Pre-Weaning Inulin Supplementation Alters the Ileal Transcriptome in Pigs Regarding Lipid Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Transcriptome Analysis
2.3. qRT-PCR Validation
2.4. Statistical Analyses
3. Results
3.1. Differentially Expressed Genes
3.2. Gene Ontology (GO) Analysis of Differential Expressed Genes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibson, G.R.; Jutkens, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janczyk, P.; Pieper, R.; Smidt, H.; Souffrant, W.B. Effect of alginate and inulin on intestinal microbial ecology of weanling pigs reared under different husbandry conditions. FEMS Microbiol. Ecol. 2010, 72, 132–142. [Google Scholar] [CrossRef]
- Mair, C.; Plitzner, C.; Domig, K.J.; Schedle, K.; Windisch, W. Impact of inulin and a multispecies probiotic formulation on performance, microbial ecology and concomitant fermentation patterns in newly weaned piglets. J. Anim. Physiol. Anim. Nutr. 2010, 94, e164–e177. [Google Scholar] [CrossRef]
- Paßlack, N.; Vahjen, W.; Zentek, J. Dietary inulin affects the intestinal microbiota in sows and their suckling piglets. BMC Vet. Res. 2015, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Eberhard, M.; Hennig, U.; Kuhla, S.; Brunner, R.M.; Kleessen, B.; Metges, C.C. Effect of inulin supplementation on selected gastric duodenal, and caecal microbiota and short chain fatty acid pattern in growing piglets. Arch. Anim. Nutr. 2007, 61, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Paßlack, N.; Zentek, J. Dietary inulin alters the intestinal absorptive and barrier function of piglet intestine after weaning. Res. Vet. Sci. 2013, 95, 249–254. [Google Scholar] [CrossRef]
- Halas, D.; Hansen, C.F.; Hampson, D.J.; Mullan, B.P.; Wilson, R.H.; Pluske, J.R. Effect of dietary supplementation with inulin and/or benzoic acid on the incidence and severity of post-weaning diarrhea in weaner pigs after experimental challenge with enterotoxigenic Escherichia coli. Arch. Anim. Nutr. 2009, 63, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Pié, S.; Awati, A.; Vida, S.; Falluel, I.; Williams, B.A.; Oswald, I.P. Effects of added fermentable carbohydrates in the diet on intestinal proinflammatory cytokine-specific mRNA content in weaning piglets. J. Anim. Sci. 2007, 85, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Wang, J.; Yu, H.; Wang, J.; Zhu, W. Changes in ileal microbial composition and microbial metabolism by an early-life galacto-oligosaccharides intervention in a neonatal porcine model. Nutrients 2019, 11, 1753. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.; Garssen, J.; Fink-Gremmels, J.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Schroyen, M.; Leblois, J.; Wavreille, J.; Soyeurt, H.; Bindelle, J.; Everaert, N. Effects of inulin supplementation to piglets in the suckling period on growth performance, postileal microbial and immunological traits in the suckling period and three weeks after weaning. Arch. Anim. Nutr. 2018, 72, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, R.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Andrew, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 1 March 2021).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, T.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3–new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.Q.; Wu, W.J.; Liu, W.H.; Zheng, R.; Li, J.L.; Zuo, B.; Xu, D.Q.; Li, F.E.; Lei, M.G.; Ni, D.B.; et al. Differential expression and effect of the porcine ANGPTL4 gene on intramuscular fat. Genet. Mol. Res. 2014, 13, 2949–2958. [Google Scholar] [CrossRef]
- Uribe, J.H.; Collado-Romero, M.; Zaldívar-López, S.; Arce, C.; Bautista, R.; Carvajal, A.; Cirera, S.; Claros, M.G.; Garrido, J.J. Transcriptional analysis of porcine intestinal mucosa infected with Salmonella Typhimurium revealed a massive inflammatory response and disruption of bile acid absorption in ileum. Vet. Res. 2016, 47, 11. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; Zhou, X.; Wang, Y.; Chen, D.; Chen, G.; Li, Y.; He, J. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. 2018, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef]
- Beylot, M. Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br. J. Nutr. 2005, 93, S163–S168. [Google Scholar] [CrossRef]
- Reis, S.A.; Conceição, L.L.; Rosa, D.D.; Dias, M.M.; Peluzio Mdo, C. Mechanisms used by inulin-type fructans to improve the lipid profile. Nutr. Hosp. 2014, 31, 528–534. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Muccioli, G.G.; Deldicque, L.; Bindels, L.B.; Pachikian, B.D.; Sohet, F.M.; et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 712–722. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Chapelet, G.; Javaudin, F.; Lepelletier, D.; Batard, E.; Montassier, E. The effects of inulin on gut microbial composition: A systematic review of evidence from human studies. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 403–413. [Google Scholar] [CrossRef]
- Ang, Z.; Er, J.Z.; Ding, J.L. The short-chain fatty acid receptor GPR43 is transcriptionally regulated by XBP1 in human monocytes. Sci. Rep. 2015, 5, 8134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.L.; Tian, H.; Li, Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef] [Green Version]
- Blædel, T.; Holm, J.B.; Sundekilde, U.K.; Schmedes, M.S.; Hess, A.L.; Lorenzen, J.K.; Kristiansen, K.; Dalsgaard, T.K.; Astrup, A.; Larsen, L.H. A randomized; controlled; crossover study of the effect of diet on angiopoietin-like protein 4 (ANGPTL4) through modification of the gut microbiome. J. Nutr. Sci. 2016, 5, e45. [Google Scholar] [CrossRef] [Green Version]
- Alex, S.; Lichtenstein, L.; Dijk, W.; Mensink, R.P.; Tan, N.S.; Kersten, S. ANGPTL4 is produced by entero-endocrine cells in the human intestinal tract. Histochem. Cell. Biol. 2014, 141, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, S.S. Probiotics and prebiotics: Present status and future perspectives on metabolic disorders. Nutrients 2016, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Delzenne, N.M.; Neyrinck, A.M.; Bäckhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrin. 2011, 7, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Stefàno, E.; Marsigliante, S. The effects of exercise training on lipid metabolism and coronary heart disease. Am. J. Physiol. Heart. Circ. Physiol. 2020, 319, H76–H88. [Google Scholar] [CrossRef] [PubMed]
- Eggerman, T.L.; Hoeg, J.M.; Meng, M.S.; Tombragel, A.; Bojanovski, D.; Brewer, H.B., Jr. Differential tissue-specific expression of human apoA-I and apoA-II. J. Lipid Res. 1991, 32, 821–828. [Google Scholar] [CrossRef]
- Tayyeb, J.Z.; Popeijus, H.E.; Mensink, R.P.; Konings, M.C.J.M.; Mulders, K.H.R.; Plat, J. The effects of short-chain fatty acids on the transcription and secretion of apolipoprotein A-I in human hepatocytes in vitro. J. Cell. Biochem. 2019, 120, 17219–17227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiu, J.H.C.; Chan, K.S.; Cheung, J.; Li, J.; Liu, Y.; Wang, Y.; Fung, W.W.L.; Cai, J.; Cheung, S.W.M.; Dorweiler, B.; et al. Gut microbiota-associated activation of TLR5 induces apolipoprotein A1 production in the liver. Circ. Res. 2020, 127, 1236–1252. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, L.; Zhu, S.; Xiao, Y.; Huang, Y.; Hua, Y.; Chu, Q.; Ren, Z. Two strains of lactobacilli effectively decrease the colonization of VRE in a mouse model. Front. Cell. Infect. Microbiol. 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I); Immunity; Inflammation and Cancer. Cancers 2019, 11, 1097. [Google Scholar] [CrossRef] [Green Version]
- Mangaraj, M.; Nanda, R.; Panda, S. Apolipoprotein A-I: A Molecule of Diverse Function. Indian. J. Clin. Biochem. 2016, 31, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunislawska, A.; Herosimczyk, A.; Lepczynski, A.; Slama, P.; Slawinska, A.; Bednarczyk, M.; Siwek, M. Molecular response in intestinal and immune tissues to in ovo administration of inulin and the combination of inulin and Lactobacillus lactis Subsp. cremoris. Front. Vet. Sci. 2021, 7, 632476. [Google Scholar] [CrossRef]
- Oikonomou, E.; Kostopoulou, E.; Rojas-Gil, A.P.; Georgiou, G.; Spiliotis, B.E. The metabolic implications of aquaporin 7 (AQP7) promoter variants in lean children and children with obesity. Hormones 2020, 19, 187–195. [Google Scholar] [CrossRef]
- Laforenza, U.; Gastaldi, G.; Grazioli, M.; Cova, E.; Tritto, S.; Faelli, A.; Calamita, G.; Ventura, U. Expression and immunolocalization of aquaporin-7 in rat gastrointestinal tract. Biol. Cell 2005, 97, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Vieira da Silva, I.; Soares, B.P.; Pimpão, C.; Pinto, R.M.A.; Costa, T.; Freire, J.P.B.; Corrent, E.; Chalvon-Demersay, T.; Prates, J.A.M.; Lopes, P.A.; et al. Glutamine and cystine-enriched diets modulate aquaporins gene expression in the small intestine of piglets. PLoS ONE 2021, 16, e0245739. [Google Scholar] [CrossRef] [PubMed]
- Ricanek, P.; Lunde, L.K.; Frye, S.A.; Støen, M.; Nygård, S.; Morth, J.P.; Rydning, A.; Vatn, M.H.; Amiry-Moghaddam, M.; Tonjum, T. Reduced expression of aquaporins in human intestinal mucosa in early stage inflammatory bowel disease. Clin. Exp. Gastroenterol. 2015, 8, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Primer Sequence (5′ → 3′) | Reference |
|---|---|---|
| ANGPTL4 | F: GGAGAAGCAGCACTTGAGAA | Ren et al., 2014 [17] |
| R: GGGTCATCTTGGGTAGTCTTT | ||
| APOA1 | F: GCAAGATGACCCGCAGTCACC | Uribe et al., 2016 [18] |
| R: GCCACTGTCTTTGATCGCATCC | ||
| AQP7 | F: GTTTGGTCTAGGCTCCGTGG | Own design |
| R: GGTCACTGTCAGCTTTCCCT | ||
| ACTB | F: TCTGGCACCACACCTTCT | Su et al., 2018 [19] |
| R: TGATCTGGGTCATCTTCTCAC | ||
| GAPDH | F: AATGGGGTGATGCTGGTGCT | Lee and Kang, 2017 [20] |
| R: GGCAGAAGGGGCAGAGATGA |
| CON Versus IN-0.50 at an FDR of 0.10 | ||||||
| Ensembl Gene ID | Gene Symbol | Average Expression | log2FC | SE log2FC | p-Value | FDR |
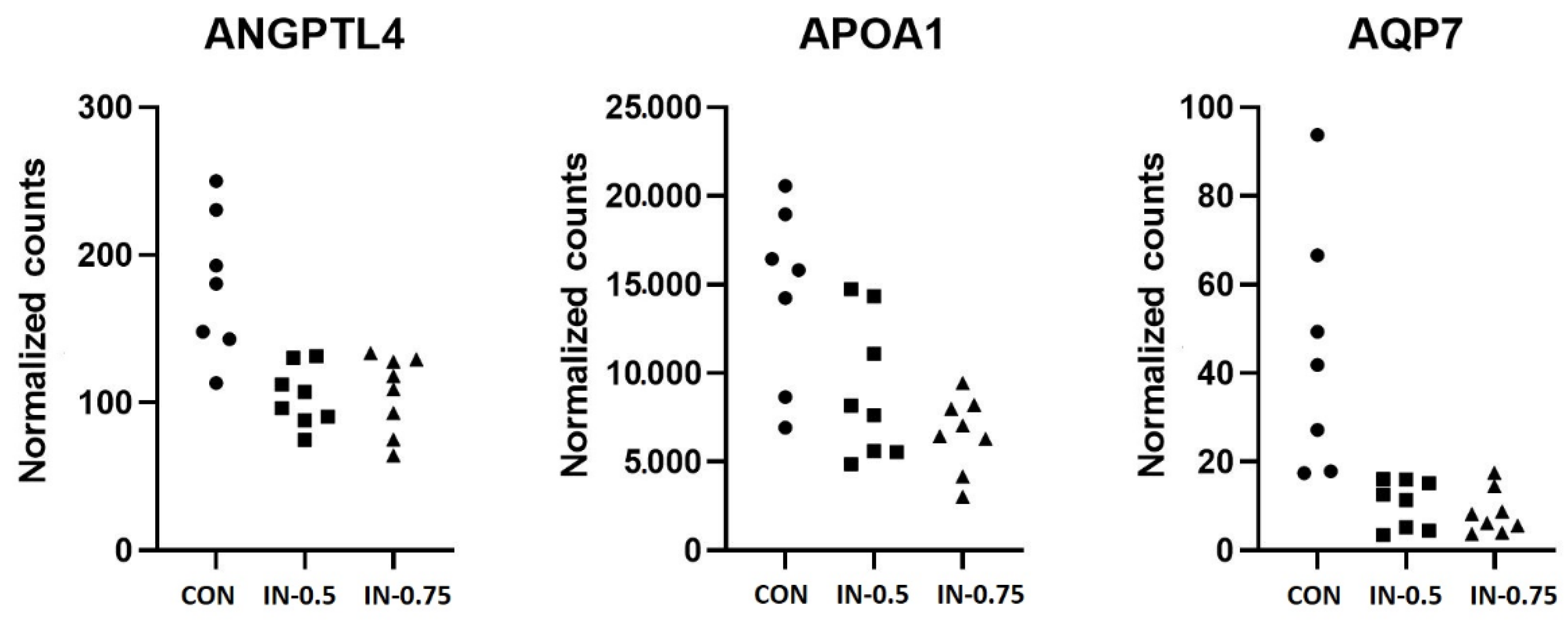
| ENSSSCG00000013599 | ANGPTL4 | 127.38 | 0.8 | 0.18 | 7.56 × 10−6 | 0.05 |
| ENSSSCG00000010992 | AQP7 | 19.83 | 2.16 | 0.41 | 1.96 × 10−7 | 0.004 |
| ENSSSCG00000008634 | ATP6V1C2 | 9.8 | 2.87 | 0.64 | 8.26 × 10−6 | 0.05 |
| CON Versus IN-0.75 at an FDR of 0.10 | ||||||
| Ensembl Gene ID | Gene Symbol | Average Expression | log2FC | SE log2FC | p-Value | FDR |
| ENSSSCG00000026585 | PROCR | 9.11 | −1.91 | 0.45 | 1.97 × 10−5 | 0.071 |
| ENSSSCG00000004232 | CLVS2 | 23.37 | −1.88 | 0.34 | 4.59 × 10−8 | 0 |
| ENSSSCG00000010166 | NTPCR | 16.45 | −1.04 | 0.25 | 3.70 × 10−5 | 0.084 |
| ENSSSCG00000007043 | GPCPD1 | 170.23 | 0.47 | 0.11 | 2.83 × 10−5 | 0.073 |
| ENSSSCG00000013599 | ANGPTL4 | 127.38 | 0.75 | 0.18 | 2.34 × 10−5 | 0.071 |
| ENSSSCG00000030921 | APOA1 | 9840.34 | 1.14 | 0.26 | 1.45 × 10−5 | 0.066 |
| ENSSSCG00000010992 | AQP7 | 19.83 | 2.47 | 0.42 | 4.52 × 10−9 | 0 |
| ENSSSCG00000008634 | ATP6V1C2 | 9.8 | 3.19 | 0.66 | 1.23 × 10−6 | 0.007 |
| Ensembl Gene ID | Gene Symbol | Expression CON | Expression IN-0.5 | Expression IN-0.75 | p-Value |
|---|---|---|---|---|---|
| ENSSSCG00000013599 | ANGPTL4 | 1.86 ± 0.36 | 1.02 ± 0.20 | 1.03 ± 0.25 | 0.071 |
| ENSSSCG00000030921 | APOA1 | 1.26 ± 0.20 | 0.76 ± 0.12 | 0.66 ± 0.17 | 0.044 |
| ENSSSCG00000010992 | AQP7 | 1.32 ± 0.20 | 0.41 ± 0.12 | 0.38 ± 0.17 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroyen, M.; Li, B.; Arévalo Sureda, E.; Zhang, Y.; Leblois, J.; Deforce, D.; Van Nieuwerburgh, F.; Wavreille, J.; Everaert, N. Pre-Weaning Inulin Supplementation Alters the Ileal Transcriptome in Pigs Regarding Lipid Metabolism. Vet. Sci. 2021, 8, 207. https://doi.org/10.3390/vetsci8100207
Schroyen M, Li B, Arévalo Sureda E, Zhang Y, Leblois J, Deforce D, Van Nieuwerburgh F, Wavreille J, Everaert N. Pre-Weaning Inulin Supplementation Alters the Ileal Transcriptome in Pigs Regarding Lipid Metabolism. Veterinary Sciences. 2021; 8(10):207. https://doi.org/10.3390/vetsci8100207
Chicago/Turabian StyleSchroyen, Martine, Bing Li, Ester Arévalo Sureda, Yuping Zhang, Julie Leblois, Dieter Deforce, Filip Van Nieuwerburgh, José Wavreille, and Nadia Everaert. 2021. "Pre-Weaning Inulin Supplementation Alters the Ileal Transcriptome in Pigs Regarding Lipid Metabolism" Veterinary Sciences 8, no. 10: 207. https://doi.org/10.3390/vetsci8100207
APA StyleSchroyen, M., Li, B., Arévalo Sureda, E., Zhang, Y., Leblois, J., Deforce, D., Van Nieuwerburgh, F., Wavreille, J., & Everaert, N. (2021). Pre-Weaning Inulin Supplementation Alters the Ileal Transcriptome in Pigs Regarding Lipid Metabolism. Veterinary Sciences, 8(10), 207. https://doi.org/10.3390/vetsci8100207






