SOD1 Gene Silencing Promotes Apoptosis and Suppresses Proliferation of Heat-Stressed Bovine Granulosa Cells via Induction of Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. The Isolation, Culture, Identification, and Treatment of Granulosa Cells
2.3. Production of siRNA and GCs Transfection
2.4. Quantitative Reverse Transcription PCR (RT-qPCR)
2.5. Protein Extraction and Western Blotting
2.6. Estimation ROS
2.7. Estimation of Apoptotic and Dead GCs
2.8. Analysis of Cell Cycle
2.9. Assessment of Mitochondrial Membrane Potential
2.10. Cell Viability Assay
2.11. E2 and P4 Levels Determination
2.12. Statistical Analysis
3. Results
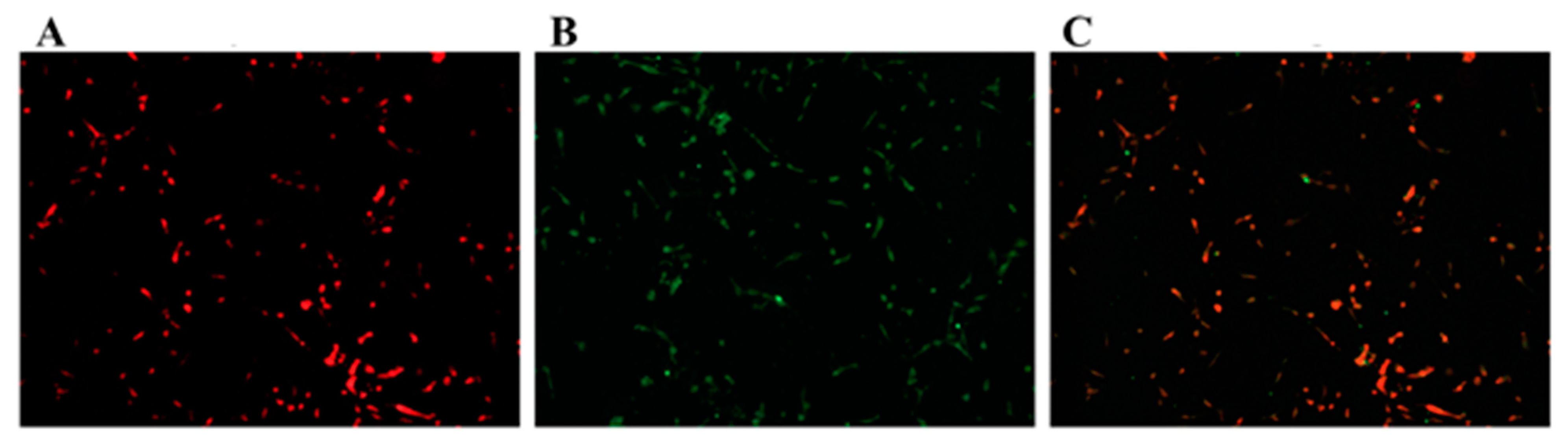
3.1. Identification of GCs
3.2. Efficacy of SOD1 Transfection
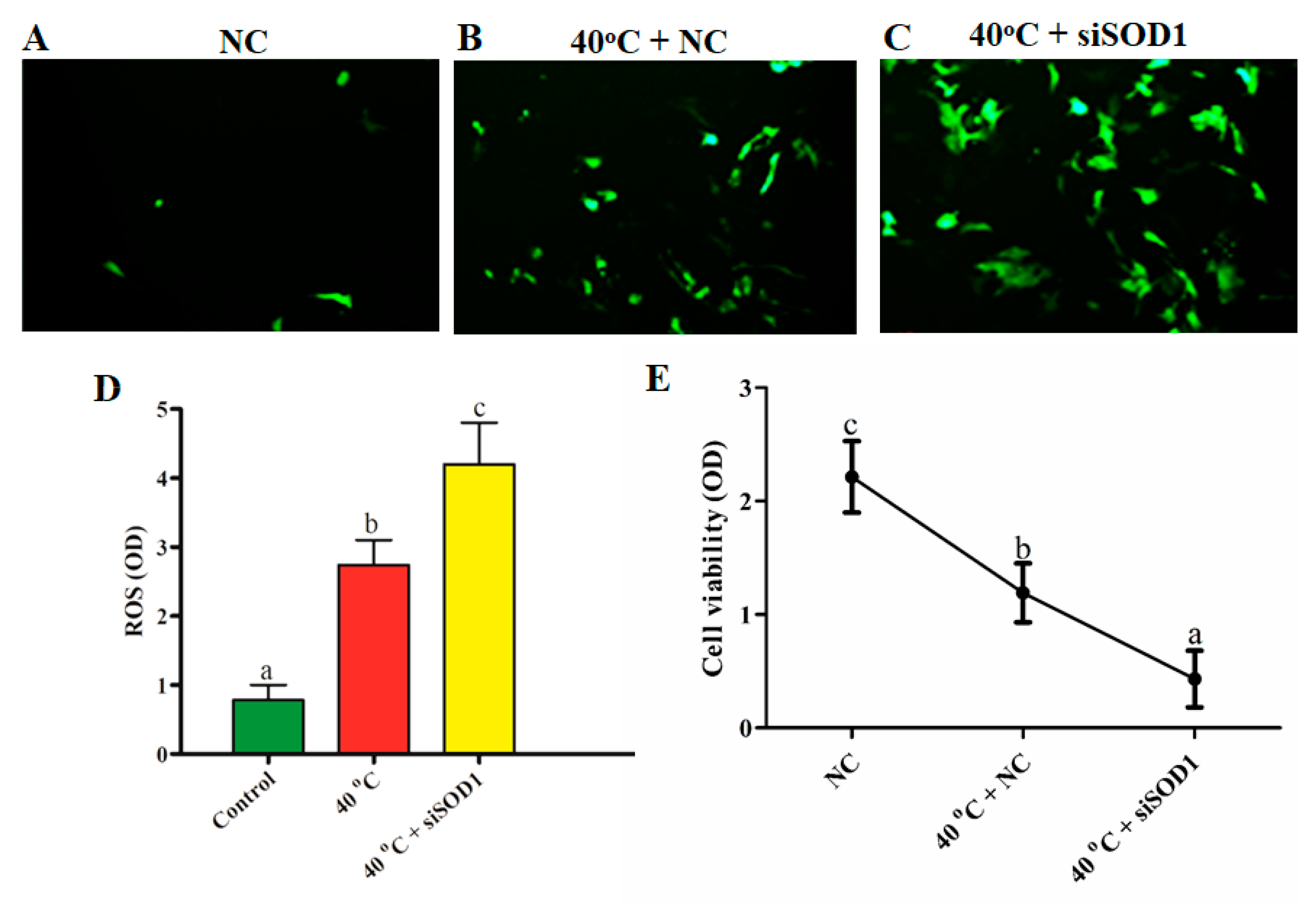
3.3. Silencing of SOD1-Induced Intracellular ROS Accumulation under Heat Stress
3.4. Silencing of SOD1-Altered Viability of GCs under Heat Stress
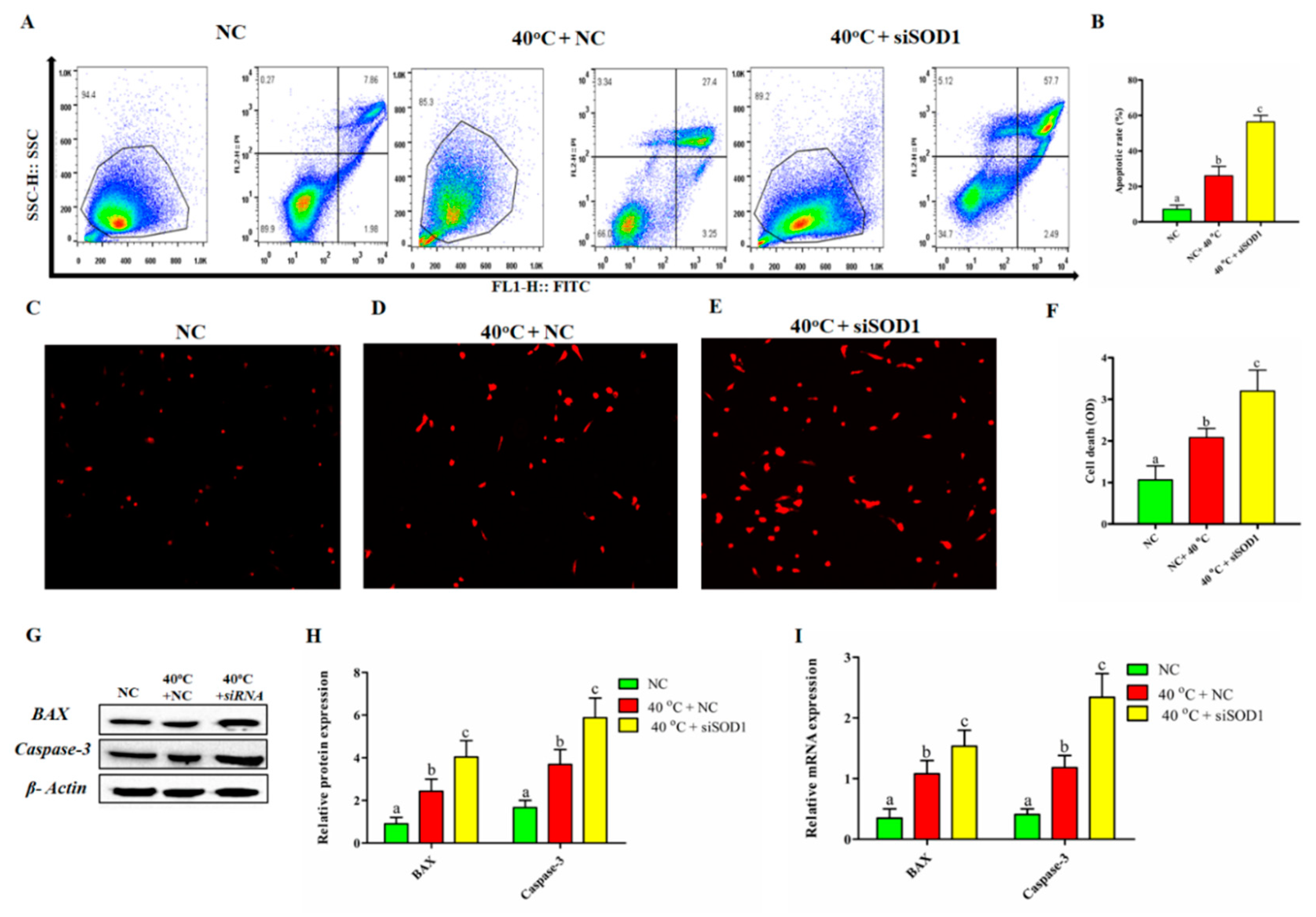
3.5. Silencing of SOD1 Promoted Apoptosis and Cell Death in GCs under Heat Stress
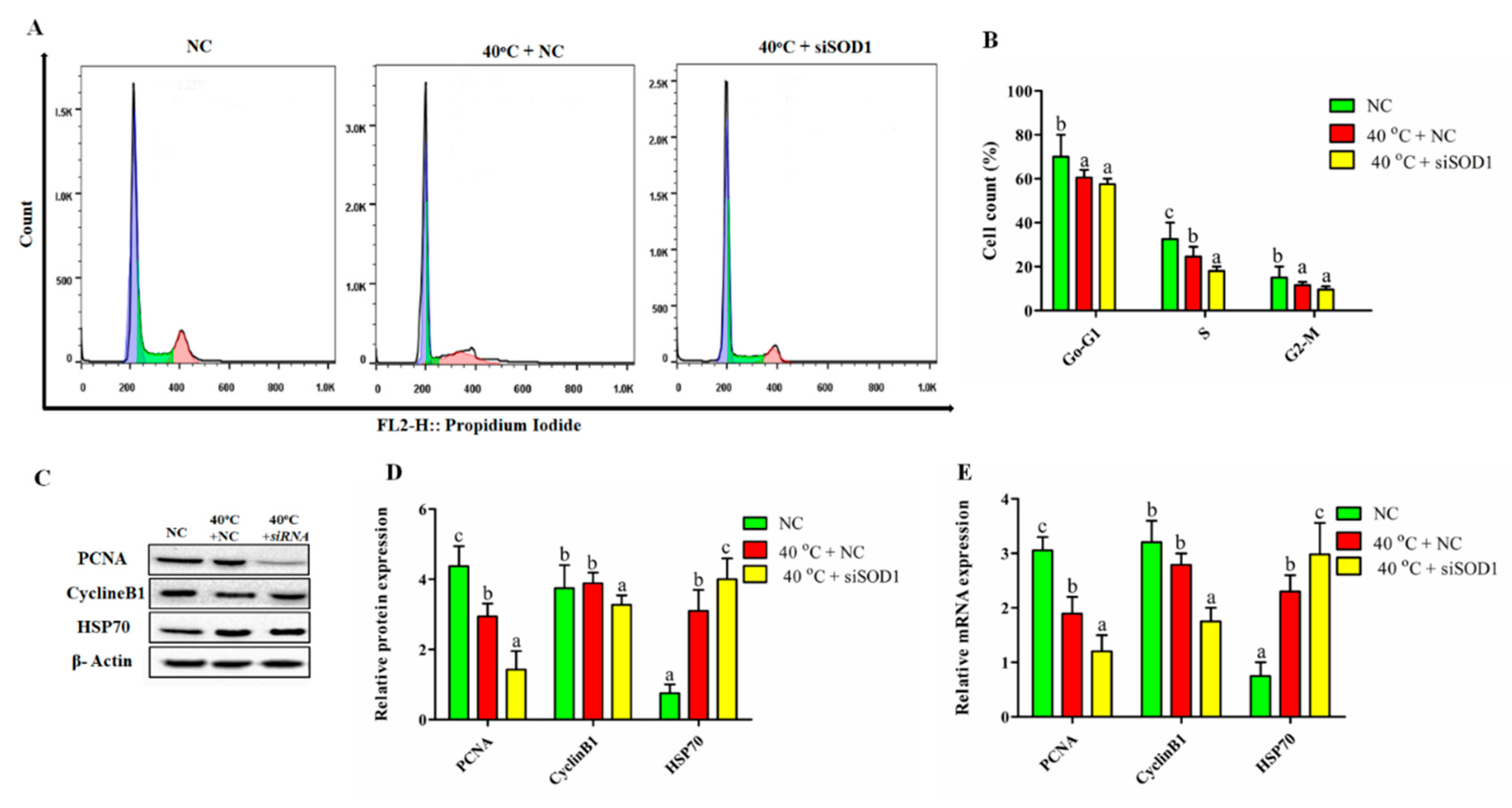
3.6. Silencing of SOD1 Altered Cell Cycle Transition in GCs under Heat Stress
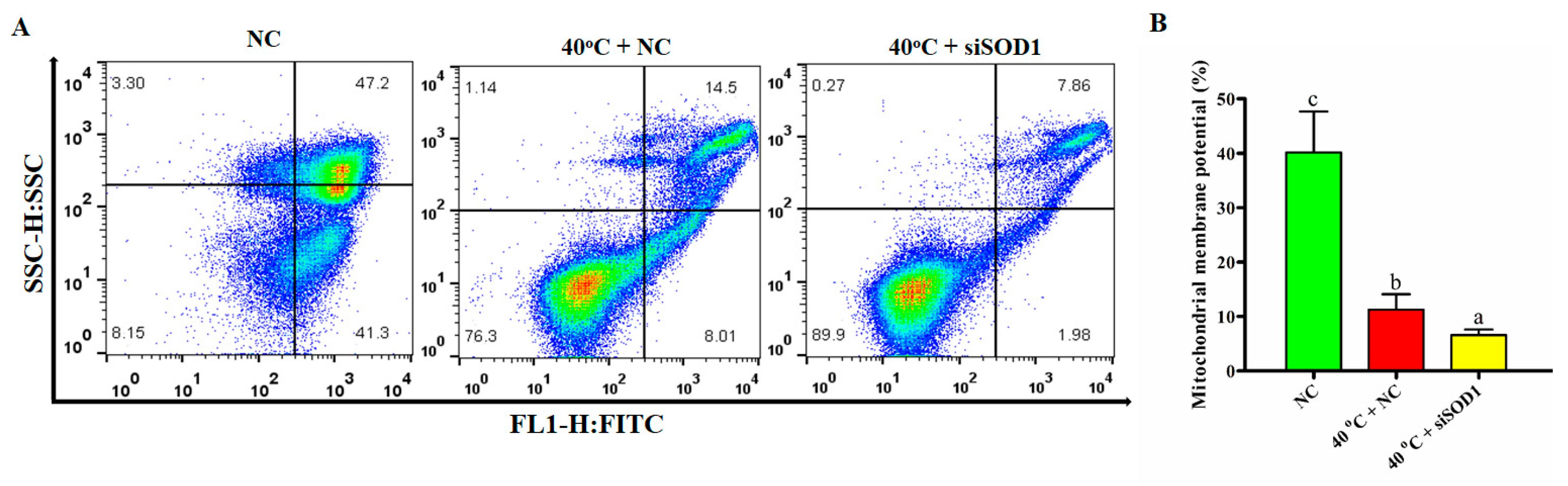
3.7. Disruption of Mitochondrial Membrane Potential of GCs under Heat Stress Caused by Silencing of SOD1
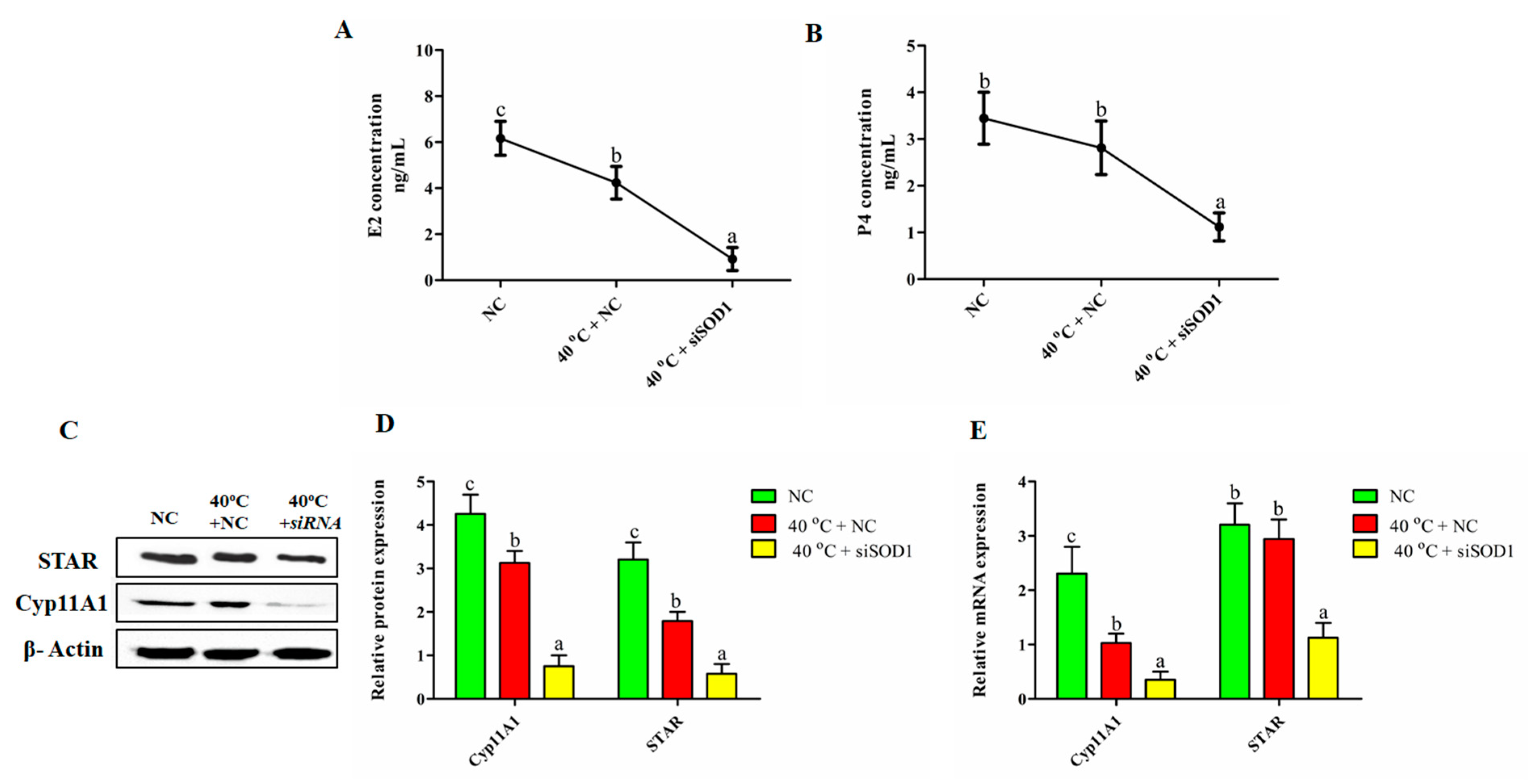
3.8. Impairment of the Synthesis of P4 and E2 in GCs under Heat Stress with SOD1 Silencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collier, R.J.; Dahl, G.E.; Vanbaale, M.J. Major advances associated with environmental effects on dairy cattle. J. Dairy Sci. 2006, 89, 1244–1253. [Google Scholar] [CrossRef]
- Wilson, S.J.; Kirby, C.J.; Koenigsfeld, A.T.; Keisler, D.H.; Lucy, M.C. Effects of Controlled Heat Stress on Ovarian Function of Dairy Cattle. 2. Heifers. J. Dairy Sci. 1998, 81, 2132–2138. [Google Scholar] [CrossRef]
- Torres-Júnior, J.R.S.; Pires, M.d.F.A.; de Sá, W.F.; Ferreira, A.d.M.; Viana, J.H.M.; Camargo, L.S.A.; Ramos, A.A.; Folhadella, I.M.; Polisseni, J.; de Freitas, C.; et al. Effect of maternal heat-stress on follicular growth and oocyte competence in Bos indicus cattle. Theriogenology 2008, 69, 155–166. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Wakamiya, K.; Kohka, M.; Yamamoto, Y.; Okuda, K. Summer heat stress affects prostaglandin synthesis in the bovine oviduct. Reproduction 2013, 146, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Shehab-El-Deen, M.A.M.M.; Leroy, J.L.M.R.; Fadel, M.S.; Saleh, S.Y.A.; Maes, D.; Van Soom, A. Biochemical changes in the follicular fluid of the dominant follicle of high producing dairy cows exposed to heat stress early post-partum. Anim. Reprod. Sci. 2010, 117, 189–200. [Google Scholar] [CrossRef]
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.Q.; Wu, X.; O’Brien, M.J.; Pendola, F.L.; Denegre, J.N.; Matzuk, M.M.; Eppig, J.J. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: Genetic evidence for an oocyte-granulosa cell regulatory loop. Dev. Biol. 2004, 276, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Khan, M.Z.; Umer, S.; Khan, I.M.; Xu, H. Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress. Animals 2020, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Morton, J.M.; Tranter, W.P.; Mayers, D.G.; Jonsson, N.N. Effects of environmental heat on conception rates in lactating dairy cows: Critical periods of exposure. J. Dairy Sci. 2007, 90, 2271–2278. [Google Scholar] [CrossRef]
- Petro, E.M.L.; Leroy, J.L.M.R.; Van Cruchten, S.J.M.; Covaci, A.; Jorssen, E.P.A.; Bols, P.E.J. Endocrine disruptors and female fertility: Focus on (bovine) ovarian follicular physiology. Theriogenology. 2012, 78, 1887–1900. [Google Scholar] [CrossRef]
- Voronina, E.; Lovasco, L.A.; Gyuris, A.; Baumgartner, R.A.; Parlow, A.F.; Freiman, R.N. Ovarian granulosa cell survival and proliferation requires the gonad-selective TFIID subunit TAF4b. Dev. Biol. 2007, 303, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Oktay, K.; Briggs, D.; Gosden, R.G. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J. Clin. Endocrinol. Metab. 1997, 82, 3748–3751. [Google Scholar] [CrossRef]
- Albertini, D.F.; Combelles, C.M.H.; Benecchi, E.; Carabatsos, M.J. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001, 121, 647–653. [Google Scholar] [CrossRef]
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.A.K.; Kikusato, M.; Sudo, S.; Amo, T.; Toyomizu, M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 157, 266–271. [Google Scholar] [CrossRef]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells. J. Anim. Sci. Biotechnol. 2020, 3, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.T.; Li, L.; Wu, F.; Zhao, P.; Yang, H.; Liu, Y.S.; Geng, Y.; Zhao, M.; Su, L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015, 5, 11497. [Google Scholar] [CrossRef] [Green Version]
- Roth, Z.; Arav, A.; Bor, A.; Zeron, Y.; Braw-Tal, R.; Wolfenson, D. Improvement of quality of oocytes collected in the autumn by enhanced removal of impaired follicles from previously heat-stressed cows. Reproduction 2001, 122, 737–744. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Tian, Z.; Wu, Y.; Wang, Y.; Fang, Y.; Lin, L.; Han, Y.; Wu, S.; Haq, I.U.; et al. Effects of chronic heat stress on granulosa cell apoptosis and follicular atresia in mouse ovary. J. Anim. Sci. Biotechnol. 2016, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Q.; Shen, M.; Wu, W.J.; Li, B.J.; Weng, Q.N.; Li, M.; Liu, H.L. Expression of PUMA in follicular granulosa cells regulated by foxo1 activation during oxidative stress. Reprod. Sci. 2015, 22, 696–705. [Google Scholar] [CrossRef]
- Peluso, J.J.; Pappalardo, A. Progesterone regulates granulosa cell viability through a protein kinase g-dependent mechanism that may involve 14-3-3σ1. Biol. Reprod. 2004, 71, 1870–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lazzari, F.; Bubacco, L.; Whitworth, A.J.; Bisaglia, M. Superoxide radical dismutation as new therapeutic strategy in Parkinson’s disease. Aging Dis. 2018, 9, 716–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, P.H.G.M.; Rossignol, R.; Dieteren, C.E.J.; Murphy, M.P.; Koopman, W.J.H. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Eskandari-Nasab, E.; Kharazi-Nejad, E.; Nakhaee, A.; Afzali, M.; Tabatabaei, S.P.; Tirgar-Fakheri, K.; Hashemi, M. 50-bp Ins/Del polymorphism of SOD1 is associated with increased risk of cardiovascular disease. Acta Med. Iran. 2014, 52, 591–595. [Google Scholar] [PubMed]
- Li, S.; Fu, L.; Tian, T.; Deng, L.; Li, H.; Xia, W.; Gong, Q. Disrupting SOD1 activity inhibits cell growth and enhances lipid accumulation in nasopharyngeal carcinoma. Cell Commun. Signal. 2018, 16, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Fang, J.; Liu, W. Superoxide dismutase coding of gene polymorphisms associated with susceptibility to Parkinson’s disease. J. Integr. Neurosci. 2019, 18, 299–303. [Google Scholar] [PubMed]
- Hammond, S.M.; Boettcher, S.; Caudy, A.A.; Kobayashi, R.; Hannon, G.J. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 2001, 293, 1146–1150. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Khan, M.Z.; Dou, J.; Umer, S.; XU, H.; Sammad, A.; Zhu, H.; Wang, Y. RNAi-Mediated silencing of catalase gene promotes apoptosis and impairs proliferation of bovine granulosa cells under heat stress. Animals 2020, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, D.T.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Alemu, T.W.; Pandey, H.O.; Wondim, D.S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 2017, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Tabayashi, D.; Latief, T.A.; Shimizu, T.; Oshima, I.; Kanai, Y. Alterations in follicular dynamics and steroidogenic abilities induced by heat stress during follicular recruitment in goats. Reproduction 2005, 129, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, J.; Luo, M.; Sun, Y.; Wang, G. The effect of heat stress on gene expression, synthesis of steroids, and apoptosis in bovine granulosa cells. Cell Stress Chaperones 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Katanani, Y.M.; Paula-Lopes, F.F.; Hansen, P.J. Effect of season and exposure to heat stress on oocyte competence in Holstein cows. J. Dairy Sci. 2002, 85, 390–396. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Ayres, H.; Chiaratti, M.R.; Ferraz, M.L.; Araújo, A.B.; Rodrigues, C.A.; Watanabe, Y.F. The low fertility of repeat-breeder cows during summer heat stress is related to a low oocyte competence to develop into blastocysts. J. Dairy Sci. 2011, 94, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, S.; Tang, Q.; Chen, P.; Yu, Y.; Tang, S. De novo assembly and characterization of transcriptome using Illumina paired-end sequencing and identification of CesA gene in ramie (Boehmeria nivea L. Gaud). BMC Genom. 2013, 14, 125. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, B.; Dong, W.; Yang, B.; Yang, Y.; Lin, X.; Gong, G. Humid heat exposure induced oxidative stress and apoptosis in cardiomyocytes through the angiotensin II signaling pathway. Heart Vessel. 2015, 30, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Tan, K.N.; Reyes-Farias, M.; De La Jara, N.; Ngo, S.T.; Garcia-Diaz, D.F.; Llanos, P.; Cires, M.J.; Borges, K. The deleterious effect of cholesterol and protection by quercetin on mitochondrial bioenergetics of pancreatic β-cells, glycemic control and inflammation: In vitro and in vivo studies. Redox Biol. 2016, 9, 229–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Luo, M.; Li, L.; Xiao, C.; Sun, Y.; Wang, G.L. Heat stress impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol. Cell. Biochem. 2016, 412, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; He, C.J.; Ji, P.Y.; Zhuo, Z.Y.; Tian, X.Z.; Wang, F.; Tan, D.X.; Liu, G.S. Effects of melatonin on the proliferation and apoptosis of sheep granulosa cells under thermal stress. Int. J. Mol. Sci. 2014, 15, 21090–21104. [Google Scholar] [CrossRef] [Green Version]
- Makarevich, A.V.; Olexiková, L.; Chrenek, P.; Kubovičová, E.; Fréharová, K.; Pivko, J. The effect of hyperthermia in vitro on vitality of rabbit preimplantation embryos. Physiol. Res. 2007, 56, 789–796. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Effect of two types of stress (heat shock/high temperature and malnutrition/serum deprivation) on porcine ovarian cell functions and their response to hormones. J. Exp. Biol. 2010, 213, 2125–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, D.; Li, K.; Wass, J.; Dolnikov, A.; Zeng, F.; Zhe, L.; Edwards, M. Heat-shock gene expression and cell cycle changes during mammalian embryonic development. Dev. Genet. 1993, 14, 127–136. [Google Scholar] [CrossRef]
- Sun, Y.L.; Zhang, J.; Ping, Z.G.; Wang, C.Q.; Sun, Y.F.; Chen, L.; Li, X.Y.; Li, C.J.; Zhu, X.L.; Liu, Z.; et al. Relationship Between Apoptosis and Proliferation in Granulosa and Theca Cells of Cystic Follicles in Sows. Reprod. Domest. Anim. 2012, 47, 601–608. [Google Scholar] [CrossRef]
- Chen, L.; Yi, K.; Sun, Y.; Sun, Y.; Tang, L.; Zhou, X. Expression of BDNF mRNA in porcine reproductive tissues during follicular phase and luteal phase and oocytes in GV and in vitro matured MII Stage. J. Anim. Vet. Adv. 2011, 10, 2571–2574. [Google Scholar]
- Scott, M.; Bonnefin, P.; Vieyra, D.; Boisvert, F.M.; Young, D.; Bazett-Jones, D.P.; Riabowol, K. UV-induced binding of ING1 to PCNA regulates the induction of apoptosis. J. Cell Sci. 2001, 114, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Ghate, N.; Das, A.; Chaudhuri, D.; Panja, S.; Mandal, N. Sundew plant, a potential source of anti-inflammatory agents, selectively induces G2/M arrest and apoptosis in MCF-7 cells through upregulation of p53 and Bax/Bcl-2 ratio. Cell Death Discov. 2016, 2, 15062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Vesco, A.P.; Gasparino, E. Production of reactive oxygen species, gene expression, and enzymatic activity in quail subjected to acute heat stress. J. Anim. Sci. 2013, 91, 582–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendelson, C.R.; Jiang, B.; Shelton, J.M.; Richardson, J.A.; Hinshelwood, M.M. Transcriptional regulation of aromatase in placenta and ovary. J. Steroid Biochem. Mol. Biol. 2005, 95, 25–33. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wu, Y.; Zhao, S.; Liu, Z.X.; Zeng, S.M.; Zhang, G.X. Lysosomes are involved in induction of steroidogenic acute regulatory protein (StAR) gene expression and progesterone synthesis through low-density lipoprotein in cultured bovine granulosa cells. Theriogenology 2015, 84, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Rekawiecki, R.; Nowik, M.; Kotwica, J. Stimulatory effect of LH, PGE2 and progesterone on StAR protein, cytochrome P450 cholesterol side chain cleavage and 3β hydroxysteroid dehydrogenase gene expression in bovine luteal cells. Prostaglandins Other Lipid Mediat. 2005, 78, 169–184. [Google Scholar] [CrossRef] [PubMed]


| Gene | Accession No. | Forward 5′→3′ | Reverse 5′→3′ |
|---|---|---|---|
| SOD1 | NM_201527.2 | TCAATAAGGAGCAGGGACGC | AAGCCGTGTATCGTGCAGTT |
| BAX | XM_003355974.2 | GGCTGGACATTGGACTTCCTTC | TGGTCACTGTCTGCCATGTGG |
| Caspase-3 | XM_005671704.1 | CTGGACTGTGGCATTGAGAC | GCAAAGGGACTGGAGAACC |
| CyclinB1 | NM_001170768.1 | AAGACGGAGCGGATCCAAAC | CCAGTGACTTCACGACCCAT |
| PCNA | NM_001291925.1 | GCGTTCATAGTCGTGTTCCG | TTCAAGATGGAGCCCTGGAC |
| STAR | NM_174189.3 | CCCATGGAGAGGCTTTATGA | TGATGACCGTGTCTTTTCCA |
| Cyp11A1 | NM_176644.2 | CTGGCATCTCCACAAAGACC | GTTCTCGATGTGGCGAAAGT |
| HSP70 | NM_001014912.1 | GGGGCCATGAAAACTGTTCG | TGGTGGAGATGTCTCAGGCT |
| GAPDH | NM_001034034.2 | GGTGCTGAGTATGTGGTGGA | GGCATTGCTGACAATCTTGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Khan, M.Z.; Dou, J.; Xu, H.; Liu, L.; Zhu, H.; Wang, Y. SOD1 Gene Silencing Promotes Apoptosis and Suppresses Proliferation of Heat-Stressed Bovine Granulosa Cells via Induction of Oxidative Stress. Vet. Sci. 2021, 8, 326. https://doi.org/10.3390/vetsci8120326
Khan A, Khan MZ, Dou J, Xu H, Liu L, Zhu H, Wang Y. SOD1 Gene Silencing Promotes Apoptosis and Suppresses Proliferation of Heat-Stressed Bovine Granulosa Cells via Induction of Oxidative Stress. Veterinary Sciences. 2021; 8(12):326. https://doi.org/10.3390/vetsci8120326
Chicago/Turabian StyleKhan, Adnan, Muhammad Zahoor Khan, Jinhuan Dou, Huitao Xu, Lei Liu, Huabin Zhu, and Yachun Wang. 2021. "SOD1 Gene Silencing Promotes Apoptosis and Suppresses Proliferation of Heat-Stressed Bovine Granulosa Cells via Induction of Oxidative Stress" Veterinary Sciences 8, no. 12: 326. https://doi.org/10.3390/vetsci8120326
APA StyleKhan, A., Khan, M. Z., Dou, J., Xu, H., Liu, L., Zhu, H., & Wang, Y. (2021). SOD1 Gene Silencing Promotes Apoptosis and Suppresses Proliferation of Heat-Stressed Bovine Granulosa Cells via Induction of Oxidative Stress. Veterinary Sciences, 8(12), 326. https://doi.org/10.3390/vetsci8120326









