A Case of Submandibular Leiomyosarcoma, Mimicking an Abscess, in a Ball Python (Python regius)
Abstract
:1. Introduction
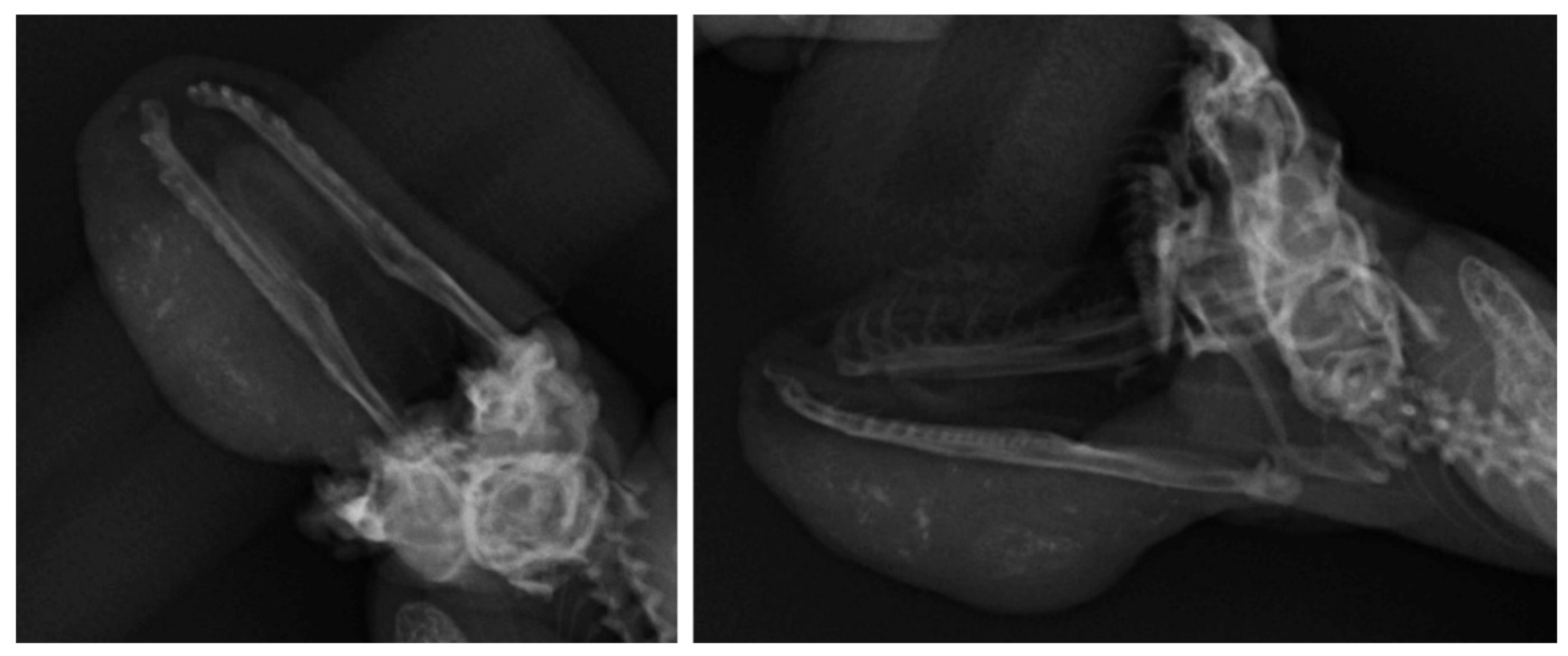
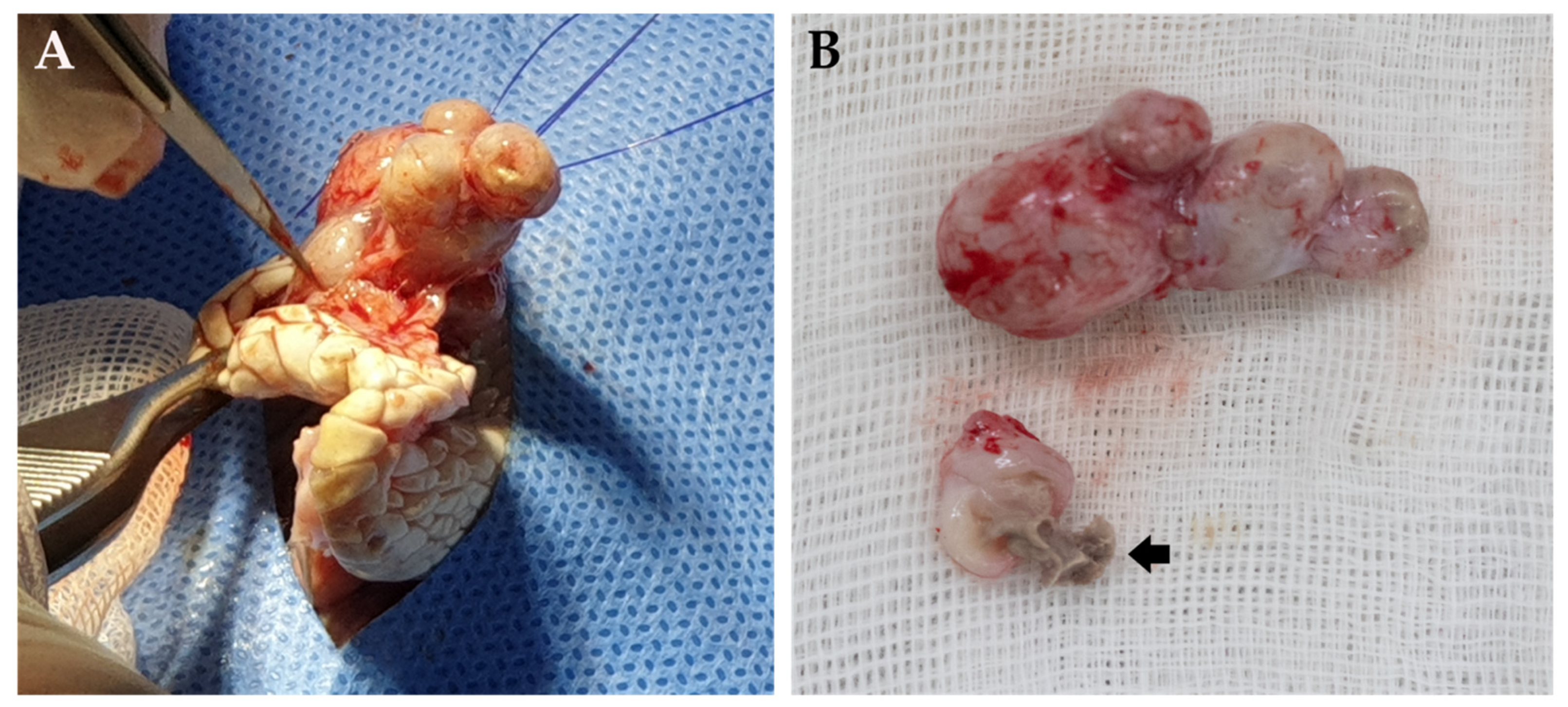
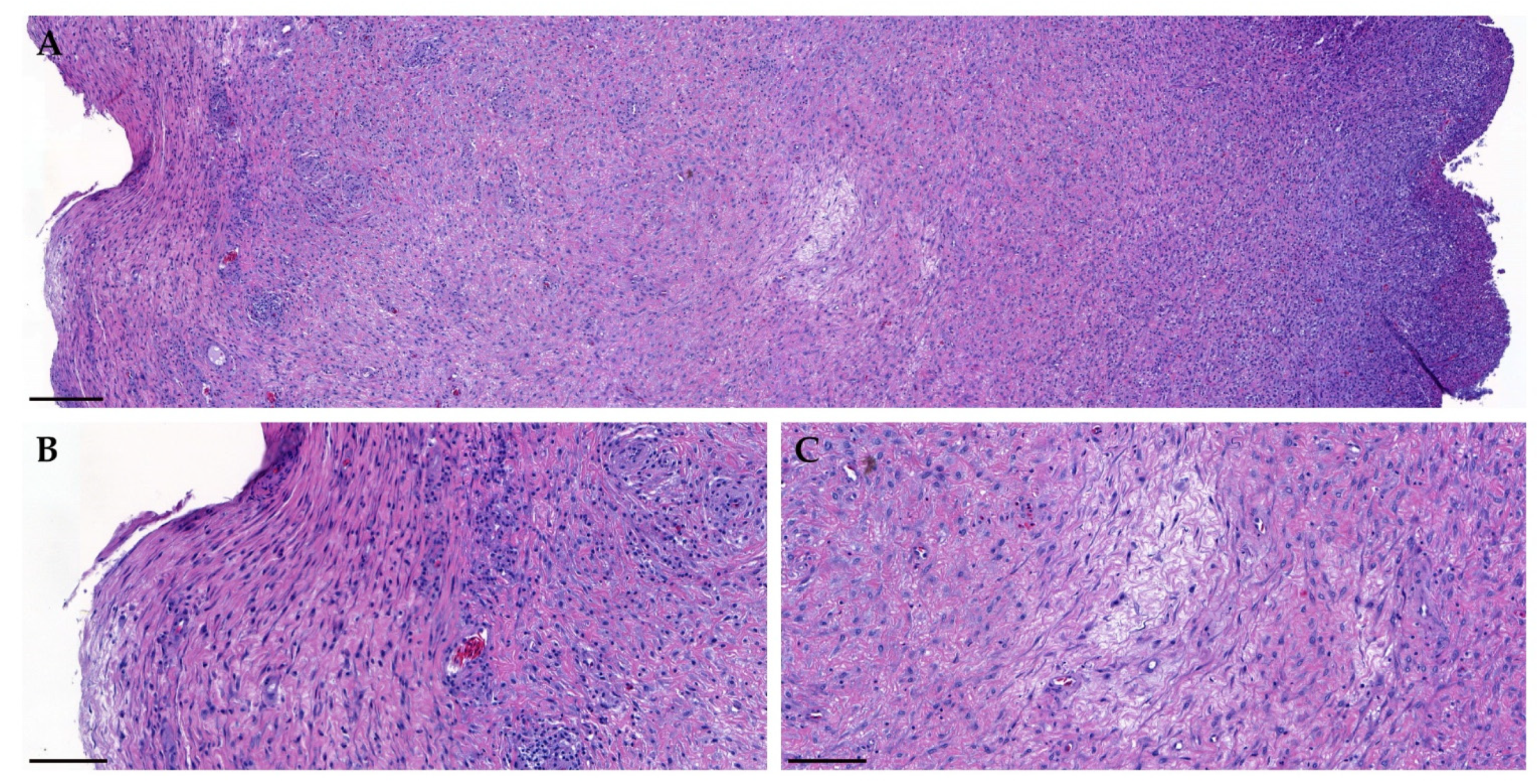
2. Case Presentation
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizzo, J.M. Captive Care and Husbandry of Ball Pythons (Python regius). J. Herpetol. Med. Surg. 2014, 24, 48–52. [Google Scholar] [CrossRef]
- D’Cruze, N.; Harrington, L.A.; Assou, D.; Green, J.; Macdonald, D.W.; Ronfot, D.; Hoinsoudé Segniagbeto, G.; Auliya, M. Betting the Farm: A Review of Ball Python and Other Reptile Trade From Togo, West Africa. Nat. Conserv. 2020, 40, 65–91. [Google Scholar] [CrossRef]
- Christman, J.; Devau, M.; Wilson-Robles, H.; Hoppes, S.; Rech, R.; Russell, K.E.; Heatley, J.J. Oncology of reptiles: Diseases, diagnosis, and treatment. Vet. Clin. Exot. Anim. Pract. 2017, 20, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.E.; Kent, M.S.; Théon, A. Current therapies in exotic animal oncology. Vet. Clin. Exot. Anim. Pract. 2004, 7, 757–781. [Google Scholar] [CrossRef]
- Serrano, C.; George, S. Leiomyosarcoma. Hematol. Oncol. Clin. N. Am. 2013, 27, 957–974. [Google Scholar] [CrossRef]
- Russell, K.N.; Mehler, S.J.; Skorupski, K.A.; Baez, J.L.; Shofer, F.S.; Goldschmidt, M.H. Clinical and Immunohistochemical Differentiation of Gastrointestinal Stromal Tumors From Leiomyosarcomas in Dogs: 42 Cases (1990–2003). J. Am. Vet. Med. Assoc. 2007, 230, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Kaddu, S.; Beham, A.; Cerroni, L.; Humer-Fuchs, U.; Salmhofer, W.; Kerl, H.; Soyer, H.P. Cutaneous Leiomyosarcoma. Am. J. Surg. Pathol. 1997, 21, 979–987. [Google Scholar] [CrossRef]
- Lin, J.Y.; Tsai, R.Y. Subcutaneous Leiomyosarcoma on the Face. Dermatol. Surg. 1999, 25, 489–491. [Google Scholar] [CrossRef]
- Salemis, N.S. Recurrent Subcutaneous Trunk Leiomyosarcoma: Management and Review of the Literature. J. Nat. Sci. Biol. Med. 2013, 4, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Avallone, G.; Rasotto, R.; Chambers, J.K.; Miller, A.D.; Behling-Kelly, E.; Monti, P.; Berlato, D.; Valenti, P.; Roccabianca, P. Review of Histological Grading Systems in Veterinary Medicine. Vet. Pathol. 2021, 58, 809–828. [Google Scholar] [CrossRef]
- Hagstrom, M.R.; Stöhr, A.C.; Cacioppo, J.A.; Anderson, A.; Wakamatsu, N.; Nevarez, J.G. Axillary Leiomyosarcoma in an Inland Bearded Dragon (Pogona vitticeps). J. Herpetol. Med. Surg. 2020, 31, 12–17. [Google Scholar]
- Vetere, A.; Bertocchi, M.; Pelizzone, I.; Moggia, E.; Gerosa, S.; Di Ianni, F. Klebsiella sp.-Related Infectious Spondylitis in a Bearded Dragon (Pogona vitticeps). BMC Vet. Res. 2021, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.A. Prevalence of Klebsiella oxytoca in Anolis Carolensis of Louisiana. Vector Borne Zoonotic Dis. 2016, 16, 800–801. [Google Scholar] [CrossRef]
- Peled, Z.; Linder, R.; Gilshtein, H.; Kakiashvili, E.; Kluger, Y. Cecal Fibromatosis (Desmoid Tumor) Mimicking Periappendicular Abscess: A Case Report. Case Rep. Oncol. 2012, 5, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Sun, C.K.; Jiang, J.S.; Tsai, M.H. Tumor-Like Liver Abscess Mimicking Malignancy With Lung Metastases in a Patient With Acute Renal Failure: A Case Report. Medicine 2016, 95, e3145. [Google Scholar] [CrossRef] [PubMed]
- Gates, L.K., Jr.; Cameron, A.J.; Nagorney, D.M.; Goellner, J.R.; Farley, D.R. Primary Leiomyosarcoma of the Liver Mimicking Liver Abscess. Am. J. Gastroenterol. 1995, 90, 649–652. [Google Scholar]
- Mavili, E.; Öztürk, M.; Yücel, T.; Yüce, I.; Çagli, S. Tongue Metastasis Mimicking an Abscess. Diagn. Interv. Radiol. 2010, 16, 27–29. [Google Scholar]
- Liu, Y.W.; Chiu, H.H.; Huang, C.C.; Tu, C.A. Retroperitoneal Schwannoma Mimicking a Psoas Abscess. Clin. Gastroenterol. Hepatol. 2007, 5, A32. [Google Scholar] [CrossRef]
- Erdogan, C.; Hakyemez, B.; Yildirim, N.; Parlak, M. Brain Abscess and Cystic Brain Tumor: Discrimination With Dynamic Susceptibility Contrast Perfusion-Weighted MRI. J. Comput. Assist. Tomogr. 2005, 29, 663–667. [Google Scholar] [CrossRef]
- Verdier, E.P.; Konsol, O.; Portillo, S. Intramedullary Cervical Abscess Mimicking a Spinal Cord Tumor in a 10-Year-Old Girl: A Case-Based Review. Childs Nerv. Syst. 2018, 34, 2143–2147. [Google Scholar] [CrossRef]
- Council, L.; Hameed, O. Differential Expression of Immunohistochemical Markers in Bladder Smooth Muscle and Myofibroblasts, and the Potential Utility of Desmin, Smoothelin, and Vimentin in Staging of Bladder Carcinoma. Mod. Pathol. 2009, 22, 639–650. [Google Scholar] [CrossRef] [Green Version]
- Santos, E.D.; Silva, J.R.; Machado, T.P.; Dau, S.L.; Rodriguez, R.; Motta, A.C.D. Oral fibrosarcoma in jararaca (Bothrops pubescens): Anatomopathological and immunohistochemical aspects. Pesqui. Vet. Bras. 2015, 35, 664–670. [Google Scholar] [CrossRef]
- Sharpe, S.; Lamm, C.G.; Killick, R. Intracoelomic anaplastic sarcoma in an intersex Madagascar tree boa (Sanzinia madagascariensis). J. Vet. Diagn. Investig. 2013, 25, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Gumber, S.; Nevarez, J.G.; Cho, D.Y. Endocardial fibrosarcoma in a reticulated python (Python reticularis). J. Vet. Diagn. Investig. 2010, 22, 1013–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haines, D.M.; Clark, E.G. Enzyme immunohistochemical staining of formalin-fixed tissues for diagnosis in veterinary pathology. Can. Vet. J. 1991, 32, 295. [Google Scholar] [PubMed]
- Mohanty, K.C.; Naik, D.R. Immunohistochemistry and Tinctorial Affinity of Adenohypophysial Cells of the Rat SnakePtyas mucosus (Colubridae). Gen. Comp. Endocrinol. 1997, 105, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Kawabata, T.; Gabe, A.; Ichi, T.; Kushi, K.; Yohena, T.; Kawasaki, H.; Yamashiro, T.; Ishikawa, K. Lung cancer mimicking lung abscess formation on CT images. Am. J. Case Rep. 2014, 15, 243. [Google Scholar] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.; Kim, S.W.; Kim, S.G.; Kim, H.J.; Lee, S.B.; Kang, J.W.; Jung, W.J.; Giri, S.S.; Lee, K.; Park, S.C. A Case of Submandibular Leiomyosarcoma, Mimicking an Abscess, in a Ball Python (Python regius). Vet. Sci. 2021, 8, 224. https://doi.org/10.3390/vetsci8100224
Kwon J, Kim SW, Kim SG, Kim HJ, Lee SB, Kang JW, Jung WJ, Giri SS, Lee K, Park SC. A Case of Submandibular Leiomyosarcoma, Mimicking an Abscess, in a Ball Python (Python regius). Veterinary Sciences. 2021; 8(10):224. https://doi.org/10.3390/vetsci8100224
Chicago/Turabian StyleKwon, Jun, Sang Wha Kim, Sang Guen Kim, Hyoun Joong Kim, Sung Bin Lee, Jeong Woo Kang, Won Joon Jung, Sib Sankar Giri, Kyunglee Lee, and Se Chang Park. 2021. "A Case of Submandibular Leiomyosarcoma, Mimicking an Abscess, in a Ball Python (Python regius)" Veterinary Sciences 8, no. 10: 224. https://doi.org/10.3390/vetsci8100224
APA StyleKwon, J., Kim, S. W., Kim, S. G., Kim, H. J., Lee, S. B., Kang, J. W., Jung, W. J., Giri, S. S., Lee, K., & Park, S. C. (2021). A Case of Submandibular Leiomyosarcoma, Mimicking an Abscess, in a Ball Python (Python regius). Veterinary Sciences, 8(10), 224. https://doi.org/10.3390/vetsci8100224







