Use of Flubendazole and Fenbendazole for Treatment of Lung Severe Infection by the Gapeworm Cyathostoma bronchialis (Nematoda: Syngamidae) in Branta hutchinsii, Anser indicus and B. leucopsis Exotic Geese: An Interesting Case
Abstract
:1. Introduction
2. Materials and Methods
2.1. Farm and Flock
2.2. Diet
2.3. Periodic Pharmacological Treatments on the Farm
3. Case History and Anamnestic Data
3.1. Acute Respiratory Syndrome: First Occurrence
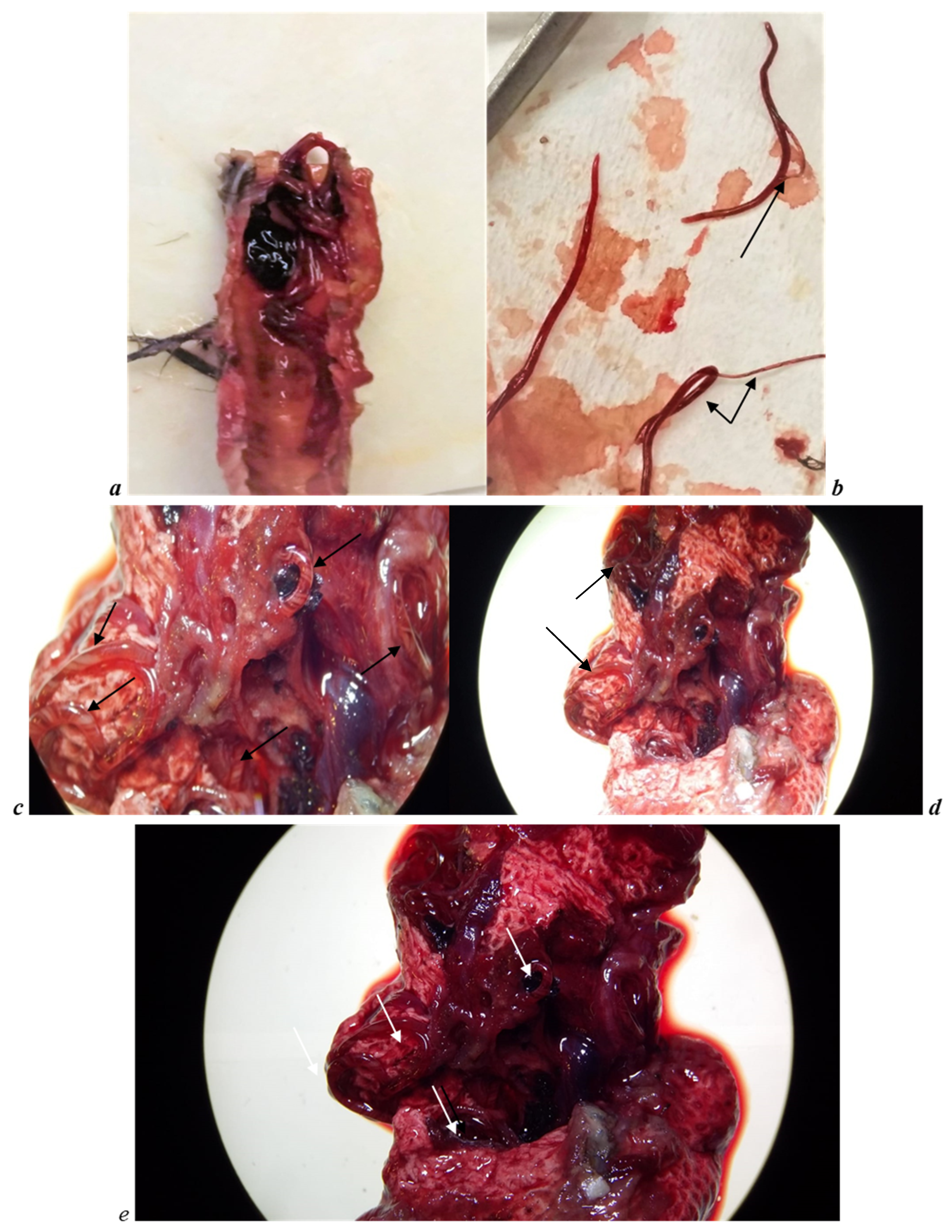
3.2. Necropsy Observations and Parasites Indentification
4. Results
4.1. Parasites Identification
4.2. Follow Up
5. Discussion
5.1. Parasite Identification and Life-Cycle Considerations
5.2. Pharmacological Treatment and Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanarek, G.; Zaleśny, G.; Sitko, J.; Blanco, A.I. Taxonomic status of Cyathostoma nematodes (Nematoda: Syngaminae) parasitizing respiratory tracts of birds of prey and owls in Europe and North America: How many species are there? Helminthologia 2016, 53, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, E. Investigations on the organization of Vermes. Ann. Sci. Nat. Zool. 1849, 3, 106–202. [Google Scholar]
- Lichtenfels, J.R. Keys to genera of the superfamily Strongyloidea. In CIH Keys to the Nematode Parasites of Vertebrates, No. 7; Anderson, R.C., Chabaud, A.G., Willmott, S., Eds.; Commonwealth Agricultural Bureaux: Buckinghamshire, UK, 1980; p. 41. [Google Scholar]
- Threlfall, W. Life-cycle of Cyathostoma Iari Blanchard, 1849 (Nematoda, Strongyloidea). Nature 1965, 206, 1167–1168. [Google Scholar] [CrossRef]
- Turemuratov, A. Syngamidae of Pelecaniform birds of Aral Sea with comments on the structure of this nematode family. Vest. Karakal. Fil. Akad. Uzb. SSR 1963, 3, 45–50. [Google Scholar]
- Ryžikov, K.M. Revision of the system of Syngamidae. Prob. Parazitol. Tez. Dokl. V Nauchnoj Konf. Ukr. Vesp. Nauchnoj Obsch. Parazitol. Kiev 1967, 184–187. [Google Scholar]
- Baruš, V.; Tenora, F. Notes on the systematics and taxonomy of the nematodes belonging to the family Syngamidae Leiper, 1912. Acta Univ. Agric. Brno 1972, 20, 275–286. [Google Scholar]
- Simpson, V.R.; Harris, E.A. Cyathostoma lari (Nematoda) infection in birds of prey. J. Zool. 1992, 277, 655–659. [Google Scholar] [CrossRef]
- Lierz, M.; Göbel, T.; Schuster, R. Research on the occurence of parasites in native birds of prey and owls. Berl. Munch. Tierarztl. Wochenschr. 2002, 115, 43–52. [Google Scholar] [PubMed]
- Ali, M.M. A review and revision of the subfamily Cyathostomatinae Nicoll, 1927 (Nematoda, Syngamidae). Acta Parasitol. Pol. 1970, 17, 237–246. [Google Scholar]
- Ryžikov, K.M.; Zavadil, R. On the identity of species of the genus Cyathostoma collected from ostrich. Acta Univ. Agric. Silvic. Brno 1958, 2, 125–132. [Google Scholar]
- Baruš, V.; Sergeeva, T.P.; Sonin, M.D.; Ryzhikov, K.M. Nematoda. In Helminths of Fisheating Birds of the Palearctic Region; Ryšavý, B., Ryzhikov, K.M., Eds.; Helminthological Laboratory Czechoslovak Academy of Sciences, Institute of Parasitology; Springer: Dordrecht, The Netherlands, 1978; p. 318. [Google Scholar]
- Fernando, M.A.; Hoover, I.J.; Ogungbade, S.G. The Migration and Development of Cyathostoma Bronchialis in Geese. J. Parasitol. 1973, 59, 980–986. [Google Scholar] [CrossRef]
- Luppi, A.; Maioli, G.; Spaggiari, B.; Gelmetti, D.; Gibelli, L.R.; Bonilauri, P.; Dottori, M. Cyathostoma bronchialis in oche di Steinbach. In Proceedings of the XI Congresso Nazionale, Parma, Italy, 30 September–2 October 2009; pp. 174–175. [Google Scholar]
- Yamaguti, S. Systema Helmintum, Volume III: The Nematodes of Vertebrates; Interscience Publishers Inc.: New York, NY, USA, 1961; p. 679. [Google Scholar]
- Thienpont, D.C.; Vanparijs, O.F.; Hermans, L.C. Mebendazole, a new potent drug against Syngamus trachea in turkeys. Poult. Sci. 1973, 52, 1712–1714. [Google Scholar] [CrossRef]
- Krone, O.; Friedrich, D.; Honisch, M. Specific status and pathogenicity of syngamid nematodes in bird species (Ciconiformes, Falconiformes, Gruiformes) from Germany. J. Helminthol. 2007, 81, 67–73. [Google Scholar] [CrossRef]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Parassitologia e Malattie Parassitarie degli Animali; Edizioni Mediche Scientifiche Internazionali: Rome, Italy, 2010; pp. 507–508. [Google Scholar]
- Hartwich, G. The Wildlife of Germany. Nematoda II. Strongylida: Strongyloidea und Ancylostomatoidea; Gustav Fisher Verlag: Jena, Germany, 1994; p. 157. [Google Scholar]
- Anderson, R.C. Nematode parasites of vertebrates: Their development and transmission: Book review. J. S. Afr. Vet. Assoc. 2000, 71, 232–239. [Google Scholar]
- Holand, H.; Jensen, H.; Tufto, J.; Sæther, B.E.; Ringsby, T.H. Temporal and spatial variation in prevalence of the parasite Syngamus trachea in a metapopulation of house sparrows (Passer domesticus). Parasitology 2013, 140, 1275–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, M.A.; Stockdale, P.H.; Remmler, O. The route of migration, development, and pathogenesis of Syngamus trachea (Montagu, 1811) Chapin, 1925, in pheasants. J. Parasitol. 1971, 57, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Chapin, E.A. Review of the nematode genera Syngamus Sieb. and Cyathostoma, E. Blanch. J. Agric. Res. 1925, 30, 557–570. [Google Scholar]
- Cram, E.B. Bird Parasites of the Nematode Suborder Strongylata, Ascaridata, and Spirurata; Bulletin of the United States National Museum, Government Printing Office: Washington, DC, USA, 1927; Volume 140, pp. 1–465.
- McDonald, M.E. Key to the Nematodes Reported in Waterfowl. Department of the Interior Bureau of Sport Fisheries and Wildlife Resource; U.S. Fish and Wildlife Service: Washington, DC, USA, 1974; Volume 122, p. 44.
- Anderson, R.C.; Chabaud, A.G.; Willmot, S. Keys to the Nematode Parasites of Vertebrates: Archival Volume; CAB International: Wallingford, UK, 2009; pp. 309–323. [Google Scholar]
- Fernando, M.A.; Barta, J.R. Tracheal worms. In Parasitic Diseases of Wild Birds; Atkinson, C.T., Thomas, N.J., Hunter, D.B., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 343–354. [Google Scholar]
- Gibbs, H.C. Hypobiosis in parasitic nematodes—An update. Adv. Parasitol. 1986, 25, 129–174. [Google Scholar]
- Love, S.; Murphy, D.; Mellor, D. Pathogenicity of cyathostome infection. Vet. Parasitol. 1999, 85, 113–122. [Google Scholar] [CrossRef]
- Gras, L.M.; Usai, F.; Stancampiano, L. Strongylosis in horses slaughteredin Italy for meat production: Epidemiology, influence of the horse origin andevidence of parasite self-regulation. Vet. Parasitol. 2011, 179, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Stancampiano, L.; Usai, F. The role of density-dependent arrested larval stages on parasite dynamics and stability: Lessons from nematodes and donkeys. Ecol. Model. 2015, 297, 69–79. [Google Scholar] [CrossRef]
- Tarbiat, B.; Jansson, D.S.; Tydén, E.; Höglund, J. Evaluation of benzimidazole resistance status in Ascaridia galli. Vet. Parasitol. 2017, 144, 1338–1345. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Veterinary Medicines and Inspections. Committee for Medicinal Products for Veterinary Use Flubendazole, EMEA/CVMP/33128/2006-FINAL, Report 4; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2006. [Google Scholar]
- European Medicines Agency (EMA). 2008. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/panacur-aquasol%20EMA/834845/2011%20EMEA/V/C/002008 (accessed on 9 April 2021).
- Vanparijs, O. Anthelmintic activity of flubendazole in naturally infected geese and the economic importance of deworming. Avian Dis. 1984, 28, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, R. Treatment of nematodiasis in poultry and game birds with fenbendazole. Avian Dis. 1984, 28, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Enigk, K.; Dey-Hazra, A. Zum Auftreten und zur Behandlung des Syngamusbefalles beim Haus- und Wildgefligel. Berl. Munch. Tierarztl. Wochenschr. 1975, 88, 166–168. [Google Scholar]
- Enigk, K.; Dey-Hazra, A. Die Behandlung des Helminthenbefalles wildlebender Saugetiere und Vogel mit Fenbendazol. Kleintierpraxis 1976, 21, 133–140. [Google Scholar]
- Enigk, K.; Dey-Hazra, A. Zur Verbreitung und Behandlung des Trichostrongylus tenuis Befalles. Berl. Munch. Tierarztl. Wochenschr. 1971, 84, 11–14. [Google Scholar]
- Enigk, K.; Dey-Hazra, A.; Batke, J. Use of mebendazole for helminthiases in chickens and geese. Avian Pathol. 1973, 2, 67–74. [Google Scholar] [CrossRef]
- Baggot, J.D.; McKellar, Q.A. The absorption, distribution and elimination of anthelmintic drugs: The role of pharmacokinetics. J. Vet. Pharmacol. Ther. 1994, 17, 409–419. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Veterinary Medicines and Inspections. Committee for Medicinal Products for Veterinary Use Flubendazole, Emea/Mrl/267/97-Final, Report 2; European Medicines Agency (EMA): Amsterdam, The Netherlands, 1997. [Google Scholar]


| Components | Nutritional Values (%) | Additives (* IU/mg/kg/Feed) |
|---|---|---|
| Corn bran–Wheat bran–Wheat–Soy flour–Brewers yeast–Soybean oil–DL-Methionine–L-Lysine | * CP: 17.1%–* CF: 3.7%–* CFi: 4.8%–Ash: 4.5%–DL-Methionine: 1.500 mg–L-Lysine: 1.500 mg–Umidity: 12.5% | Vit. A: 9.950 IU–Vit. D3: 2.701 IU–Vit. E 38 mg–Vit.K3: 3.25 mg–Vit.B1: 3.25 mg–Vit. B2: 7.8 mg–Vit. B6: 5.2 mg–Vit. B12: 0.02 mg–Niacin: 39 mg–Ca-D-pantotenate: 10 mg–Folic acid: 0.65 mg–Biotin: 0.1 mg–Cu: 5 mg–Mn: 50 mg–Se: 0.13 mg–Zn: 50 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, A.; Carminati, A.; Stancampiano, L.; Roncada, P.; Frasnelli, M. Use of Flubendazole and Fenbendazole for Treatment of Lung Severe Infection by the Gapeworm Cyathostoma bronchialis (Nematoda: Syngamidae) in Branta hutchinsii, Anser indicus and B. leucopsis Exotic Geese: An Interesting Case. Vet. Sci. 2021, 8, 147. https://doi.org/10.3390/vetsci8080147
Guerrini A, Carminati A, Stancampiano L, Roncada P, Frasnelli M. Use of Flubendazole and Fenbendazole for Treatment of Lung Severe Infection by the Gapeworm Cyathostoma bronchialis (Nematoda: Syngamidae) in Branta hutchinsii, Anser indicus and B. leucopsis Exotic Geese: An Interesting Case. Veterinary Sciences. 2021; 8(8):147. https://doi.org/10.3390/vetsci8080147
Chicago/Turabian StyleGuerrini, Alessandro, Andrea Carminati, Laura Stancampiano, Paola Roncada, and Matteo Frasnelli. 2021. "Use of Flubendazole and Fenbendazole for Treatment of Lung Severe Infection by the Gapeworm Cyathostoma bronchialis (Nematoda: Syngamidae) in Branta hutchinsii, Anser indicus and B. leucopsis Exotic Geese: An Interesting Case" Veterinary Sciences 8, no. 8: 147. https://doi.org/10.3390/vetsci8080147
APA StyleGuerrini, A., Carminati, A., Stancampiano, L., Roncada, P., & Frasnelli, M. (2021). Use of Flubendazole and Fenbendazole for Treatment of Lung Severe Infection by the Gapeworm Cyathostoma bronchialis (Nematoda: Syngamidae) in Branta hutchinsii, Anser indicus and B. leucopsis Exotic Geese: An Interesting Case. Veterinary Sciences, 8(8), 147. https://doi.org/10.3390/vetsci8080147






