The Role of Galectin-3 in 1α,25(OH)2D3-Regulated Osteoclast Formation from White Leghorn Chickens In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation of BMSCs and BMMs
2.3. Transfection
2.4. Overexpression of Gal-3 Plasmids
2.5. Co-Culture of BMSCs and BMMs
2.6. Identification of Osteoclastogenesis
2.7. Identification of Bone Resorption
2.8. qRT-PCR
2.9. ELISA
2.10. Statistical Analysis
3. Results
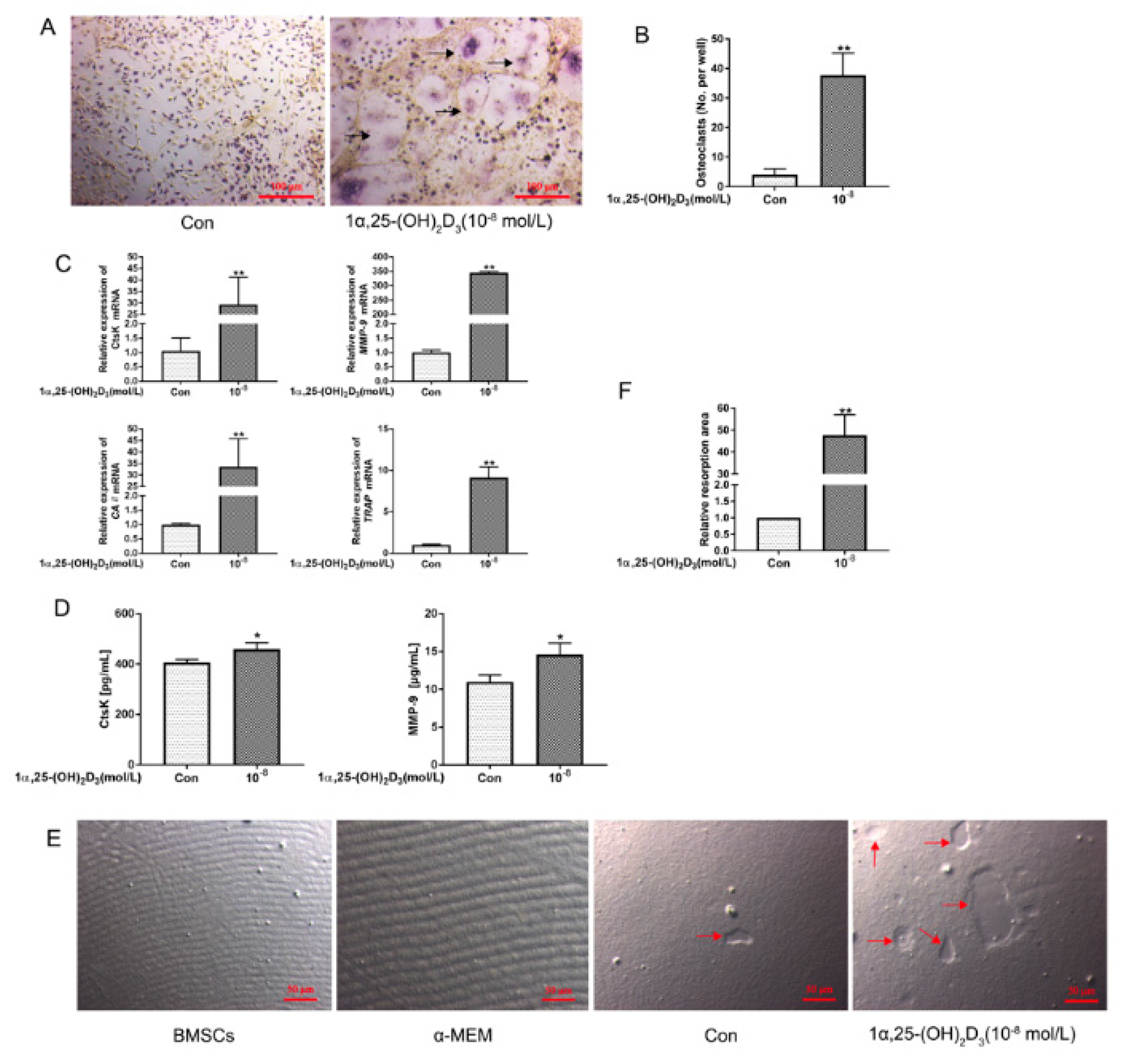
3.1. 1α,25(OH)2D3 Promoted Osteoclastogenesis and Bone Resorption
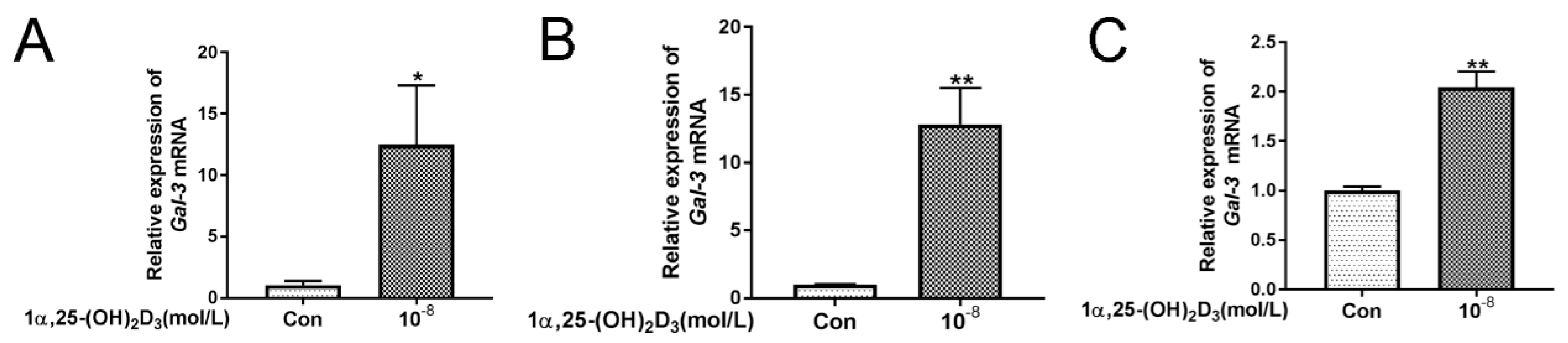
3.2. 1α,25(OH)2D3 Promoted the Expression of Gal-3
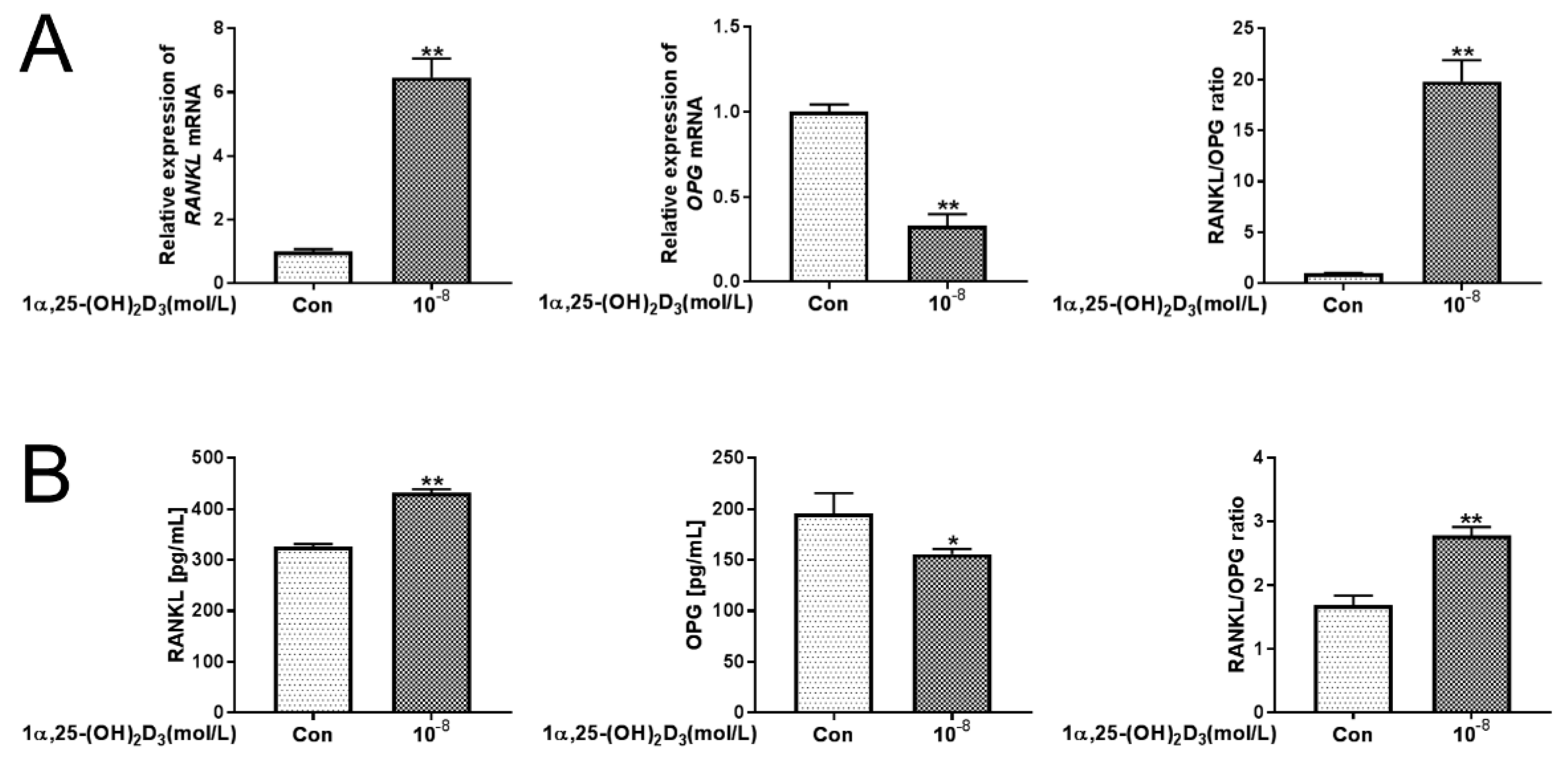
3.3. 1α,25(OH)2D3 Promoted RANKL Expression and Inhibited OPG Expression
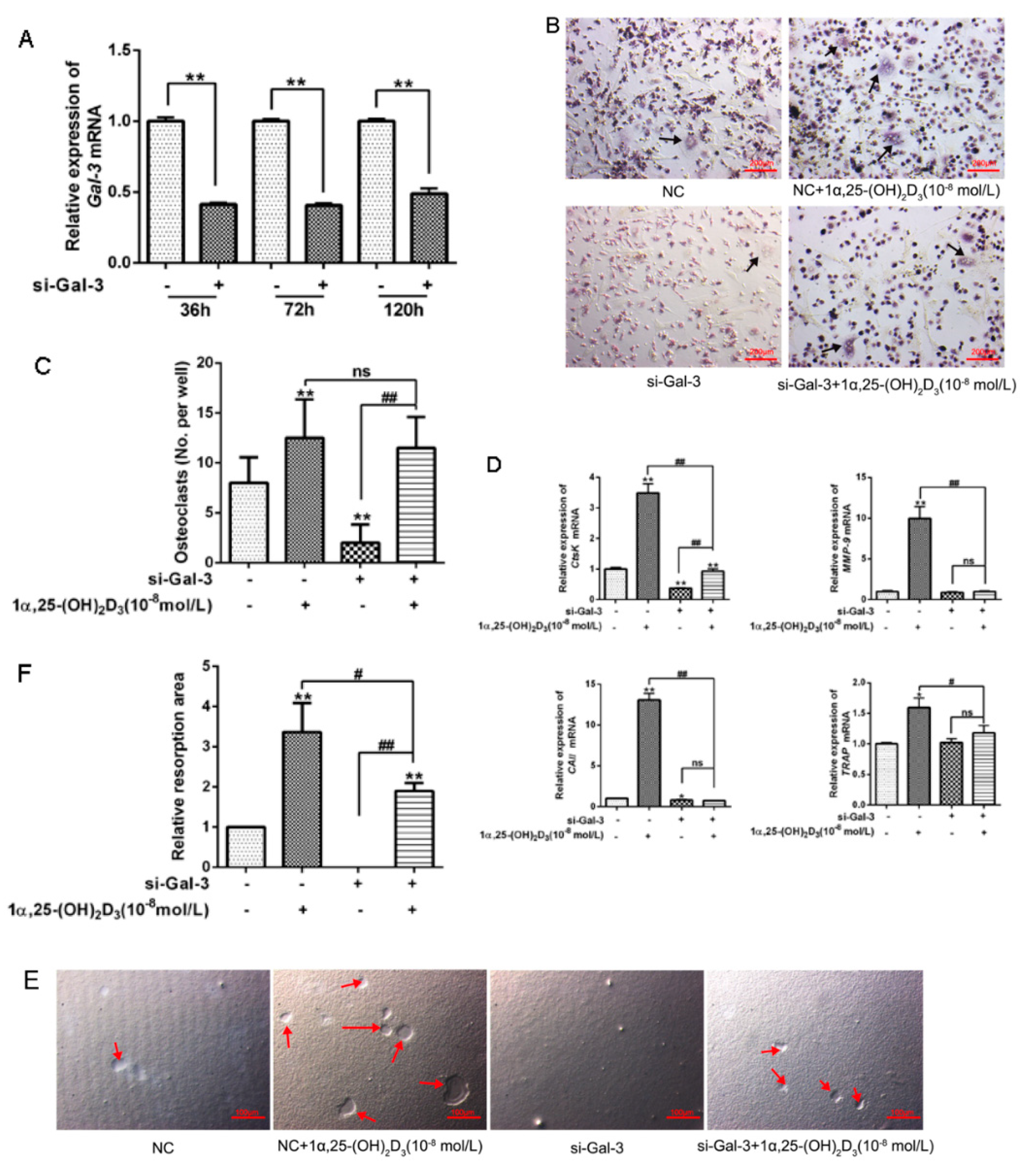
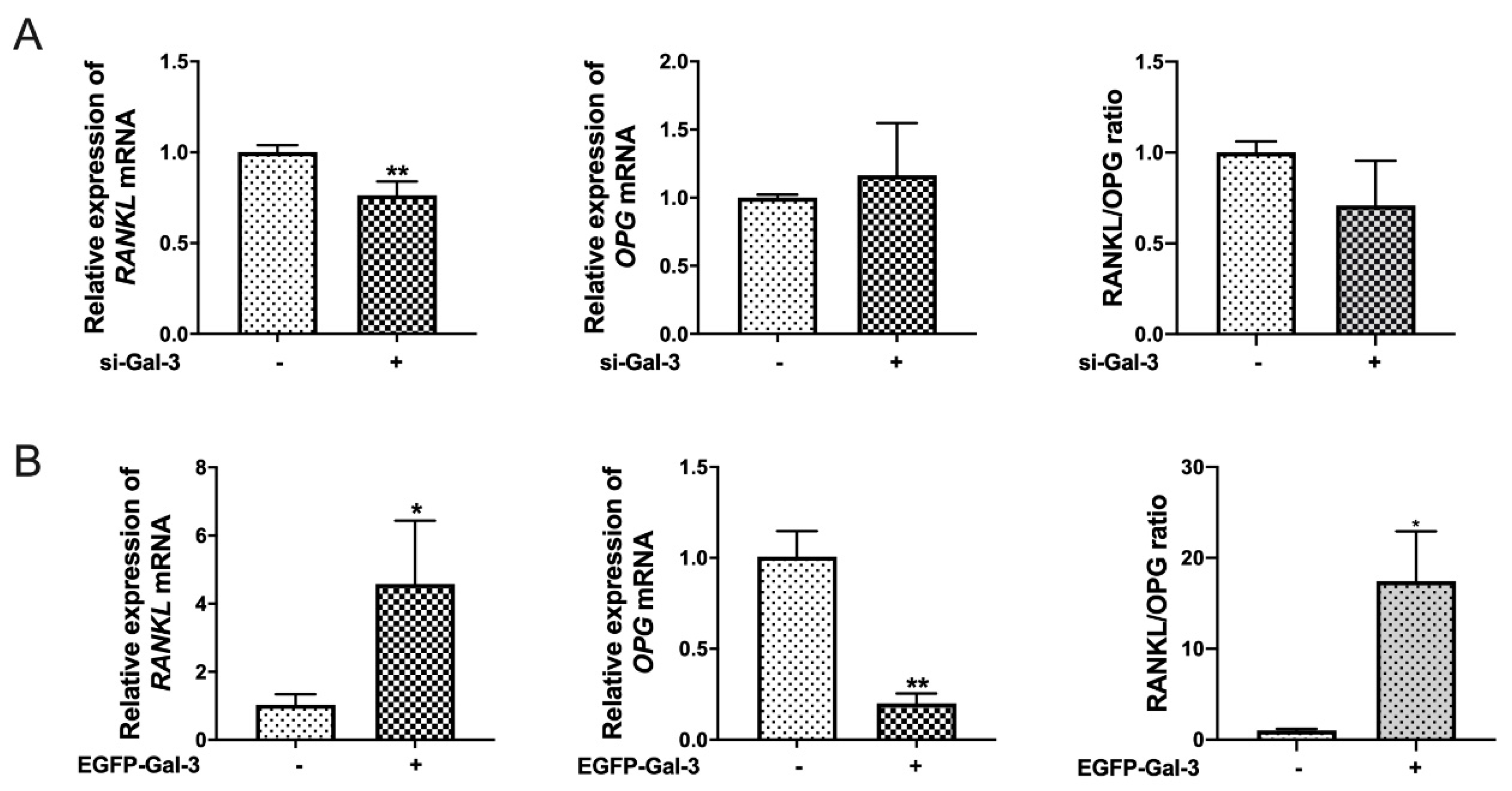
3.4. Knockdown of Gal-3 Inhibited Osteoclastogenesis
3.5. EGFP-Gal-3 Promoted OC Differentiation
3.6. Effects of Si-Gal-3 and EGFP-Gal-3 on RANKL Expression in BMSCs
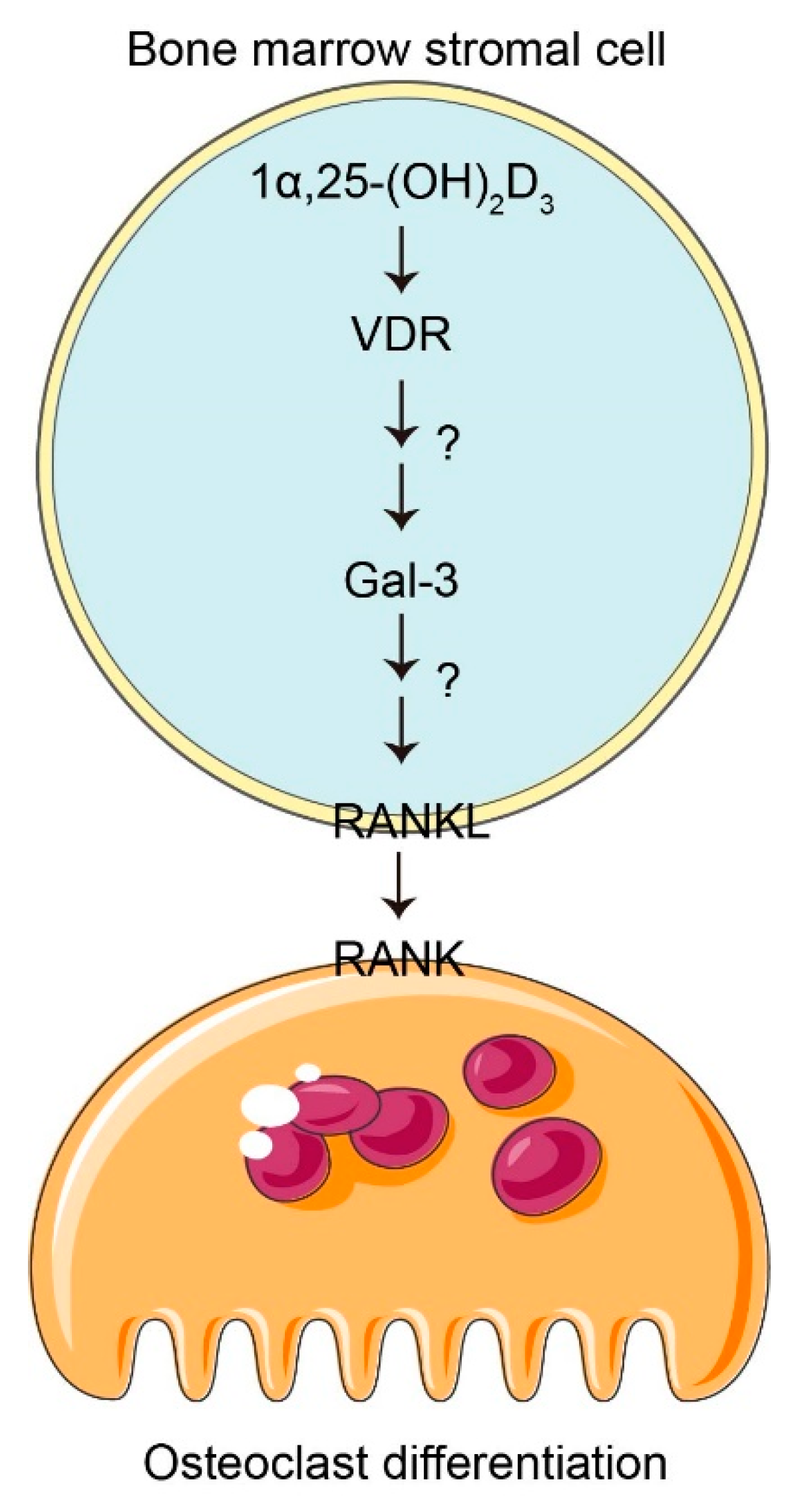
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sims, N.A.; Gooi, J.H. Bone remodeling: Multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.-I.; Rodan, G.A. Control of osteoblast function and regulation of bone mass. Nat. Cell Biol. 2003, 423, 349–355. [Google Scholar] [CrossRef]
- Blair, H.C.; Athanasou, N.A. Recent advances in osteoclast biology and pathological bone resorption. Histol. Histopathol. 2004, 19, 189–199. [Google Scholar]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nat. Cell Biol. 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Bolland, M.J. Controversies in medicine: the role of calcium and vitamin D supplements in adults. Med. J. Aust. 2019, 211, 468–473. [Google Scholar] [CrossRef]
- Kitazawa, S.; Kajimoto, K.; Kondo, T.; Kitazawa, R. Vitamin D3 supports osteoclastogenesis via functional vitamin D response element of human RANKL gene promoter. J. Cell. Biochem. 2003, 89, 771–777. [Google Scholar] [CrossRef]
- Rubin, J.; Murphy, T.C.; Fan, X.; Goldschmidt, M.; Taylor, W.R. Activation of Extracellular Signal-Regulated Kinase Is Involved in Mechanical Strain Inhibition of RANKL Expression in Bone Stromal Cells. J. Bone Miner. Res. 2002, 17, 1452–1460. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Rabinovich, G.A.; Liu, F.-T. Galectins: structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef] [PubMed]
- Inohara, H.; Akahani, S.; Raz, A. Galectin-3 Stimulates Cell Proliferation. Exp. Cell Res. 1998, 245, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Fantauzzi, C.B.; Pugliese, G.; Menini, S. Role of Galectin-3 in Bone Cell Differentiation, Bone Pathophysiology and Vascular Osteogenesis. Int. J. Mol. Sci. 2017, 18, 2481. [Google Scholar] [CrossRef] [Green Version]
- Guevremont, M.; Martel-Pelletier, J.; Boileau, C.; Liu, F.-T.; Richard, M.; Fernandes, J.-C.; Reboul, P. Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann. Rheum. Dis. 2004, 63, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Fantauzzi, C.B.; Bedini, R.; Pecci, R.; Bartolazzi, A.; Amadio, B.; Pesce, C.; Pugliese, G.; Menini, S. Galectin-3 is essential for proper bone cell differentiation and activity, bone remodeling and biomechanical competence in mice. Metabolism 2018, 83, 149–158. [Google Scholar] [CrossRef]
- Stock, M.; Schäfer, H.; Stricker, S.; Gross, G.; Mundlos, S.; Otto, F. Expression of Galectin-3 in Skeletal Tissues Is Controlled by Runx2. J. Biol. Chem. 2003, 278, 17360–17367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Xia, Y.; Liu, L.; Huang, L.; Liu, Y.; Zhang, X.; Xu, K.; Wei, J.; Hu, Y.; Mu, Y.; et al. Galectin-3 Enhances Migration of Minature Pig Bone Marrow Mesenchymal Stem Cells Through Inhibition of RhoA-GTP Activity. Sci. Rep. 2016, 6, 26577. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.E.; Gupta, A.K.; Bhargava, U.; Turksen, K. Expression and regulation of galectin 3 in rat osteoblastic cells. J. Cell. Physiol. 1996, 169, 468–480. [Google Scholar] [CrossRef]
- Simon, D.; Derer, A.; Andes, F.T.; Lezuo, P.; Bozec, A.; Schett, G.; Herrmann, M.; Harre, U. Galectin-3 as a novel regulator of osteoblast-osteoclast interaction and bone homeostasis. Bone 2017, 105, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Aota, Y.; Terayama, H.; Saito, T.; Itoh, M. Pinealectomy in a broiler chicken model impairs endochondral ossification and induces rapid cancellous bone loss. Spine J. 2013, 13, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Narbaitz, R.; Tsang, C.P.W. Vitamin D deficiency in the chick embryo: Effects on prehatching motility and on the growth and differentiation of bones, muscles, and parathyroid glands. Calcif. Tissue Int. 1989, 44, 348–355. [Google Scholar] [CrossRef]
- Hancox, N.M. The survival of transplanted embryo bone grafted to chorioallantoic membrane, and subsequent osteogenesis. J. Physiol. 1947, 106, 279–285. [Google Scholar] [CrossRef]
- Gu, J.; Min, W.; Zhou, J.; Zhao, Y.; Bian, J.; Liu, X.; Yuan, Y.; Liu, Z. . Vitamin D Regulates OC Formation in the Co-culture System of Chicken BMSCs and BMMs. Acta Vet. Zootech. Sin. 2019, 50, 1509–1517. [Google Scholar] [CrossRef]
- Tong, X.; Gu, J.; Chen, M.; Wang, T.; Zou, H.; Song, R.; Zhao, H.; Bian, J.; Liu, Z. p53 positively regulates osteoprotegerin-mediated inhibition of osteoclastogenesis by downregulating TSC2-induced autophagy in vitro. Differentiation 2020, 114, 58–66. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, C.; Wang, D.; Song, R.; Ma, Y.; Cao, Y.; Zhao, H.; Bian, J.; Gu, J.; Liu, Z. Suppression of AMP-activated protein kinase reverses osteoprotegerin-induced inhibition of osteoclast differentiation by reducing autophagy. Cell Prolif. 2020, 53, e12714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Li, S.; Wang, G.; Zhang, X.; Yuan, Y.; Liu, X.; Bian, J.; Tong, X.; Liu, Z. Cadmium Toxicity on Chondrocytes and the Palliative Effects of 1α, 25-Dihydroxy Vitamin D3 in White Leghorns Chicken’s Embryo. Front. Veter-Sci. 2021, 8, 637369. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Yoshizawa, T.; Fukuda, T.; Shirode-Fukuda, Y.; Yu, T.; Sekine, K.; Sato, T.; Kawano, H.; Aihara, K.-I.; Nakamichi, Y.; et al. Vitamin D Receptor in Osteoblasts Is a Negative Regulator of Bone Mass Control. Endocrinology 2013, 154, 1008–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Liu, K.; Anderson, J.; Patrene, K.; Lentzsch, S.; Roodman, G.D.; Ouyang, H. Expression of XBP1s in bone marrow stromal cells is critical for myeloma cell growth and osteoclast formation. Blood 2012, 119, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Boyle, W.J.; Simonet, W.S.; Kostenuik, P.J.; Dougall, W.C.; Sullivan, J.K.; Martin, J.S.; Dansey, R. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 2012, 11, 401–419. [Google Scholar] [CrossRef]
- Kitazawa, R.; Mori, K.; Yamaguchi, A.; Kondo, T.; Kitazawa, S. Modulation of mouse RANKL gene expression by Runx2 and vitamin D3. J. Cell. Biochem. 2008, 105, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Ortega, N.; Behonick, D.; Colnot, C.; Cooper, D.N.; Werb, Z. Galectin-3 Is a Downstream Regulator of Matrix Metalloproteinase-9 Function during Endochondral Bone Formation. Mol. Biol. Cell 2005, 16, 3028–3039. [Google Scholar] [CrossRef]
- Nakajima, K.; Kho, D.H.; Yanagawa, T.; Harazono, Y.; Hogan, V.; Chen, W.; Ali-Fehmi, R.; Mehra, R.; Raz, A. Galectin-3 Cleavage Alters Bone Remodeling: Different Outcomes in Breast and Prostate Cancer Skeletal Metastasis. Cancer Res. 2016, 76, 1391–1402. [Google Scholar] [CrossRef] [Green Version]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]

| Name | Gene Number | Length (bp) | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|---|---|
| CtsK (chicken) en) | 395818 | 161 | CATCATTGACGGAGCGATGC | TTTCGTCCTCCTTGCCGTTG |
| TRAP (chicken) | 107057619 | 110 | CCGCTTCTTCTATGGGGCT | GGTACAGAACTCTCCCGTTGG |
| MMP-9 (chicken) | 395387 | 182 | CTTCTGCCCAGACAGAGGTT | AGCCACGACCATAGAGGTACT |
| CAII (chicken) | 396257 | 188 | GGCGTGAAGTACGATGCAGA | GCTTGTTTCCCCTTGGTTTGAA |
| RANKL (chicken) | 428067 | 116 | GCCCACTTCTTGGAAACAGC | TACAACGTGGCCCTGTGAAG |
| OPG (chicken) | 378803 | 90 | CTGCACGCTTGTGCTCTTGG | GATGTCCCCGGGTCGTAATG |
| Galectin-3 (chicken) | 373917 | 112 | GCCATATCCTGGAGGACCAAC | GGTTATGAGCAGTCGAGGCA |
| GAPDH (chicken) | 374193 | 137 | GCCCAGAACATCATCCCAG | CGGCAGGTCAGGTCAACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Min, W.; Zhao, Y.; Zhang, X.; Yuan, Y.; Liu, X.; Bian, J.; Tong, X.; Liu, Z. The Role of Galectin-3 in 1α,25(OH)2D3-Regulated Osteoclast Formation from White Leghorn Chickens In Vitro. Vet. Sci. 2021, 8, 234. https://doi.org/10.3390/vetsci8100234
Gu J, Min W, Zhao Y, Zhang X, Yuan Y, Liu X, Bian J, Tong X, Liu Z. The Role of Galectin-3 in 1α,25(OH)2D3-Regulated Osteoclast Formation from White Leghorn Chickens In Vitro. Veterinary Sciences. 2021; 8(10):234. https://doi.org/10.3390/vetsci8100234
Chicago/Turabian StyleGu, Jianhong, Wenyan Min, Yutian Zhao, Xueqing Zhang, Yan Yuan, Xuezhong Liu, Jianchun Bian, Xishuai Tong, and Zongping Liu. 2021. "The Role of Galectin-3 in 1α,25(OH)2D3-Regulated Osteoclast Formation from White Leghorn Chickens In Vitro" Veterinary Sciences 8, no. 10: 234. https://doi.org/10.3390/vetsci8100234
APA StyleGu, J., Min, W., Zhao, Y., Zhang, X., Yuan, Y., Liu, X., Bian, J., Tong, X., & Liu, Z. (2021). The Role of Galectin-3 in 1α,25(OH)2D3-Regulated Osteoclast Formation from White Leghorn Chickens In Vitro. Veterinary Sciences, 8(10), 234. https://doi.org/10.3390/vetsci8100234







