Prolonged Infection of Canine Distemper Virus in a Mixed-Breed Dog
Abstract
1. Introduction
2. Materials and Methods
2.1. Case History
2.2. PCR Reaction and Sequencing
2.3. Phylogenetic Analysis
3. Results
3.1. PCR Detection and Sequencing
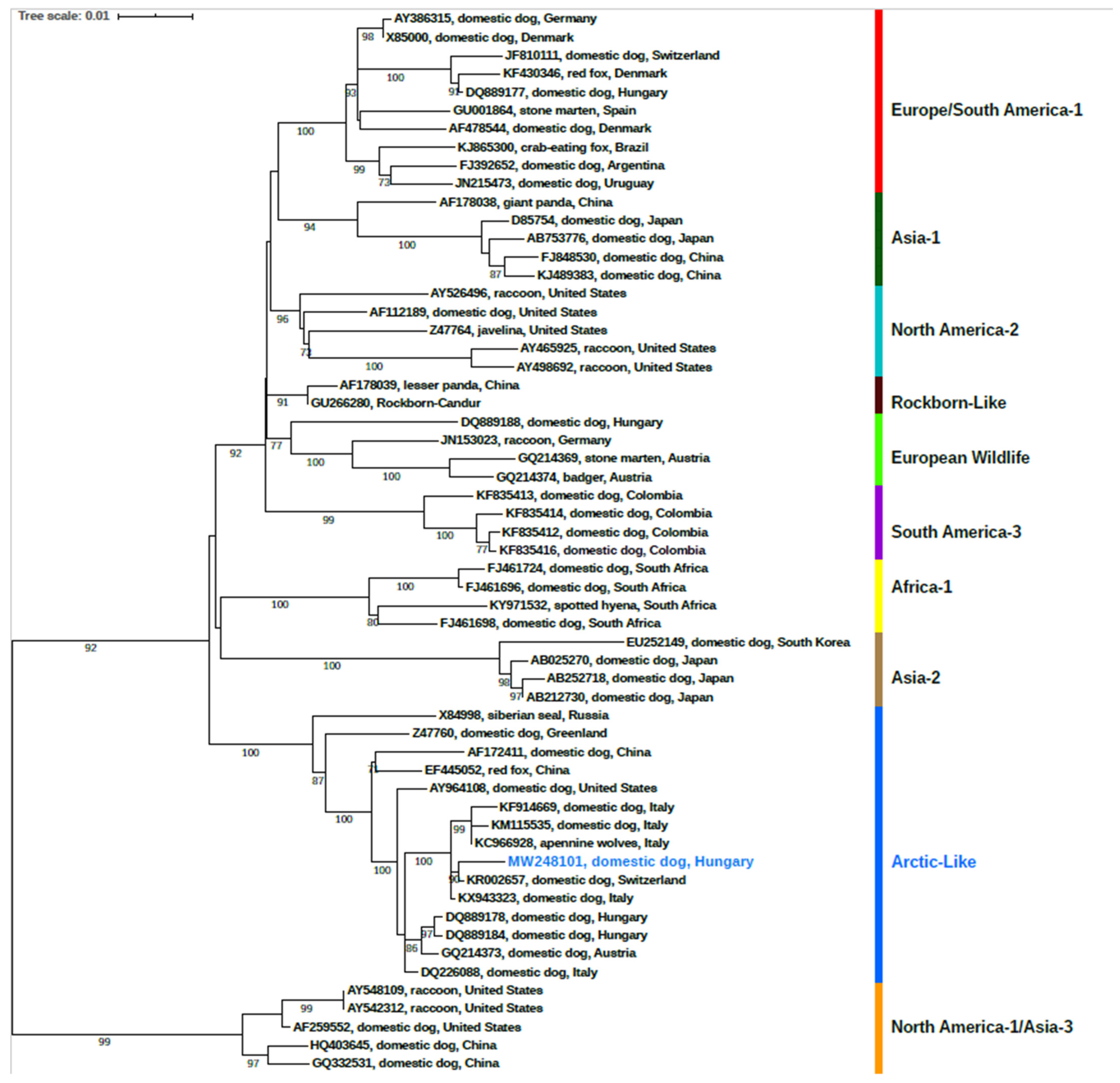
3.2. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapil, S.; Yeary, T.J. Canine distemper spillover in domestic dogs from urban wildlife. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1069–1086. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systematic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef]
- Greene, C.E. Infectious Diseases of the Dog and Cat, 4th ed.; Elsevier/Saunders: St. Louis, MO, USA, 2012; pp. 25–42. [Google Scholar]
- Willi, B.; Spiri, A.M.; Meli, M.L.; Grimm, F.; Beatrice, L.; Riond, B.; Bley, T.; Jordi, R.; Dannler, M.; Hofmann-Lehmann, R. Clinical and molecular investigation of a canine distemper outbreak and vector-borne infections in a group of rescue dogs imported from Hungary to Switzerland. BMC Vet. Res. 2015, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.C.; Wilkes, R.P. Sequencing of emerging canine distemper virus strain reveals new distinct genetic lineage in the United States associated with disease in wildlife and domestic canine populations. Virol. J. 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Martella, V.; Elia, G.; Buonavoglia, C. Canine distemper virus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.E.; Kelman, M.; Ward, M.P. Epidemiology and clinical presentation of canine distemper disease in dogs and ferrets in Australia, 2006–2014. Aust. Vet. J. 2016, 94, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E. Canine and Feline Infectious Diseases, 1st ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; pp. 152–165. [Google Scholar]
- Chvala, S.; Benetka, V.; Möstl, K.; Zeugswetter, F.; Spergser, J.; Weissenböck, H. Simultaneous canine distemper virus, canine adenovirus type 2, and Mycoplasma cynos infection in a dog with pneumonia. Vet. Pathol. 2007, 44, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Liu, R.; He, Y.; Xiao, X.; Xiao, W.; Zheng, Q.; Lin, X.; Tao, P.; Zhou, P.; Li, S. Multiplex PCR methods for detection of several viruses associated with canine respiratory and enteric diseases. PLoS ONE 2019, 14, e0213295. [Google Scholar] [CrossRef]
- De Vries, R.D.; Duprex, W.P.; De Swart, R.L. Morbillivirus infections: An introduction. Viruses 2015, 7, 699–706. [Google Scholar] [CrossRef]
- Duque-Valencia, J.; Forero-Muñoz, N.R.; Díaz, F.J.; Martins, E.; Barato, P.; Ruiz-Saenz, J. Phylogenetic evidence of the intercontinental circulation of a Canine distemper virus lineage in the Americas. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Elia, G.; Decaro, N.; Martella, V.; Cirone, F.; Lucente, M.S.; Lorusso, E.; Trani, L.D.; Buonavoglia, C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods 2006, 136, 171–176. [Google Scholar] [CrossRef]
- Demeter, Z.; Lakatos, B.; Palade, E.A.; Kozma, T.; Forgách, P.; Rusvai, M. Genetic diversity of Hungarian canine distemper virus strains. Vet. Microbiol. 2007, 122, 258–269. [Google Scholar] [CrossRef]
- Demeter, Z.; Palade, E.A.; Rusvai, M. Canine distemper: Still a major concern in Central Europe. Lucr. Stiint. Univ. Stiint. Agric. Banat. Timis. Med. Vet. 2009, 42, 136–150. [Google Scholar]
- Bolt, G.; Jensen, T.D.; Gottschalck, E.; Arctander, P.; Appel, M.J.; Buckland, R.; Blixenkrone-Møller, M. Genetic diversity of the attachment (H) protein gene of current field isolates of canine distemper virus. J. Gen. Virol. 1997, 78, 367–372. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Tokiyoshi, S.; Hirayama, N.; Nakamura, K.; Ohashi, K.; Wakasa, C.; Mikami, T.; Kai, C. Antigenic differences in the H proteins of canine distemper viruses. Vet. Microbiol. 2000, 71, 281–286. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Shaw, M.A.; Goodman, S.J. Pathogen evolution and disease emergence in carnivores. Proc. R. Soc. B 2007, 274, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- Romanutti, C.; Calderón, M.G.; Keller, L.; Mattion, N.; La Torre, J. RT-PCR and sequence analysis of the full-length fusion protein of canine distemper virus from domestic dogs. J. Virol. Methods 2016, 228, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Cirone, F.; Elia, G.; Lorusso, E.; Decaro, N.; Campolo, M.; Desario, C.; Lucente, M.S.; Bellacicco, A.L.; Blixenkrone-Møller, M.; et al. Heterogeneity within the hemagglutinin genes of canine distemper virus (CDV) strains detected in Italy. Vet. Microbiol. 2006, 116, 301–309. [Google Scholar] [CrossRef]
- Lan, N.T.; Yamaguchi, R.; Inomata, A.; Furuya, Y.; Uchida, K.; Sugano, S.; Tateyama, S. Comparative analyses of canine distemper viral isolates from clinical cases of canine distemper in vaccinated dogs. Vet. Microbiol. 2006, 115, 32–42. [Google Scholar] [CrossRef]
- Espinal, M.A.; Díaz, F.J.; Ruiz-Saenz, J. Phylogenetic evidence of a new canine distemper virus lineage among domestic dogs in Colombia, South America. Vet. Microbiol. 2014, 172, 168–176. [Google Scholar] [CrossRef]
- Budaszewski, R.F.; Pinto, L.D.; Weber, M.N.; Caldart, E.T.; Alves, C.D.B.T.; Martella, V.; Ikuta, N.; Lunge, V.R.; Canal, C.W. Genotyping of canine distemper virus strains circulating in Brazil from 2008 to 2012. Virus Res. 2014, 180, 76–83. [Google Scholar] [CrossRef]
- Shin, Y.J.; Cho, K.O.; Cho, H.S.; Kang, S.K.; Kim, H.J.; Kim, Y.H.; Park, H.S.; Park, N.Y. Comparison of one-step RT-PCR and a nested PCR for the detection of canine distemper virus in clinical samples. Aust. Vet. J. 2004, 82, 83–86. [Google Scholar] [CrossRef]
- Saito, T.B.; Alfieri, A.A.; Wosiacki, S.R.; Negrao, F.J.; Morais, H.S.A.; Alfieri, A.F. Detection of canine distemper virus by reverse transcriptase-polymerase chain reaction in the urine of dogs with clinical signs of distemper encephalitis. Res. Vet. Sci. 2006, 80, 116–119. [Google Scholar] [CrossRef]
- Elia, G.; Camero, M.; Losurdo, M.; Lucente, M.S.; Larocca, V.; Martella, V.; Decaro, N.; Buonavoglia, C. Virological and serological findings in dogs with naturally occurring distemper. J. Virol. Methods 2015, 213, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Sekulin, K.; Hafner-Marx, A.; Kolodziejek, J.; Janik, D.; Schmidt, P.; Nowotny, N. Emergence of canine distemper in Bavarian wildlife associated with a specific amino acid exchange in the haemagglutinin protein. Vet. J. 2011, 187, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Modarelli, J.J.; Ferro, P.J.; Esteve-Gasent, M.D. Development and application of a canine endogenous internal positive control for use in real-time PCR assays. J. Vet. Diagn. Investig. 2018, 30, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Mira, F.; Purpari, G.; Di Bella, S.; Vicari, D.; Schirò, G.; Di Marco, P.; Macaluso, G.; Battilani, M.; Guercio, A. Update on canine distemper virus (CDV) strains of Arctic-like lineage detected in dogs in Italy. Vet. Ital. 2018, 54, 225–236. [Google Scholar]
- Di Sabatino, D.; Lorusso, A.; Di Francesco, C.E.; Gentile, L.; Di Pirro, V.; Bellacicco, A.L.; Giovannini, A.; Di Francesco, G.; Marruchella, G.; Marsilio, F.; et al. Arctic lineage-canine distemper virus as a cause of death in Apennine wolves (Canis lupus) in Italy. PLoS ONE 2014, 9, e82356. [Google Scholar] [CrossRef]
- Monne, I.; Fusaro, A.; Valastro, V.; Citterio, C.; Pozza, M.D.; Obber, F.; Trevisiol, K.; Cova, M.; De Benedictis, P.; Bregoli, M.; et al. A distinct CDV genotype causing a major epidemic in Alpine wildlife. Vet. Microbiol. 2011, 150, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Demeter, Z.; Palade, E.A.; Hornyák, Á.; Rusvai, M. Controversial results of the genetic analysis of a canine distemper vaccine strain. Vet. Microbiol. 2010, 142, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Headley, S.A.; Graça, D.L.; da Costa, M.M.; de Vargas, A.C. Canine distemper virus infection with secondary Bordetella bronchiseptica pneumonia in dogs. Cienc. Rural 1999, 29, 741–743. [Google Scholar] [CrossRef]
- Jacsó, O.; Mándoki, M.; Majoros, G.; Pétsch, M.; Mortarino, M.; Genchi, C.; Fok, E. First autochthonous Dirofilaria immitis (Leidy, 1856) infection in a dog in Hungary. Helminthologia 2009, 46, 159–161. [Google Scholar] [CrossRef]
- Bacsadi, Á.; Papp, A.; Szeredi, L.; Tóth, G.; Nemes, C.; Imre, V.; Tolnai, Z.; Széll, Z.; Sréter, T. Retrospective study on the distribution of Dirofilaria immitis in dogs in Hungary. Vet. Parasitol. 2016, 220, 83–86. [Google Scholar] [CrossRef]
- Osterhaus, A.D.M.E.; Groen, J.; de Vries, P.; UytdeHaag, F.G.C.M.; Klingeborn, B.; Zarnke, R. Canine distemper virus in seals. Nature 1988, 335, 403–404. [Google Scholar] [CrossRef]
- Osterhaus, A.D.M.E.; Groen, J.; UytdeHaag, F.G.C.M.; Visser, I.K.G.; Bildt, M.W.G.V.D.; Bergman, A.; Klingeborn, B. Distemper virus in Baikal seals. Nature 1989, 338, 209–210. [Google Scholar] [CrossRef]
- Visser, I.K.G.; Kumarev, V.P.; Örvell, C.; De Vries, P.; Broeders, H.W.J.; Van de Bildt, M.W.G.; Groen, J.; Teppema, J.S.; Burger, M.C.; UytdeHaag, F.G.C.M.; et al. Comparison of two morbilliviruses isolated from seals during outbreaks of distemper in North West Europe and Siberia. Arch. Virol. 1990, 111, 149–164. [Google Scholar] [CrossRef]
- Mamaev, L.V.; Denikina, N.N.; Belikov, S.I.; Volchkov, V.E.; Visser, I.K.G.; Fleming, M.; Kai, C.; Harder, T.C.; Liess, B.; Osterhaus, A.D.M.E.; et al. Characterisation of morbilliviruses isolated from Lake Baikal seals (Phoca sibirica). Vet. Microbiol. 1995, 44, 251–259. [Google Scholar] [CrossRef]
- Kapil, S.; Allison, R.W.; Johnston, L.; Murray, B.L.; Holland, S.; Meinkoth, J.; Johnson, B. Canine distemper virus strains circulating among North American dogs. Clin. Vaccine Immunol. 2008, 15, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Yan, X.J.; Chai, X.L.; Martella, V.; Luo, G.L.; Zhang, H.L.; Gao, H.; Liu, Y.X.; Bai, X.; Zhang, L.; et al. Phylogenetic analysis of the haemagglutinin gene of canine distemper virus strains detected from breeding foxes, raccoon dogs and minks in China. Vet. Microbiol. 2010, 140, 34–42. [Google Scholar] [CrossRef]
- Namroodi, S.; Rostami, A.; Majidzadeh-Ardebili, K.; Langroudi, A.G.; Morovvati, A. Detection of Arctic and European cluster of canine distemper virus in north and center of Iran. Vet. Res. Forum 2015, 6, 199–204. [Google Scholar] [PubMed]
- Ricci, I.; Cersini, A.; Manna, G.; Marcario, G.A.; Conti, R.; Brocherel, G.; Grifoni, G.; Eleni, C.; Scicluna, M.T. A Canine Distemper Virus Retrospective Study Conducted from 2011 to 2019 in Central Italy (Latium and Tuscany Regions). Viruses 2021, 13, 272. [Google Scholar] [CrossRef]
- Benetka, V.; Leschnik, M.; Affenzeller, N.; Möstl, K. Phylogenetic analysis of Austrian canine distemper virus strains from clinical samples from dogs and wild carnivores. Vet. Rec. 2011, 168, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, D.; Di Francesco, G.; Zaccaria, G.; Malatesta, D.; Brugnola, L.; Marcacci, M.; Portanti, O.; De Massis, F.; Savini, G.; Teodori, L.; et al. Lethal distemper in badgers (Meles meles) following epidemic in dogs and wolves. Infect. Genet. Evol. 2016, 46, 130–137. [Google Scholar] [CrossRef] [PubMed]
| 2019 | 2020 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| February | March | April | May | July | August | September | October | November | January | February | June | |
| Ct value | 18.48 | 22.66 | 20.37 | 23.9 | 32.66 | 39.75 | 38.3 | 39.3 | 40 | 39.39 | 40 | 41 |
| cRNA (n copies/μL) | 2,510,955 | 207,819 | 813,841 | 99,235 | 536 | <100 | <100 | <100 | <100 | <100 | <100 | <100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanszki, Z.; Zana, B.; Zeghbib, S.; Jakab, F.; Szabó, N.; Kemenesi, G. Prolonged Infection of Canine Distemper Virus in a Mixed-Breed Dog. Vet. Sci. 2021, 8, 61. https://doi.org/10.3390/vetsci8040061
Lanszki Z, Zana B, Zeghbib S, Jakab F, Szabó N, Kemenesi G. Prolonged Infection of Canine Distemper Virus in a Mixed-Breed Dog. Veterinary Sciences. 2021; 8(4):61. https://doi.org/10.3390/vetsci8040061
Chicago/Turabian StyleLanszki, Zsófia, Brigitta Zana, Safia Zeghbib, Ferenc Jakab, Nikoletta Szabó, and Gábor Kemenesi. 2021. "Prolonged Infection of Canine Distemper Virus in a Mixed-Breed Dog" Veterinary Sciences 8, no. 4: 61. https://doi.org/10.3390/vetsci8040061
APA StyleLanszki, Z., Zana, B., Zeghbib, S., Jakab, F., Szabó, N., & Kemenesi, G. (2021). Prolonged Infection of Canine Distemper Virus in a Mixed-Breed Dog. Veterinary Sciences, 8(4), 61. https://doi.org/10.3390/vetsci8040061







