Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bird Collections and Fecal Samplings
2.2. Coprological Techniques
2.2.1. Mini-FLOTAC
2.2.2. McMaster
2.2.3. Fecal Cultures
2.3. Statistical Analysis
3. Results
3.1. Epidemiological Results with Mini-FLOTAC in Domestic and Exotic Birds
3.2. McMaster and Mini-FLOTAC Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papini, R.; Girivetto, M.; Marangi, M.; Mancianti, F.; Giangaspero, A. Endoparasite infections in pet and zoo birds in Italy. Sci. World J. 2012, 2012, 253127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ederli, N.B.; Rodrigues de Oliveira, F.C. Gastrointestinal nematodes in ostriches, Struthio camelus, in different regions of the state of Rio de Janeiro, Brazil. Braz. J. Vet. Parasitol. 2015, 24, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Lozano, J.; Anaya, A.; Palomero Salinero, A.; Lux Hoppe, E.G.; Gomes, L.; Paz-Silva, A.; Teresa Rebelo, M.; Madeira de Carvalho, L. Gastrointestinal parasites of free-range chickens—A worldwide issue. Bull. UASVM Vet. Med. 2019, 76, 110–117. [Google Scholar] [CrossRef]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Carrera-Játiva, P.D.; Morgan, E.R.; Barrows, M.; Jiménez-Uzcátegui, G.; Tituaña, J.R.A. Free-ranging avifauna as a source of generalist parasites for captive birds in zoological settings: An overview of parasite records and potential for cross-transmission. J. Adv. Vet. Anim. Res. 2020, 7, 482–500. [Google Scholar] [CrossRef]
- Carrisosa, M.; Jin, S.; McCrea, B.A.; Macklin, K.S.; Dormitorio, T.; Hauck, R. Prevalence of select intestinal parasites in Alabama backyard poultry flocks. Animals 2021, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Titilincu, A.; Mircean, V.; Bejan, A.; Iovu, A.; Ungureanu, R.; Cozma, V. Prevalence of endoparasites in peacocks (Pavo cristatus). Sci. Parasitol. 2009, 10, 101–105. [Google Scholar]
- Jaiswal, A.K.; Sudan, V.; Shanker, D.; Kumar, P. Endoparasitic infections in Indian peacocks (Pavo cristatus) of Veterinary College Campus, Mathura. J. Parasit. Dis. 2013, 37, 26–28. [Google Scholar] [CrossRef] [Green Version]
- Prakashbabu, B.C.; Thenmozhi, V.; Limon, G.; Kundu, K.; Kumar, S.; Garg, R.; Clark, E.L.; Srinivasa Rao, A.S.R.; Raj, D.G.; Raman, M.; et al. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet. Parasitol. 2017, 233, 62–72. [Google Scholar] [CrossRef]
- Lolli, S.; Grilli, G.; Ferrari, L.; Ferrari, P.; Ferrante, V. Effect of range use on endo- and ectoparasite infestation in italian organic egg production. Ital. J. Anim. Sci. 2019, 18, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Ilić, T.; Becskei, Z.; Gajić, B.; Özvegy, J.; Stepanović, P.; Nenadović, K.; Dimitrijević, S. Prevalence of endoparasitic infections of birds in zoo gardens in Serbia. Acta Parasitol. 2018, 63, 134–146. [Google Scholar] [CrossRef]
- Jansson, D.S.; Christensson, D. Gastrointestinala parasiter hos strutsfåglar i Sverige. Sven. Vet. Tidn. 2000, 52, 621–626. [Google Scholar]
- Ponce Gordo, F.; Herrera, S.; Castro, A.T.; García Durán, B.; Martínez Díaz, R.A. Parasites from farmed ostriches (Struthio camelus) and rheas (Rhea americana) in Europe. Vet. Parasitol. 2002, 107, 137–160. [Google Scholar] [CrossRef]
- McKenna, P.B. Libyostrongylus infections in ostriches—A brief review with particular reference to their detection in New Zealand. N. Z. Vet. J. 2005, 53, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Kummrow, M.S. Ratites or Struthioniformes: Struthiones, Rheae, Cassuarii, Apteryges (Ostriches, Rheas, Emus, Cassowaries, and Kiwis), and Tinamiformes (Tinamous). In Fowler’s Zoo and Wild Animal Medicine; Eric Miller, R., Fowler, M.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 8, pp. 75–82. [Google Scholar]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef]
- Capasso, M.; Maurelli, M.P.; Ianniello, D.; Alves, L.C.; Amadesi, A.; Laricchiuta, P.; Silvestre, P.; Campolo, M.; Cringoli, G.; Rinaldi, L. Use of Mini-FLOTAC and Fill-FLOTAC for rapidly diagnosing parasitic infections in zoo mammals. Braz. J. Vet. Parasitol. 2019, 28, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Maurelli, M.P.; Martins, O.M.; Morgan, E.R.; Charlier, J.; Cringoli, G.; Mateus, T.L.; Bacescu, B.; Chartier, C.; Claerebout, E.; De Waal, T.; et al. A Qualitative Market Analysis Applied to Mini-FLOTAC and Fill-FLOTAC for Diagnosis of Helminth Infections in Ruminants. Front. Vet. Sci. 2020, 7, 580649. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, C.; Paras, K.L.; Applegate, T.J.; Verocai, G.G. Comparison between McMaster and Mini-FLOTAC methods for the enumeration of Eimeria maxima oocysts in poultry excreta. Vet. Parasitol. 2018, 254, 21–25. [Google Scholar] [CrossRef]
- Coker, S.M.; Pomroy, W.E.; Howe, L.; McInnes, K.; Vallee, E.; Morgan, K.J. Comparing the Mini-FLOTAC and centrifugal faecal flotation for the detection of coccidia (Eimeria spp.) in kiwi (Apteryx mantelli). Parasitol. Res. 2020, 119, 4287–4290. [Google Scholar] [CrossRef]
- Daş, G.; Klauser, S.; Stehr, M.; Tuchscherer, A.; Metges, C.C. Accuracy and precision of McMaster and Mini-FLOTAC egg counting techniques using egg-spiked faeces of chickens and two different flotation fluids. Vet. Parasitol. 2020, 283, 109158. [Google Scholar] [CrossRef]
- Lozano, J.; Anaya, A.; Rinaldi, L.; Cringoli, G.; Gomes, L.; Oliveira, M.; Paz-Silva, A.; Teresa Rebelo, M.; Madeira de Carvalho, L. Diagnosis of coccidiosis by Eimeria spp. in free-range chickens using Mini-FLOTAC and McMaster techniques—Preliminary results. Sci. Parasitol. 2021, 22, 13–18. [Google Scholar]
- Zajac, A.M.; Conboy, G.A. Fecal Exam Procedures. In Veterinary Clinical Parasitology, 8th ed.; Zajac, A.M., Conboy, G.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 4–15. [Google Scholar]
- Gordon, H.; Whitlock, H.V. A new technique for counting nematode eggs in sheep faeces. J. Coun. Sci. Industr. Res. 1939, 12, 50–52. [Google Scholar]
- Cringoli, G.; Rinaldi, L.; Maurelli, M.P.; Utzinger, J. FLOTAC: New multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010, 5, 503–515. [Google Scholar] [CrossRef]
- Ederli, N.B.; Bonadiman, S.F.; Almeida de Moraes Neto, A.H.; DaMatta, R.A.; Santos, C.P. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet. Parasitol. 2008, 151, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Ederli, N.B.; Rodrigues de Oliveira, F.C. Comparative morphology of the species of Libyostrongylus and Codiostomum, parasites from ostriches, Struthio camelus, with a identification key to the species. Braz. J. Vet. Parasitol. 2014, 23, 291–300. [Google Scholar] [CrossRef] [Green Version]
- López-Osorio, S.; Chaparro-Gutiérrez, J.J.; Gómez-Osorio, L.M. Overview of poultry Eimeria life cycle and host-parasite interactions. Front. Vet. Sci. 2020, 7, 384. [Google Scholar] [CrossRef]
- Shamim, A.; Hassan, M.U.; Yousaf, A.; Iqbal, M.F.; Zafar, M.A.; Siddique, R.M.; Abubakar, M. Occurrence and identification of Eimeria species in broiler rearing under traditional system. J. Anim. Sci. Technol. 2015, 57, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaboudi, K.; Umar, S.; Munir, M.T. Prevalence of coccidiosis in free-range chicken in Sidi Thabet, Tunisia. Scientifica 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Thapa, S.; Hinrichsen, L.K.; Brenninkmeyer, C.; Gunnarsson, S.; Heerkens, J.L.T.; Verwer, C.; Niebuhr, K.; Willett, A.; Grilli, G.; Thamsborg, S.M.; et al. Prevalence and magnitude of helminth infections in organic laying hens (Gallus gallus domesticus) across Europe. Vet. Parasitol. 2015, 214, 118–124. [Google Scholar] [CrossRef]
- Saraiva, D.J.; Campina, A.C.C.; Gonçalves, F.C.S.; Melo-Viegas, D.; Santos, A.C.G.; Nogueira, R.M.S.; Costa, A.P. Gastrointestinal parasites in free-range chicken raised under extensive system from the northeast of Brazil. Braz. J. Poult. Sci. 2020, 23, 1–4. [Google Scholar] [CrossRef]
- Yazwinski, T.A.; Tucker, C.A. Nematodes and Acanthocephalans. In Diseases of Poultry, 12th ed.; Saif, Y.M., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2008; pp. 1025–1056. [Google Scholar]
- Mariño-González, G.A.; Ramírez-Hernández, A.; Cortés-Vecino, J.A. Libyostrongylus douglassii (Strongylida: Trichostrongylidae) in ostrich (Struthio camelus) farms from Colombia. Vet. Parasitol. 2017, 235, 53–56. [Google Scholar] [CrossRef]
- Eslami, A.; Rahmat, H.; Meshgi, B.; Ranjbar-Bahadori, S. Gastrointestinal parasites of ostrich (Struthio camelus domesticus) raised in Iran. Iran. J. Vet. Res. 2007, 8, 80–82. [Google Scholar]
- Barton, N.J.; Seward, D.A. Detection of Libyostrongylus douglassii in ostriches in Australia. Aust. Vet. J. 1993, 70, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Button, C.; Barton, N.J.; Veale, P.I.; Overend, D.J. A survey of Libyostrongylus douglassii on ostrich farms in eastern Victoria. Aust. Vet. J. 1993, 70, 76. [Google Scholar] [CrossRef] [PubMed]
- More, S.J. The performance of farmed ostrich hens in eastern Australia. Prev. Vet. Med. 1996, 29, 107–120. [Google Scholar] [CrossRef]
- Mukaratirwa, S.; Cindzi, Z.M.; Maononga, D.B. Prevalence of Libyostrongylus douglassii in commercially reared ostriches in the highveld region of Zimbabwe. J. Helminthol. 2004, 78, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Jansson, D.S.; Christensson, D.A.; Christensson, B.E. Winter survival in Sweden of L3-stage larvae of the ostrich wireworm Libyostrongylus douglassii. Vet. Parasitol. 2002, 106, 69–74. [Google Scholar] [CrossRef]



| Period | Collection | Species | No. of Birds | Age | Samples | Outdoor Area |
|---|---|---|---|---|---|---|
| October–December 2020 | Bird collection 1 (Lisbon) | Peacocks | 20 | 3 months–9 years | 29 | 9600 m2 |
| April 2021 | Bird collection 2 (Lisbon) | Peacocks | 40 | 3 months–19 years | 25 | 6000 m2 |
| September 2020–February 2021 | Bird collection 3 (Abrantes) | Ostriches | 2 | 4 years | 9 | 50,000 m2 |
| Emus | 6 | 7–14 years | 19 | |||
| Peacocks | 3 | 3–6 years | 14 | 6000 m2 | ||
| July–November 2020 | Poultry farm (Lourinhã) | Organic layers | 200 | 16 months | 46 | 1700 m2 |
| Collection | Bird Species | GI Parasites | OPG|EPG (Min–Max) | Prevalence (%) |
|---|---|---|---|---|
| Bird collection 1 | Peacocks | Eimeria spp. | 107 (0–750) | 29 |
| Helminths | 145 (0–2000) | 14 (Capillaria spp.) | ||
| 14 (S. pavonis) | ||||
| Bird collection 2 | Peacocks | Eimeria spp. | 502 (0–1800) | 25 |
| Helminths | 66 (0–1000) | 8 (T. tenuis) | ||
| 4 (S. pavonis) | ||||
| Bird collection 3 | Ostriches | L. douglassii | 2731 (500–5700) | 100 |
| Emus | 60 (0–420) | 32 | ||
| Peacocks | Eimeria spp. | 9 (0–30) | 43 | |
| Poultry farm | Organic layers | Eimeria spp. | 24 (0–300) | 41 |
| Average Eimeria spp., Galliformes | 160 (0–1800) | 35 | ||
| Average Helminths, Peacocks | 70 (0–2000) | 15 | ||
| Average Helminths, Ratites | 1396 (0–5700) | 66 | ||
| Bird Groups | GI Parasites | Mini-FLOTAC EPG|OPG (Min–Max) | McMaster EPG | OPG (Min–Max) |
|---|---|---|---|
| Galliformes | Eimeria spp. | 160 (0–1800) | 188 (0–5100) |
| Peacocks (Collections 1 and 2) | Helminths | 105 (0–2000) | 16 (0–400) |
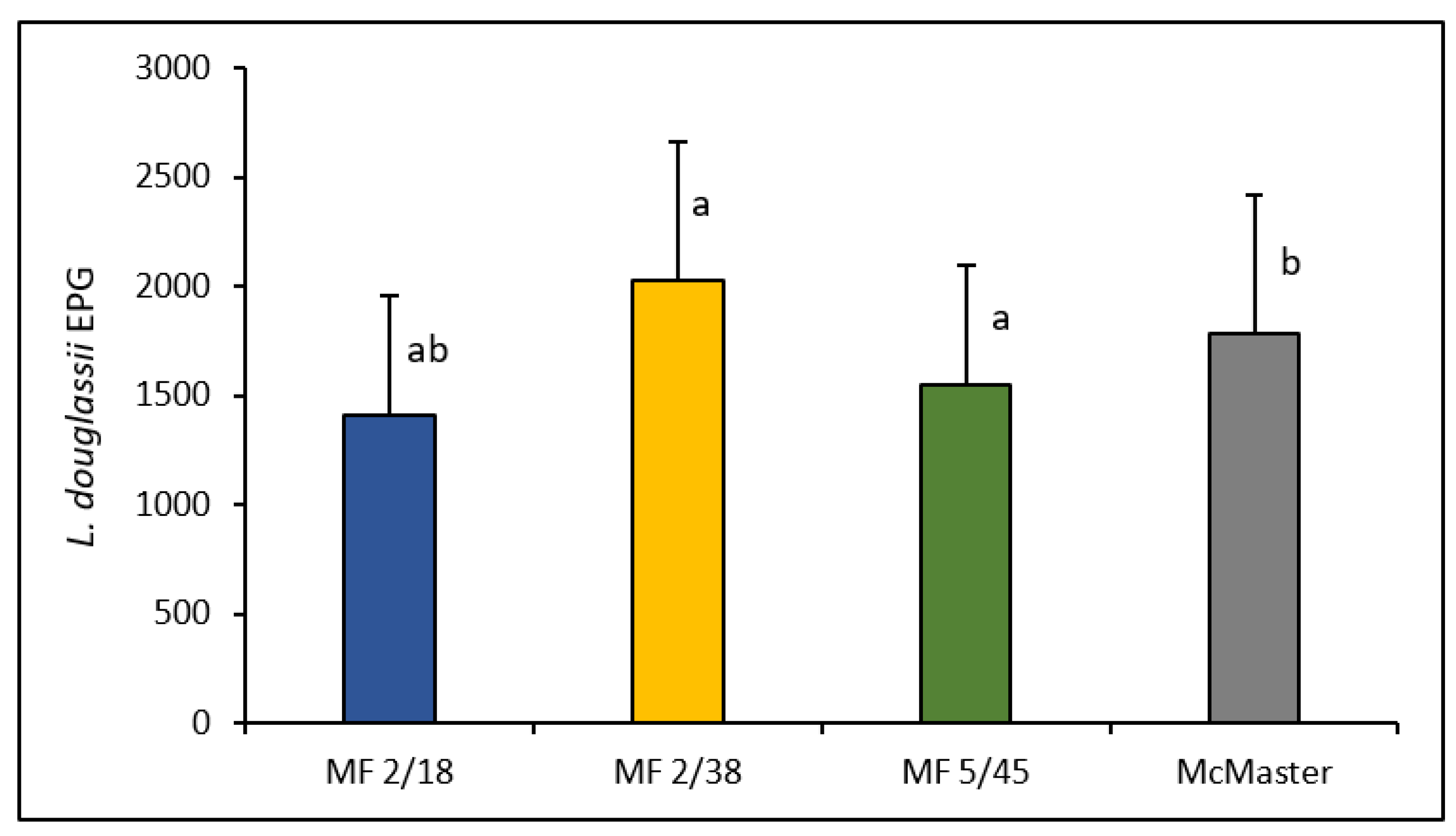
| Ratites | L. douglassii | 1396 (0–5700) | 1647 (0–10,000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, J.; Almeida, C.; Victório, A.C.; Melo, P.; Rodrigues, J.P.; Rinaldi, L.; Cringoli, G.; Gomes, L.; Oliveira, M.; Paz-Silva, A.; et al. Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds. Vet. Sci. 2021, 8, 160. https://doi.org/10.3390/vetsci8080160
Lozano J, Almeida C, Victório AC, Melo P, Rodrigues JP, Rinaldi L, Cringoli G, Gomes L, Oliveira M, Paz-Silva A, et al. Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds. Veterinary Sciences. 2021; 8(8):160. https://doi.org/10.3390/vetsci8080160
Chicago/Turabian StyleLozano, João, Cristina Almeida, Ana Cláudia Victório, Pedro Melo, João Paulo Rodrigues, Laura Rinaldi, Giuseppe Cringoli, Lídia Gomes, Manuela Oliveira, Adolfo Paz-Silva, and et al. 2021. "Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds" Veterinary Sciences 8, no. 8: 160. https://doi.org/10.3390/vetsci8080160
APA StyleLozano, J., Almeida, C., Victório, A. C., Melo, P., Rodrigues, J. P., Rinaldi, L., Cringoli, G., Gomes, L., Oliveira, M., Paz-Silva, A., & Madeira de Carvalho, L. (2021). Implementation of Mini-FLOTAC in Routine Diagnosis of Coccidia and Helminth Infections in Domestic and Exotic Birds. Veterinary Sciences, 8(8), 160. https://doi.org/10.3390/vetsci8080160









