Sarcoplasmic Reticulum from Horse Gluteal Muscle Is Poised for Enhanced Calcium Transport
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Purification of SR Vesicles from Horse and Rabbit Muscle
2.4. SDS-PAGE and Coomassie Densitometry
2.5. Oxalate-Facilitated 45Ca2+ Transport Assay
2.6. Proteinase K Assay of SERCA Conformational States
2.7. Experimental Design, Statistical Analysis, and Data Presentation
3. Results
3.1. Horse SR Vesicles Contain an Abundant Amount of SERCA Protein, Although at a Lower Level Than Rabbit SR Vesicles
3.2. Horse SR Vesicles Contain an Abundant Level of CASQ Protein, Similar to Rabbit SR Vesicles
3.3. Horse SR Vesicles Show Greater ATP-Dependent Ca2+ Transport Than Rabbit SR Vesicles
3.4. Horse and Rabbit SERCA Show Similar Temperature-Dependence of Ca2+-Activated ATPase Activity
3.5. Horse and Rabbit SERCA Show Similar Ca2+-Dependent Cleavage by Proteinase K
4. Discussion
4.1. Analysis of SERCA Ca2+ Transport and ATPase Activities in SR Vesicles from Horse and Rabbit Muscle
4.2. The Relative Ratio of Ca2+ Transport to ATP Hydrolysis Is Greater for Horse SR Vesicles Than Rabbit SR Vesicles
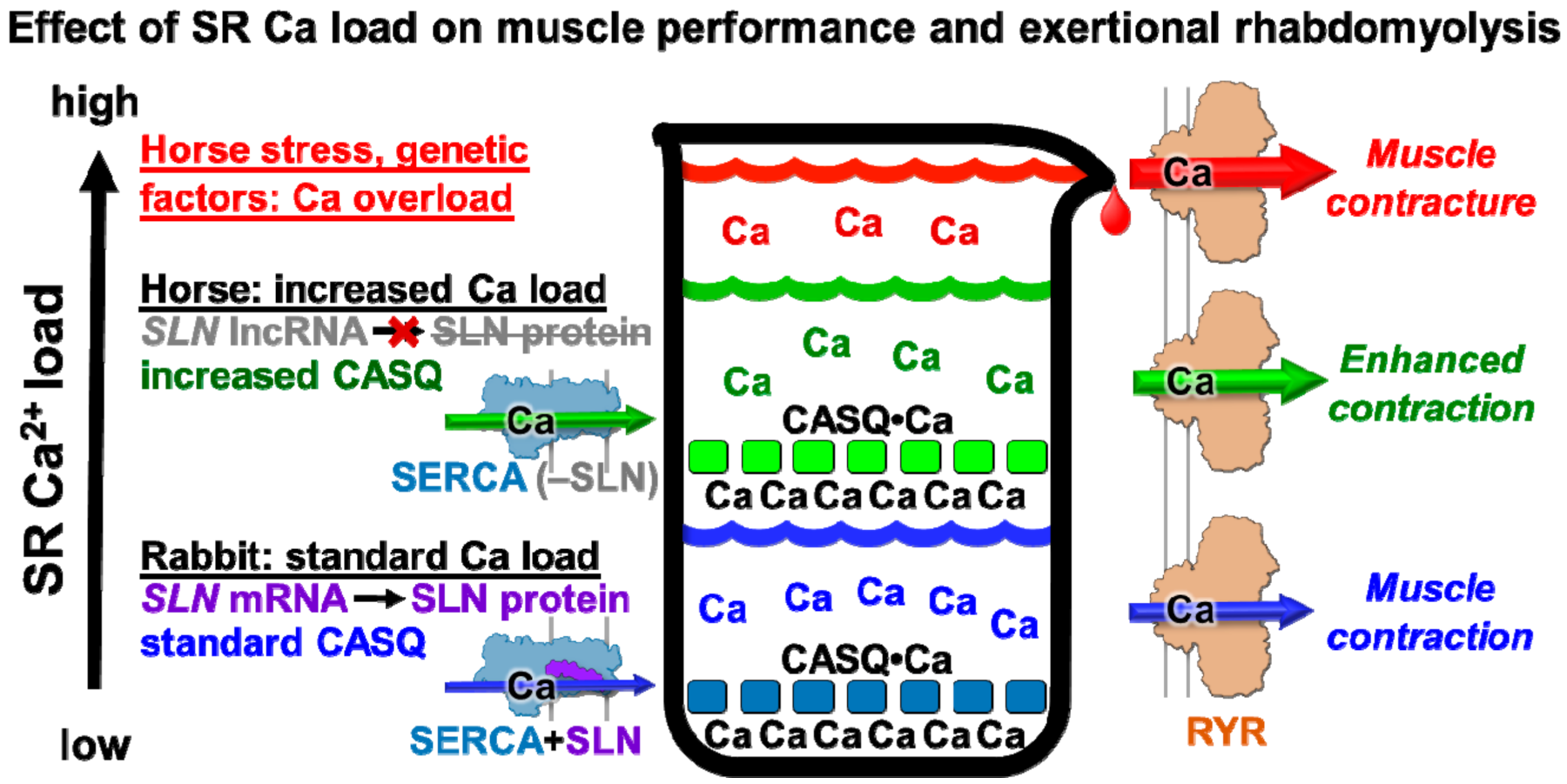
4.3. Proposed Physiological Effects of Minimal Expression of SLN and Abundant Expression of CASQ on Horse Muscular Performance
4.4. Proposed Role of SLN Expression in Horse Exertional Rhabdomyolysis and Relationship to Human Muscular Dystrophies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valberg, S.J. Muscle conditions affecting sport horses. Vet. Clin. N. Am. Equine Pract. 2018, 34, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Mickelson, J.R.; Valberg, S.J. The genetics of skeletal muscle disorders in horses. Annu. Rev. Anim. Biosci. 2015, 3, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Isgren, C.M.; Upjohn, M.M.; Fernandez-Fuente, M.; Massey, C.; Pollott, G.; Verheyen, K.L.; Piercy, R.J. Epidemiology of exertional rhabdomyolysis susceptibility in standardbred horses reveals associated risk factors and underlying enhanced performance. PLoS ONE 2010, 5, e11594. [Google Scholar] [CrossRef] [PubMed]
- MacLeay, J.M.; Sorum, S.A.; Valberg, S.J.; Marsh, W.E.; Sorum, M.D. Epidemiologic analysis of factors influencing exertional rhabdomyolysis in Thoroughbreds. Am. J. Vet. Res. 1999, 60, 1562–1566. [Google Scholar]
- Ward, T.L.; Valberg, S.J.; Gallant, E.M.; Mickelson, J.R. Calcium regulation by skeletal muscle membranes of horses with recurrent exertional rhabdomyolysis. Am. J. Vet. Res. 2000, 61, 242–247. [Google Scholar] [CrossRef]
- Mlekoday, J.A.; Mickelson, J.R.; Valberg, S.J.; Horton, J.H.; Gallant, E.M.; Thompson, L.V. Calcium sensitivity of force production and myofibrillar ATPase activity in muscles from Thoroughbreds with recurrent exertional rhabdomyolysis. Am. J. Vet. Res. 2001, 62, 1647–1652. [Google Scholar] [CrossRef]
- Lentz, L.R.; Valberg, S.J.; Mickelson, J.R.; Gallant, E.M. In vitro contractile responses and contracture testing of skeletal muscle from Quarter Horses with exertional rhabdomyolysis. Am. J. Vet. Res. 1999, 60, 684–688. [Google Scholar]
- Aldrich, K.; Velez-Irizarry, D.; Fenger, C.; Schott, M.; Valberg, S.J. Pathways of calcium regulation, electron transport, and mitochondrial protein translation are molecular signatures of susceptibility to recurrent exertional rhabdomyolysis in Thoroughbred racehorses. PLoS ONE 2021, 16, e0244556. [Google Scholar] [CrossRef]
- Valberg, S.J.; Soave, K.; Williams, Z.J.; Perumbakkam, S.; Schott, M.; Finno, C.J.; Petersen, J.L.; Fenger, C.; Autry, J.M.; Thomas, D.D. Coding sequences of sarcoplasmic reticulum calcium ATPase regulatory peptides and expression of calcium regulatory genes in recurrent exertional rhabdomyolysis. J. Vet. Intern. Med. 2019, 33, 933–941. [Google Scholar] [CrossRef]
- Autry, J.M.; Karim, C.B.; Perumbakkam, S.; Finno, C.J.; McKenzie, E.C.; Thomas, D.D.; Valberg, S.J. Sarcolipin Exhibits Abundant RNA Transcription and Minimal Protein Expression in Horse Gluteal Muscle. Vet. Sci. 2020, 7, 178. [Google Scholar] [CrossRef]
- Autry, J.M.; Karim, C.B.; Cocco, M.; Carlson, S.F.; Thomas, D.D.; Valberg, S.J. Purification of sarcoplasmic reticulum vesicles from horse gluteal muscle. Anal. Biochem. 2020, 610, 113965. [Google Scholar] [CrossRef]
- Montigny, C.; Decottignies, P.; Le Marechal, P.; Capy, P.; Bublitz, M.; Olesen, C.; Møller, J.V.; Nissen, P.; le Maire, M. S-palmitoylation and s-oleoylation of rabbit and pig sarcolipin. J. Biol. Chem. 2014, 289, 33850–33861. [Google Scholar] [CrossRef]
- Vangheluwe, P.; Schuermans, M.; Zador, E.; Waelkens, E.; Raeymaekers, L.; Wuytack, F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem. J. 2005, 389, 151–159. [Google Scholar] [CrossRef]
- Fajardo, V.A.; Bombardier, E.; Vigna, C.; Devji, T.; Bloemberg, D.; Gamu, D.; Gramolini, A.O.; Quadrilatero, J.; Tupling, A.R. Co-expression of SERCA isoforms, phospholamban and sarcolipin in human skeletal muscle fibers. PLoS ONE 2013, 8, e84304. [Google Scholar] [CrossRef]
- Babu, G.J.; Bhupathy, P.; Carnes, C.A.; Billman, G.E.; Periasamy, M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J. Mol. Cell. Cardiol. 2007, 43, 215–222. [Google Scholar] [CrossRef]
- Qaisar, R.; Bhaskaran, S.; Premkumar, P.; Ranjit, R.; Natarajan, K.S.; Ahn, B.; Riddle, K.; Claflin, D.R.; Richardson, A.; Brooks, S.V.; et al. Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J. Cachexia Sarcopenia Muscle 2018, 9, 1003–1017. [Google Scholar] [CrossRef]
- Odermatt, A.; Becker, S.; Khanna, V.K.; Kurzydlowski, K.; Leisner, E.; Pette, D.; MacLennan, D.H. Sarcolipin regulates the activity of SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 1998, 273, 12360–12369. [Google Scholar] [CrossRef]
- Smith, W.S.; Broadbridge, R.; East, J.M.; Lee, A.G. Sarcolipin uncouples hydrolysis of ATP from accumulation of Ca2+ by the Ca2+-ATPase of skeletal-muscle sarcoplasmic reticulum. Biochem. J. 2002, 361, 277–286. [Google Scholar] [CrossRef]
- Gorski, P.A.; Glaves, J.P.; Vangheluwe, P.; Young, H.S. Sarco(endo)plasmic reticulum calcium ATPase (SERCA) inhibition by sarcolipin is encoded in its luminal tail. J. Biol. Chem. 2013, 288, 8456–8467. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Shaikh, S.A.; Sopariwala, D.H.; Bal, N.C.; Bruhn, D.S.; Kopec, W.; Khandelia, H.; Periasamy, M. The N terminus of sarcolipin plays an important role in uncoupling sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) ATP hydrolysis from Ca2+ transport. J. Biol. Chem. 2015, 290, 14057–14067. [Google Scholar] [CrossRef]
- Autry, J.M.; Thomas, D.D.; Espinoza-Fonseca, L.M. Sarcolipin Promotes Uncoupling of the SERCA Ca(2+) Pump by Inducing a Structural Rearrangement in the Energy-Transduction Domain. Biochemistry 2016, 55, 6083–6086. [Google Scholar] [CrossRef] [PubMed]
- Barbot, T.; Beswick, V.; Montigny, C.; Quiniou, E.; Jamin, N.; Mouawad, L. Deciphering the Mechanism of Inhibition of SERCA1a by Sarcolipin Using Molecular Simulations. Front. Mol. Biosci. 2020, 7, 606254. [Google Scholar] [CrossRef] [PubMed]
- Montigny, C.; Huang, D.L.; Beswick, V.; Barbot, T.; Jaxel, C.; le Maire, M.; Zheng, J.S.; Jamin, N. Sarcolipin alters SERCA1a interdomain communication by impairing binding of both calcium and ATP. Sci. Rep. 2021, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Gramolini, A.O.; Trivieri, M.G.; Oudit, G.Y.; Kislinger, T.; Li, W.; Patel, M.M.; Emili, A.; Kranias, E.G.; Backx, P.H.; Maclennan, D.H. Cardiac-specific overexpression of sarcolipin in phospholamban null mice impairs myocyte function that is restored by phosphorylation. Proc. Natl. Acad. Sci. USA 2006, 103, 2446–2451. [Google Scholar] [CrossRef]
- Bhupathy, P.; Babu, G.J.; Ito, M.; Periasamy, M. Threonine-5 at the N-terminus can modulate sarcolipin function in cardiac myocytes. J. Mol. Cell. Cardiol. 2009, 47, 723–729. [Google Scholar] [CrossRef]
- Barbot, T.; Montigny, C.; Decottignies, P.; le Maire, M.; Jaxel, C.; Jamin, N.; Beswick, V. Functional and structural insights into sarcolipin, a regulator of the sarco-endoplasmic reticulum Ca2+-ATPases. In Regulation of Ca2+-ATPases, V-ATPases and F-ATPases, 14th ed.; Chakraborti, S., Dhalla, N.S., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 153–186. [Google Scholar]
- Tupling, A.R.; Bombardier, E.; Gupta, S.C.; Hussain, D.; Vigna, C.; Bloemberg, D.; Quadrilatero, J.; Trivieri, M.G.; Babu, G.J.; Backx, P.H.; et al. Enhanced Ca2+ transport and muscle relaxation in skeletal muscle from sarcolipin-null mice. Am. J. Physiol.-Cell Physiol. 2011, 301, C841–C849. [Google Scholar] [CrossRef]
- Shanmugam, M.; Molina, C.E.; Gao, S.; Severac-Bastide, R.; Fischmeister, R.; Babu, G.J. Decreased sarcolipin protein expression and enhanced sarco(endo)plasmic reticulum Ca2+ uptake in human atrial fibrillation. Biochem. Biophys. Res. Commun. 2011, 410, 97–101. [Google Scholar] [CrossRef]
- Molina, C.E.; Abu-Taha, I.H.; Wang, Q.; Rosello-Diez, E.; Kamler, M.; Nattel, S.; Ravens, U.; Wehrens, X.H.T.; Hove-Madsen, L.; Heijman, J.; et al. Profibrotic, electrical, and calcium-handling remodeling of the atria in heart failure patients with and without atrial fibrillation. Front. Physiol. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Wakizaka, M.; Eshima, H.; Tanaka, Y.; Shirakawa, H.; Poole, D.C.; Kano, Y. In vivo Ca(2+) dynamics induced by Ca(2+) injection in individual rat skeletal muscle fibers. Physiol. Rep. 2017, 5, e13180. [Google Scholar] [CrossRef]
- Murayama, T.; Kurebayashi, N.; Ogawa, H.; Yamazawa, T.; Oyamada, H.; Suzuki, J.; Kanemaru, K.; Oguchi, K.; Iino, M.; Sakurai, T. Genotype-phenotype correlations of malignant hyperthermia and central core disease mutations in the central region of the RYR1 channel. Hum. Mutat. 2016, 37, 1231–1241. [Google Scholar] [CrossRef]
- Kong, H.; Wang, R.; Chen, W.; Zhang, L.; Chen, K.; Shimoni, Y.; Duff, H.J.; Chen, S.R. Skeletal and cardiac ryanodine receptors exhibit different responses to Ca2+ overload and luminal Ca2+. Biophys. J. 2007, 92, 2757–2770. [Google Scholar] [CrossRef]
- Kim, E.; Youn, B.; Kemper, L.; Campbell, C.; Milting, H.; Varsanyi, M.; Kang, C. Characterization of human cardiac calsequestrin and its deleterious mutants. J. Mol. Biol. 2007, 373, 1047–1057. [Google Scholar] [CrossRef]
- Sanchez, E.J.; Lewis, K.M.; Munske, G.R.; Nissen, M.S.; Kang, C. Glycosylation of skeletal calsequestrin: Implications for its function. J. Biol. Chem. 2012, 287, 3042–3050. [Google Scholar] [CrossRef]
- McDonald, A.G.; Boyce, S.; Tipton, K.F. ExplorEnz: The primary source of the IUBMB enzyme list. Nucleic Acids Res. 2009, 37, D593–D597. [Google Scholar] [CrossRef]
- Ikemoto, N.; Sreter, F.A.; Gergely, J. Structural features of the surface of the vesicles of FSR--lack of functional role in Ca2+ uptake and ATPase activity. Arch. Biochem. Biophys. 1971, 147, 571–582. [Google Scholar] [CrossRef]
- Autry, J.M.; Jones, L.R. Functional co-expression of the canine cardiac Ca2+ pump and phospholamban in Spodoptera frugiperda (Sf21) cells reveals new insights on ATPase regulation. J. Biol. Chem. 1997, 272, 15872–15880. [Google Scholar] [CrossRef]
- Jones, L.R.; Besch, H.R., Jr.; Watanabe, A.M. Regulation of the calcium pump of cardiac sarcoplasmic reticulum. Interactive roles of potassium and ATP on the phosphoprotein intermediate of the (K+,Ca2+)-ATPase. J. Biol. Chem. 1978, 253, 1643–1653. [Google Scholar] [CrossRef]
- Danko, S.; Yamasaki, K.; Daiho, T.; Suzuki, H. Membrane perturbation of ADP-insensitive phosphoenzyme of Ca(2+)-ATPase modifies gathering of transmembrane helix M2 with cytoplasmic domains and luminal gating. Sci. Rep. 2017, 7, 41172. [Google Scholar] [CrossRef]
- Møller, J.V.; Lenoir, G.; Marchand, C.; Montigny, C.; le Maire, M.; Toyoshima, C.; Juul, B.S.; Champeil, P. Calcium transport by sarcoplasmic reticulum Ca(2+)-ATPase. Role of the A domain and its C-terminal link with the transmembrane region. J. Biol. Chem. 2002, 277, 38647–38659. [Google Scholar] [CrossRef]
- Autry, J.M.; Rubin, J.E.; Svensson, B.; Li, J.; Thomas, D.D. Nucleotide activation of the Ca-ATPase. J. Biol. Chem. 2012, 287, 39070–39082. [Google Scholar] [CrossRef]
- Shutova, A.N.; Storey, K.B.; Lopina, O.D.; Rubtsov, A.M. Comparative characteristics of sarcoplasmic reticulum preparations from skeletal muscles of the ground squirrel Spermophilus undulatus, rats, and rabbits. Biochem. C/C Biokhimiia 1999, 64, 1250–1257. [Google Scholar]
- Kumar, S.; Li, C.; Montigny, C.; le Maire, M.; Barth, A. Conformational changes of recombinant Ca2+-ATPase studied by reaction-induced infrared difference spectroscopy. FEBS J. 2013, 280, 5398–5407. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Smyth, N.; Broadbridge, R.; Council, C.E.; Lee, A.G.; Stocker, C.J.; Hislop, D.C.; Arch, J.R.; Cawthorne, M.A.; Malcolm East, J. The effects of sarcolipin over-expression in mouse skeletal muscle on metabolic activity. Arch. Biochem. Biophys. 2015, 569, 26–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murphy, R.M.; Larkins, N.T.; Mollica, J.P.; Beard, N.A.; Lamb, G.D. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J. Physiol. 2009, 587, 443–460. [Google Scholar] [CrossRef]
- Cala, S.E.; Jones, L.R. Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J. Biol. Chem. 1983, 258, 11932–11936. [Google Scholar] [CrossRef]
- Kozlowski, L.P. IPC—Isoelectric point calculator. Biol. Direct 2016, 11, 55. [Google Scholar] [CrossRef]
- Jones, L.R.; Besch, H.R., Jr.; Watanabe, A.M. Monovalent cation stimulation of Ca2+ uptake by cardiac membrane vesicles. J. Biol. Chem. 1977, 252, 3315–3323. [Google Scholar] [CrossRef]
- Jones, L.R.; Besch, H.R., Jr.; Fleming, J.W.; McConnaughey, M.M.; Watanabe, A.M. Separation of vesicles of cardiac sarcolemma from vesicles of cardiac sarcoplasmic reticulum. Comparative biochemical analysis of component activities. J. Biol. Chem. 1979, 254, 530–539. [Google Scholar] [CrossRef]
- Jones, L.R.; Cala, S.E. Biochemical evidence for functional heterogeneity of cardiac sarcoplasmic reticulum vesicles. J. Biol. Chem. 1981, 256, 11809–11818. [Google Scholar] [CrossRef]
- Borchman, D.; Simon, R.; Bicknell-Brown, E. Variation in the lipid composition of rabbit muscle sarcoplasmic reticulum membrane with muscle type. J. Biol. Chem. 1982, 257, 14136–14139. [Google Scholar] [CrossRef]
- Smith, G.L.; Eisner, D.A. Calcium Buffering in the Heart in Health and Disease. Circulation 2019, 139, 2358–2371. [Google Scholar] [CrossRef]
- Inesi, G.; Tadini-Buoninsegni, F. Ca(2+)/H (+) exchange, lumenal Ca(2+) release and Ca (2+)/ATP coupling ratios in the sarcoplasmic reticulum ATPase. J. Cell Commun. Signal. 2014, 8, 5–11. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Holland, P.C. Calcium transport in sarcoplasmic reticulum. Annu. Rev. Biophys. Bioeng. 1975, 4, 377–404. [Google Scholar] [CrossRef]
- Sagara, Y.; Inesi, G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J. Biol. Chem. 1991, 266, 13503–13506. [Google Scholar] [CrossRef]
- Byrd, S.K.; McCutcheon, L.J.; Hodgson, D.R.; Gollnick, P.D. Altered sarcoplasmic reticulum function after high-intensity exercise. J. Appl. Physiol. 1989, 67, 2072–2077. [Google Scholar] [CrossRef]
- Beech, J.; Lindborg, S.; Fletcher, J.E.; Lizzo, F.; Tripolitis, L.; Braund, K. Caffeine contractures, twitch characteristics and the threshold for Ca(2+)-induced Ca2+ release in skeletal muscle from horses with chronic intermittent rhabdomyolysis. Res. Vet. Sci. 1993, 54, 110–117. [Google Scholar] [CrossRef]
- Wilson, J.A.; Kronfeld, D.S.; Gay, L.; Wilson, T.M. Isolating equine sarcoplasmic reticulum: Its function during high intensity repeated springs. Equine Vet. J. 1995, 27 (Suppl. 18), 252–255. [Google Scholar] [CrossRef]
- Wilson, J.A.; Kronfeld, D.S.; Gay, L.S.; Williams, J.H.; Wilson, T.M.; Lindinger, M.I. Sarcoplasmic reticulum responses to repeated sprints are affected by conditioning of horses. J. Anim. Sci. 1998, 76, 3065–3071. [Google Scholar] [CrossRef]
- Marlin, D.J.; Scott, C.M.; Roberts, C.A.; Casas, I.; Holah, G.; Schroter, R.C. Post exercise changes in compartmental body temperature accompanying intermittent cold water cooling in the hyperthermic horse. Equine Vet. J. 1998, 30, 28–34. [Google Scholar] [CrossRef]
- Ramirez-Soto, I.; Rodriguez, E.; Alvarez, R.; Quiroz, E.; Ortega, A. Intracellular effect of beta3-adrenoceptor agonist Carazolol on skeletal muscle, a direct interaction with SERCA. Cell Calcium 2019, 79, 20–26. [Google Scholar] [CrossRef]
- Toyoshima, C.; Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 2002, 418, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.V.; Olesen, C.; Winther, A.M.; Nissen, P. The sarcoplasmic Ca2+-ATPase: Design of a perfect chemi-osmotic pump. Q Rev. Biophys. 2010, 43, 501–566. [Google Scholar] [CrossRef] [PubMed]
- Tsunekawa, N.; Ogawa, H.; Tsueda, J.; Akiba, T.; Toyoshima, C. Mechanism of the E2 to E1 transition in Ca(2+) pump revealed by crystal structures of gating residue mutants. Proc. Natl. Acad. Sci. USA 2018, 115, 12722–12727. [Google Scholar] [CrossRef] [PubMed]
- Autry, J.M.; Svensson, B.; Espinoza-Fonseca, L.M.; Valberg, S.J.; Thomas, D.D. Homology structural modeling and comparative sequence analysis of the horse SERCA calcium pump and sarcolipin inhibitory subunit. 2021. submitted. [Google Scholar]
- Drogemuller, C.; Drogemuller, M.; Leeb, T.; Mascarello, F.; Testoni, S.; Rossi, M.; Gentile, A.; Damiani, E.; Sacchetto, R. Identification of a missense mutation in the bovine ATP2A1 gene in congenital pseudomyotonia of Chianina cattle: An animal model of human Brody disease. Genomics 2008, 92, 474–477. [Google Scholar] [CrossRef]
- Sacchetto, R.; Testoni, S.; Gentile, A.; Damiani, E.; Rossi, M.; Liguori, R.; Drogemuller, C.; Mascarello, F. A defective SERCA1 protein is responsible for congenital pseudomyotonia in Chianina cattle. Am. J. Pathol. 2009, 174, 565–573. [Google Scholar] [CrossRef]
- Grunberg, W.; Sacchetto, R.; Wijnberg, I.; Neijenhuis, K.; Mascarello, F.; Damiani, E.; Drogemuller, C. Pseudomyotonia, a muscle function disorder associated with an inherited ATP2A1 (SERCA1) defect in a Dutch Improved Red and White cross-breed calf. Neuromuscul. Disord. 2010, 20, 467–470. [Google Scholar] [CrossRef]
- Fajardo, V.A.; Rietze, B.A.; Chambers, P.J.; Bellissimo, C.; Bombardier, E.; Quadrilatero, J.; Tupling, A.R. Effects of sarcolipin deletion on skeletal muscle adaptive responses to functional overload and unload. Am. J. Physiol.-Cell Physiol. 2017, 313, C154–C161. [Google Scholar] [CrossRef]
- Guerrero-Hernandez, A.; Sanchez-Vazquez, V.H.; Martinez-Martinez, E.; Sandoval-Vazquez, L.; Perez-Rosas, N.C.; Lopez-Farias, R.; Dagnino-Acosta, A. Sarco-Endoplasmic Reticulum Calcium Release Model Based on Changes in the Luminal Calcium Content. Adv. Exp. Med. Biol. 2020, 1131, 337–370. [Google Scholar] [CrossRef]
- Batiste, S.M.; Blackwell, D.J.; Kim, K.; Kryshtal, D.O.; Gomez-Hurtado, N.; Rebbeck, R.T.; Cornea, R.L.; Johnston, J.N.; Knollmann, B.C. Unnatural verticilide enantiomer inhibits type 2 ryanodine receptor-mediated calcium leak and is antiarrhythmic. Proc. Natl. Acad. Sci. USA 2019, 116, 4810–4815. [Google Scholar] [CrossRef]
- Nikolaienko, R.; Bovo, E.; Rebbeck, R.T.; Kahn, D.; Thomas, D.D.; Cornea, R.L.; Zima, A.V. The functional significance of redox-mediated intersubunit cross-linking in regulation of human type 2 ryanodine receptor. Redox Biol. 2020, 37, 101729. [Google Scholar] [CrossRef]
- Lentz, L.R.; Valberg, S.J.; Balog, E.M.; Mickelson, J.R.; Gallant, E.M. Abnormal regulation of muscle contraction in horses with recurrent exertional rhabdomyolysis. Am. J. Vet. Res. 1999, 60, 992–999. [Google Scholar]
- Lentz, L.R.; Valberg, S.J.; Herold, L.V.; Onan, G.W.; Mickelson, J.R.; Gallant, E.M. Myoplasmic calcium regulation in myotubes from horses with recurrent exertional rhabdomyolysis. Am. J. Vet. Res. 2002, 63, 1724–1731. [Google Scholar] [CrossRef]
- Cole, F.L.; Mellor, D.J.; Hodgson, D.R.; Reid, S.W. Prevalence and demographic characteristics of exertional rhabdomyolysis in horses in Australia. Vet. Rec. 2004, 155, 625–630. [Google Scholar] [CrossRef]
- Lopez, J.R.; Linares, N.; Cordovez, G.; Terzic, A. Elevated myoplasmic calcium in exercise-induced equine rhabdomyolysis. Pflüg. Arch. 1995, 430, 293–295. [Google Scholar] [CrossRef]
- Voit, A.; Patel, V.; Pachon, R.; Shah, V.; Bakhutma, M.; Kohlbrenner, E.; McArdle, J.J.; Dell’Italia, L.J.; Mendell, J.R.; Xie, L.H.; et al. Reducing sarcolipin expression mitigates Duchenne muscular dystrophy and associated cardiomyopathy in mice. Nat. Commun. 2017, 8, 1068. [Google Scholar] [CrossRef]
- Niranjan, N.; Mareedu, S.; Tian, Y.; Kodippili, K.; Fefelova, N.; Voit, A.; Xie, L.H.; Duan, D.; Babu, G.J. Sarcolipin overexpression impairs myogenic differentiation in Duchenne muscular dystrophy. Am. J. Physiol.-Cell Physiol. 2019, 317, C813–C824. [Google Scholar] [CrossRef]
- Fajardo, V.A.; Chambers, P.J.; Juracic, E.S.; Rietze, B.A.; Gamu, D.; Bellissimo, C.; Kwon, F.; Quadrilatero, J.; Russell Tupling, A. Sarcolipin deletion in mdx mice impairs calcineurin signalling and worsens dystrophic pathology. Hum. Mol. Genet. 2018, 27, 4094–4102. [Google Scholar] [CrossRef]
- Valberg, S.J. Muscle Anatomy: Adaptations to Exercise and Training. In The Athletic Horse: Principles and Practice of Equine Sports Medicine, 2nd ed.; Hodgson, D.R., McKeever, K.H., McGowan, C.M., Eds.; Saunders: New York, NY, USA, 2014; pp. 174–201. [Google Scholar]
- Valberg, S.J.; Perumbakkam, S.; McKenzie, E.C.; Finno, C.J. Proteome and transcriptome profiling of equine myofibrillar myopathy identifies diminished peroxiredoxin 6 and altered cysteine metabolic pathways. Physiol. Genom. 2018, 50, 1036–1050. [Google Scholar] [CrossRef]

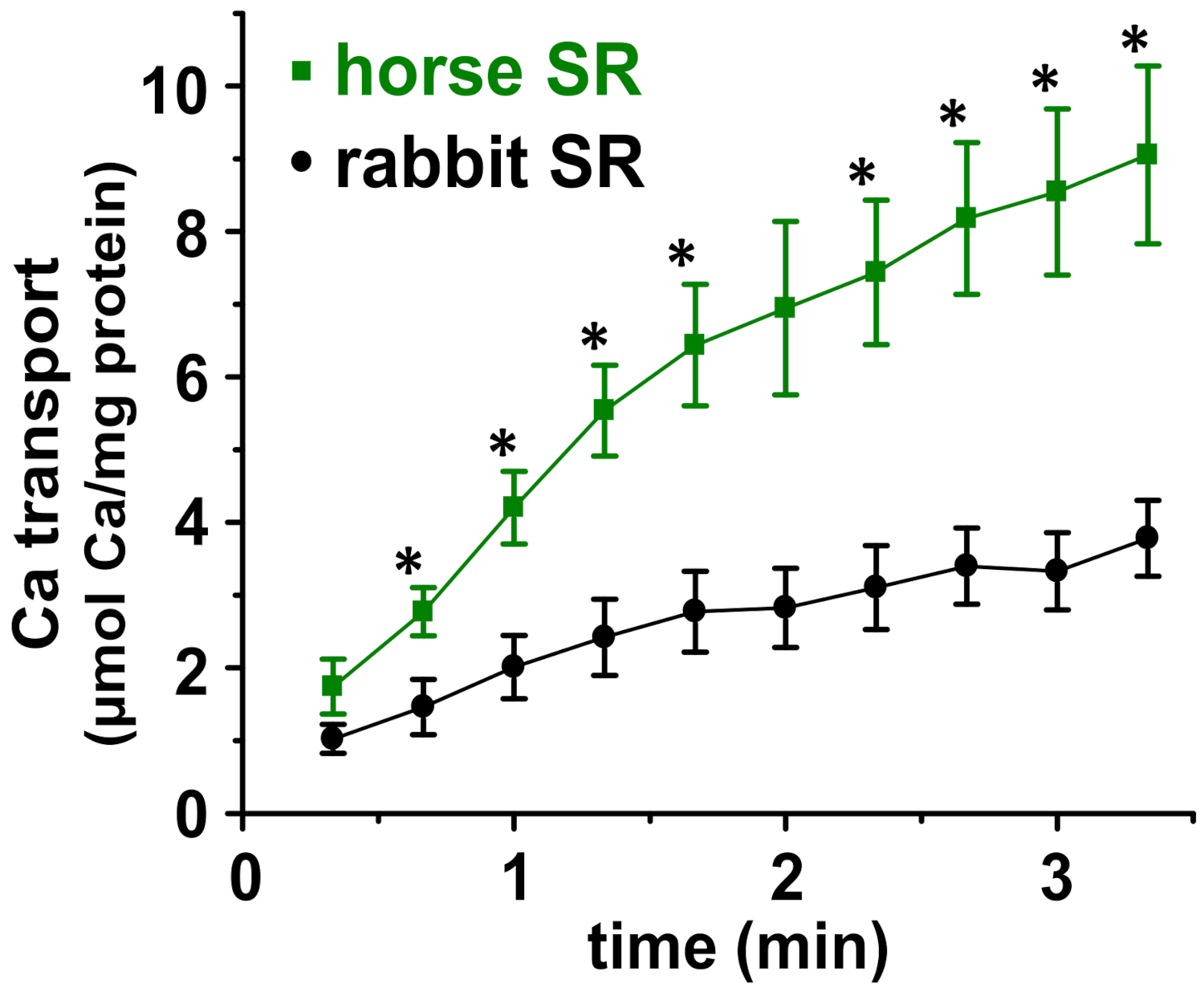
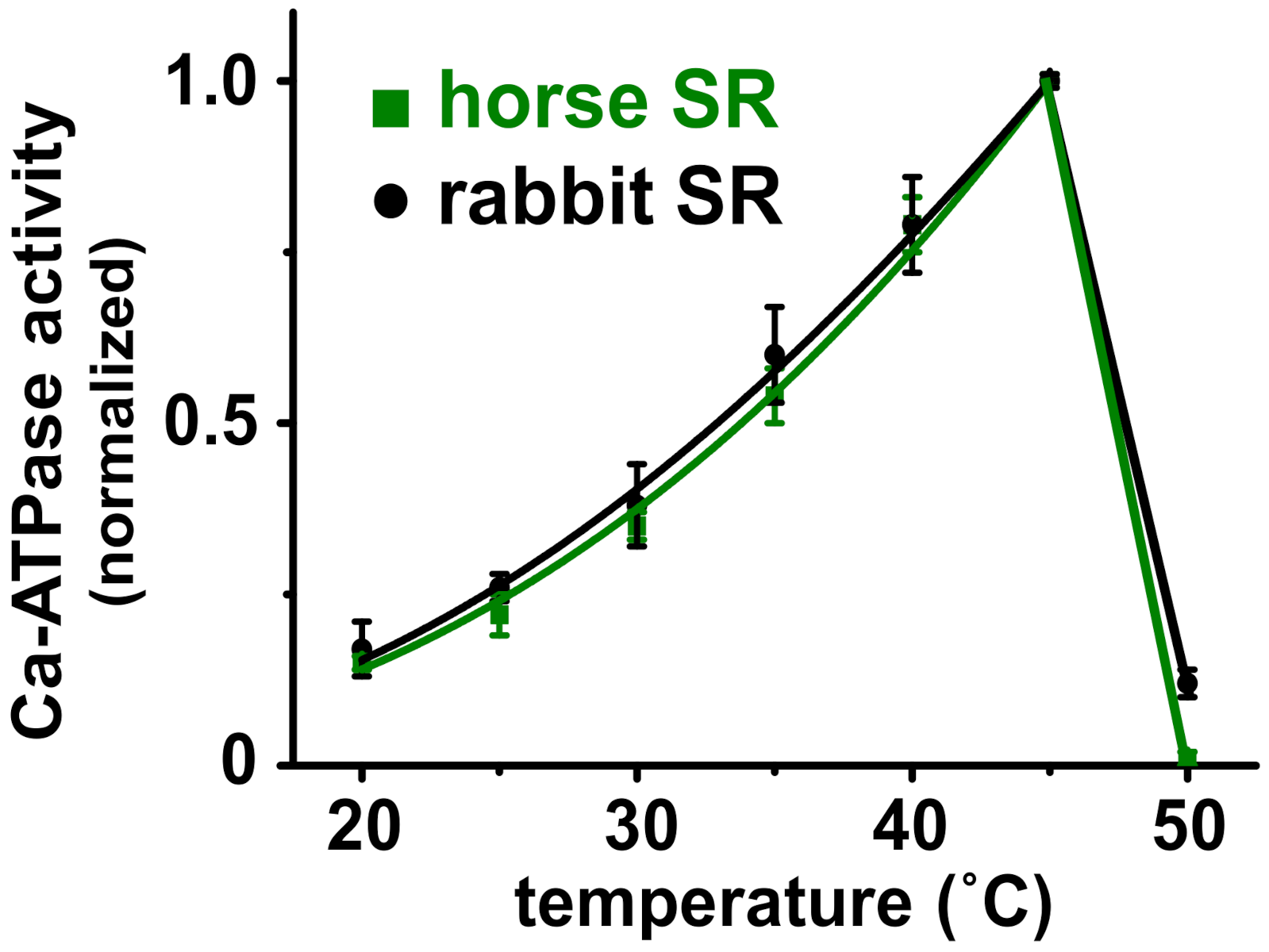
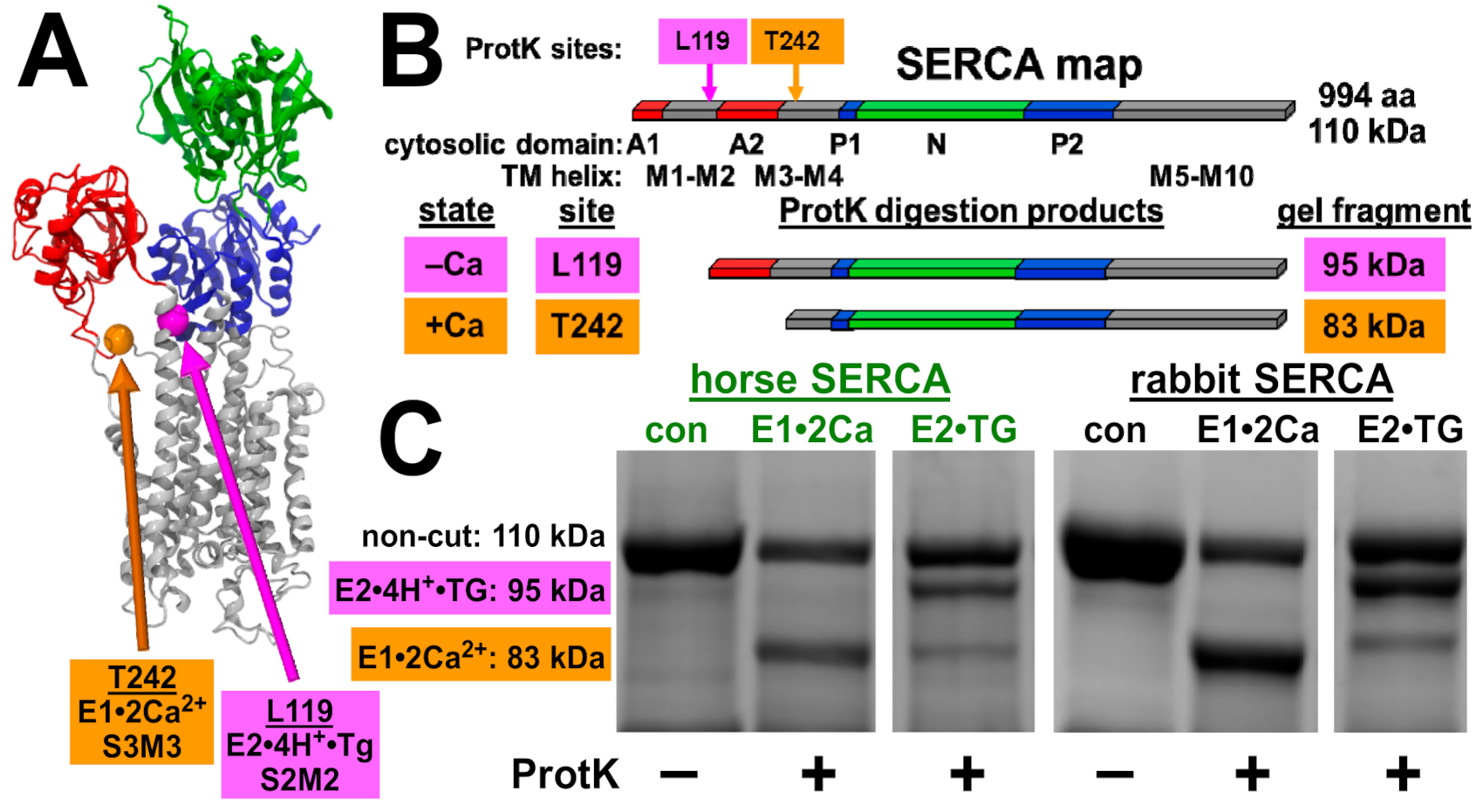
| SR | Ca2+ Transport (IU) + Oxalate a | Ca2+-ATPase (IU) + A23187 b | Transport/ATPase (IU/IU) |
|---|---|---|---|
| Horse (Anal. Biochem. 2020 c, this study d) | 4.2 ± 0.7 d | 4.0 ± 0.4 c | 1.05 ± 0.20 |
| Horse (Eq. Vet. J. Suppl. 1998 e) | 0.18 ± 0.02 | 0.16 ± 0.01 | 1.13 ± 0.13 |
| Horse (J. Anim. Sci. 1995 f) | 0.19 ± 0.02 | 0.16 ± 0.01 | 1.19 ± 0.12 |
| Horse (J. Appl. Physiol. 1989 g) | 0.55 ± 0.16 | 0.73 ± 0.14 | 0.75 ± 0.35 |
| Rabbit h | 1.8 ± 0.5 d | 7.6 ± 0.5 c | 0.24 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Autry, J.M.; Svensson, B.; Carlson, S.F.; Chen, Z.; Cornea, R.L.; Thomas, D.D.; Valberg, S.J. Sarcoplasmic Reticulum from Horse Gluteal Muscle Is Poised for Enhanced Calcium Transport. Vet. Sci. 2021, 8, 289. https://doi.org/10.3390/vetsci8120289
Autry JM, Svensson B, Carlson SF, Chen Z, Cornea RL, Thomas DD, Valberg SJ. Sarcoplasmic Reticulum from Horse Gluteal Muscle Is Poised for Enhanced Calcium Transport. Veterinary Sciences. 2021; 8(12):289. https://doi.org/10.3390/vetsci8120289
Chicago/Turabian StyleAutry, Joseph M., Bengt Svensson, Samuel F. Carlson, Zhenhui Chen, Razvan L. Cornea, David D. Thomas, and Stephanie J. Valberg. 2021. "Sarcoplasmic Reticulum from Horse Gluteal Muscle Is Poised for Enhanced Calcium Transport" Veterinary Sciences 8, no. 12: 289. https://doi.org/10.3390/vetsci8120289
APA StyleAutry, J. M., Svensson, B., Carlson, S. F., Chen, Z., Cornea, R. L., Thomas, D. D., & Valberg, S. J. (2021). Sarcoplasmic Reticulum from Horse Gluteal Muscle Is Poised for Enhanced Calcium Transport. Veterinary Sciences, 8(12), 289. https://doi.org/10.3390/vetsci8120289






