, 199, 13–20. [Google Scholar] [CrossRef]
- Huijgen, H.J.; Sanders, G.T.B.; Koster, R.W.; Vreeken, J.; Bossuyt, P.M.M. The Clinical Value of Lactate Dehydrogenase in Serum: A Quantitative Review. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Karp, J.E.; Emadi, A. Elevated lactate dehydrogenase (LDH) can be a marker of immune suppression in cancer: Interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer Biomark. 2017, 19, 353–363. [Google Scholar] [CrossRef]
- Klein, R.; Nagy, O.; Tóthová, C.; Chovanová, F. Clinical and Diagnostic Significance of Lactate Dehydrogenase and Its Isoenzymes in Animals. Vet. Med. Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Preus, M.; Karsten, B.; Bhargava, A.S. Serum Isoenzyme Pattern of Creatine Kinase and Lactate Dehydrogenase in Various Animal Species. J. Clin. Chem. Clin. Biochem. 1989, 27, 787–790. [Google Scholar] [CrossRef]
- Yasuda, J.; Tateyama, K.; Syuto, B.; Too, K. Lactate dehydrogenase and creatine phosphokinase isoenzymes in tissues of laboratory animals. Jpn. J. Vet. Res. 1990, 38, 19–29. [Google Scholar]
- Heinová, D.; Blahovec, J.; Rosival, I. Lactate Dehydrogenase Isoenzyme Patterns in Bird, Carp and Mammalian Sera. Clin. Chem. Lab. Med. 1996, 34, 91–96. [Google Scholar] [CrossRef][Green Version]
- Crofton, P.M. Biochemistry of alkaline phosphatase isoenzymes. Crit. Rev. Clin. Lab. Sci. 1982, 16, 161–194. [Google Scholar] [CrossRef]
- Meyer-Sabellek, W.; Sinha, P.; Köttgen, E. Alkaline phosphatase. Laboratory and clinical implications. J. Chromatogr. 1988, 429, 419–444. [Google Scholar] [CrossRef]
- Allen, M.J. Biochemical markers of bone metabolism in animals: Uses and limitations. Vet. Clin. Pathol. 2003, 32, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Price, C.P. Multiple forms of human serum alkaline phosphatase: Detection and quantitation. Ann. Clin. Biochem. 1993, 30, 355–372. [Google Scholar] [CrossRef]
- Linder, C.H.; Englund, U.H.; Narisawa, S.; Millán, J.L.; Magnusson, P. Isozyme profile and tissue-origin of alkaline phosphatases in mouse serum. Bone 2013, 53, 399–408. [Google Scholar] [CrossRef]
- Hatayama, K.; Nishihara, Y.; Kimura, S.; Goto, K.; Nakamura, D.; Wakita, A.; Urasoko, Y. Alkaline phosphatase isoenzymes in mouse plasma detected by polyacrylamide-gel disk electrophoresis. J. Toxicol. Sci. 2011, 36, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.E.; Everds, N.; Pignatello, M.; Solter, P.F. Automated and semiautomated analysis of rat alkaline phosphatase isoenzymes. Toxicol. Pathol. 1994, 22, 633–638. [Google Scholar] [CrossRef]
- Japan Society of Clinical Chemistry. Japan Society of Clinical Chemistry JSCC Document: Recommendation for measuring enzyme activity in human serum (LD). Jpn J. Clin. Chem 2003, 32, 86–97. [Google Scholar]
- Schumann, G.; Bonora, R.; Ceriotti, F.; Clerc-Renaud, P.; Ferrero, C.A.; Férard, G.; Franck, P.F.H.; Gella, F.-J.; Hoelzel, W.; Jørgensen, P.J.; et al. IFCC Primary Reference Procedures for the Measurement of Catalytic Activity Concentrations of Enzymes at 37 °C. Part 3. Reference Procedure for the Measurement of Catalytic Concentration of Lactate Dehydrogenase. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Scientific Division, Committee on Reference Systems of Enzymes (C-RSE). Clin. Chem. Lab. Med. 2002, 40, 643–648. [Google Scholar] [CrossRef]
- Japan Society of Clinical Chemistry Document: Recommendation for measuring enzyme activity in human serum (ALP). Japan Soc. Clin. Chem. 1990, 19, 209–227.
- Schumann, G.; Klauke, R.; Canalias, F.; Bossert-Reuther, S.; Franck, P.F.H.; Gella, F.J.; Jørgensen, P.J.; Kang, D.; Lessinger, J.M.; Panteghini, M.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 9: Reference procedure for the measurement of catalytic concentration of alkaline phosphatase. International Federation of Clinical Chemistr. Clin. Chem. Lab. Med. 2011, 49, 1439–1446. [Google Scholar] [CrossRef]
- Hata, A.; Fujitani, N.; Takeshita, M.; Tanaka, C.; Matsuda, N.; Takaishi, M.; Miyama, T.S.; Hoshi, F. Comparison of regression for blood ALP levels using methods of the Japan Society of Clinical Chemistry and the International Federation of Clinical Chemistry and Laboratory Medicine in bovine, canine, feline, and human testing. PLoS ONE 2021, 16, e0253396. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, H. Alkaline phosphatase. I. Kinetics and inhibition by levamisole of purified isoenzymes from humans. Am. Assoc. Clin. Chem. 1976, 22, 972–976. [Google Scholar] [CrossRef]
- Japan Society of Clinical Chemistry Guidance on Changing ALP/LD Measurement Method (for Medical Professionals). ver. 1.0 (21 November 2019) (In Japanese). Available online: http://jscc-jp.gr.jp/file/2019/alpld2.pdf (accessed on 25 April 2021).
- Fountain, J.A.; Parks, M.E.; Dickey, A.; McKee, R.W. Lactate Dehydrogenase Isoenzymes in Tissues of Normal and Ehrlich-Lettré Ascites Tumor-bearing Swiss Mice. Cancer Res. 1970, 30, 998–1002. [Google Scholar]
- Tateyama, K. Lactate dehydrogenase and creatine phosphokinase isozymes in tissues and sera of experimental animals. Jpn J. Vet. Res. 1989, 37, 136. [Google Scholar]
- Heinová, D.; Kostecká, Z.; Petrovová, E. Chapter 5, Lactate Dehydrogenase Isoenzyme Electrophoretic Pattern in Serum and Tissues of Mammalian and Bird Origin. In Electrophoresis-Life Sciences Practical Applications; Boldura, O.M., Balta, C., Eds.; Intech Open: London, UK, 2018; pp. 81–90. [Google Scholar]
- Harris, H. The human alkaline phosphatases: What we know and what we don’t know. Clin. Chim. Acta 1990, 186, 133–150. [Google Scholar] [CrossRef]
- Matsushita, M.; Irino, T.; Stigbrand, T.; Nakajima, T.; Komoda, T. Changes in intestinal alkaline phosphatase isoforms in healthy subjects bearing the blood group secretor and non-secretor. Clin. Chim. Acta 1998, 277, 13–24. [Google Scholar] [CrossRef]
- Menahan, L.A.; Sobocinski, K.A.; Peter Austin, B. The origin of plasma alkaline phosphatase activity in mice and rats. Comp. Biochem. Physiol. Part B Biochem. 1984, 79, 279–283. [Google Scholar] [CrossRef]
- Rightti, A.B.-B.; Kaplan, M.M. The origin of the serum alkaline phosphatase in nomal rats. Biochim. Biophys Acta 1971, 230, 504–509. [Google Scholar]
- Hirata, M.; Nomura, M.; Yamada, M. Influence factors to be considered on establishment of the reference data of ALT, AST and ALP in rats. Rinsho Kagaku 2001, 30, 142–152. (In Japanese) [Google Scholar]
- Neafsey, P.J.; Schwartz, R. Serum and duodenal alkaline phosphatase levels in fed and fasted magnesium deficient rats. J. Nutr. 1977, 107, 1061–1067. [Google Scholar] [CrossRef]
| Sample | Fed Animals | Fasted Animals | Total | |
|---|---|---|---|---|
| Mouse | Male | 10 | 10 | 20 |
| Female | 11 | 10 | 21 | |
| Rat | Male | 8 | 10 | 18 |
| Female | 7 | 10 | 17 | |
| Total | 36 | 40 | 76 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furumoto, K.; Fujitani, N.; Nohara, M.; Hata, A. Comparison of Analytical Values after Changing to the International Standardized Method for Lactate Dehydrogenase and Alkaline Phosphatase Measurements in Mouse and Rat. Vet. Sci. 2022, 9, 595. https://doi.org/10.3390/vetsci9110595
Furumoto K, Fujitani N, Nohara M, Hata A. Comparison of Analytical Values after Changing to the International Standardized Method for Lactate Dehydrogenase and Alkaline Phosphatase Measurements in Mouse and Rat. Veterinary Sciences. 2022; 9(11):595. https://doi.org/10.3390/vetsci9110595
Chicago/Turabian StyleFurumoto, Kayo, Noboru Fujitani, Masakatsu Nohara, and Akihisa Hata. 2022. "Comparison of Analytical Values after Changing to the International Standardized Method for Lactate Dehydrogenase and Alkaline Phosphatase Measurements in Mouse and Rat" Veterinary Sciences 9, no. 11: 595. https://doi.org/10.3390/vetsci9110595
APA StyleFurumoto, K., Fujitani, N., Nohara, M., & Hata, A. (2022). Comparison of Analytical Values after Changing to the International Standardized Method for Lactate Dehydrogenase and Alkaline Phosphatase Measurements in Mouse and Rat. Veterinary Sciences, 9(11), 595. https://doi.org/10.3390/vetsci9110595






