Rational Use of Bethanechol in Dogs and Cats with Bladder Dysfunction
Simple Summary
Abstract
1. Introduction
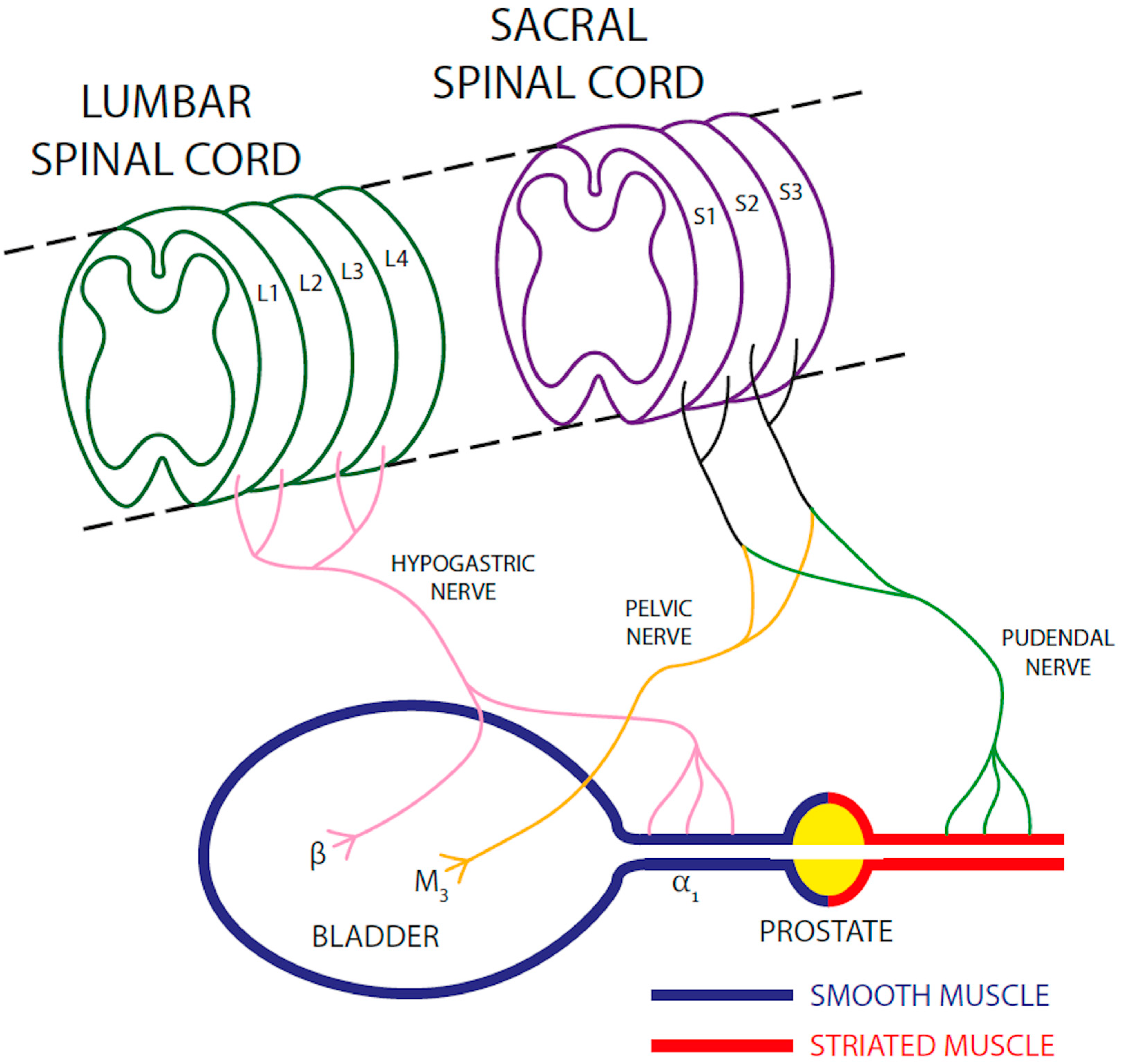
2. Anatomical and Physiological Basis of Micturition
3. Etiopathogenesis of Detrusor Contraction Deficit
4. Pharmacological Properties of Bethanechol
5. Effects of Bethanechol on the Bladder
6. Effects of Bethanechol on the Urethra
7. Indications for a Rational Use of Bethanechol
7.1. Total or Partial Suprasacral Lesions (UMNB)
7.2. Total or Partial Injuries Involving Sacral Segments, Cauda Equina, or the Pelvic Nerve (LMNB)
7.3. Reflex Dyssynergia
7.4. Myopathic Decompensated Bladder
8. Bethanechol Dosage and Route of Administration
9. Bethanechol Side Effects
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raezer, D.M.; Wein, A.J.; Jacobowitz, D.; Corriere, J.N., Jr. Autonomic innervation of canine urinary bladder. Cholinergic and adrenergic contributions and interaction of sympathetic and parasympathetic nervous systems in bladder function. Urology 1973, 2, 211–221. [Google Scholar] [CrossRef]
- Moro, C.; Phelps, C.; Veer, V.; Clark, J.; Glasziou, P.; Tikkinen, K.A.O.; Scott, A.M. The effectiveness of parasympathomimetics for treating underactive bladder: A systematic review and meta-analysis. Neurourol. Urodyn. 2022, 41, 127–139. [Google Scholar] [CrossRef]
- Mangel, A.W.; Stavorski, J.R.; Pendleton, R.G. Effects of bethanechol, metoclopramide, and domperidone on antral contractions in cats and dogs. Digestion 1983, 28, 205–209. [Google Scholar] [CrossRef]
- Levin, R.M.; Longhurst, P.A.; Kato, K.; McGuire, E.J.; Elbadawi, A.; Wein, A.J. Comparative physiology and pharmacology of the cat and rabbit urinary bladder. J. Urol. 1990, 143, 848–852. [Google Scholar] [CrossRef]
- Noel, S.; Massart, L.; Hamaide, A. Urodynamic investigation by telemetry in Beagle dogs: Validation and effects of oral administration of current urological drugs: A pilot study. BMC Vet. Res. 2013, 9, 197. [Google Scholar] [CrossRef]
- Kendall, A.; Byron, J.K.; Westropp, J.L.; Coates, J.R.; Vaden, S.; Adin, C.; Oetelaar, G.; Bartges, J.W.; Foster, J.D.; Adams, L.G.; et al. ACVIM consensus statement on diagnosis and management of urinary incontinence in dogs. J. Vet. Intern. Med. 2024, 38, 878–903. [Google Scholar] [CrossRef]
- Edvardsen, P.; Setekleiv, J. Distribution of adrenergic receptors in the urinary bladder of cats, rabbits, and guinea-pigs. Acta Pharmacol. Toxicol. 1968, 26, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Nergardh, A.; Boreus, L.O. The functional role of cholinergic receptors in the outlet region of the urinary bladder: An in vitro study in the cat. Acta Pharmacol. Toxicol. 1973, 32, 467–480. [Google Scholar] [CrossRef]
- Li, X.; Liao, L. Updates of underactive bladder: A review of the recent literature. Int. Urol. Nephrol. 2016, 48, 919–930. [Google Scholar] [CrossRef]
- Padda, I.S.; Derian, A. Bethanechol. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hegde, S.S. Muscarinic receptors in the bladder: From basic research to therapeutics. Br. J. Pharmacol. 2006, 147 (Suppl. S2), S80–S87. [Google Scholar] [CrossRef]
- Izumi, K.; Maolake, A.; Maeda, Y.; Shigehara, K.; Namiki, M. Effects of bethanechol chloride and distigmine bromide on postvoiding residual volume in patients with underactive bladder. Minerva Urol. Nefrol. 2014, 66, 241–247. [Google Scholar]
- Schneider, T.; Fetscher, C.; Krege, S.; Michel, M.C. Signal transduction underlying carbachol-induced contraction of human urinary bladder. J. Pharmacol. Exp. Ther. 2004, 309, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Twiddy, D.A.; Downie, J.W.; Awad, S.A. Response of the bladder to bethanechol after acute spinal cord transection in cats. J. Pharmacol. Exp. Ther. 1980, 215, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Downie, J.W. The autonomic pharmacology of the urinary bladder and urethra: A neglected area. Trends Pharmacol. Sci. 1981, 2, 3. [Google Scholar] [CrossRef]
- Downie, J.W. Bethanechol Chloride in Urology-A Discussion of Issues. Neurourol. Urodyn. 1984, 3, 12. [Google Scholar] [CrossRef]
- El-Salmy, S. The Dependence of Sustained Bladder Contractions Provoked by Filling or by Subcutaneous Bethanechol Upon Sacral Afferent Pathways. Neurourol. Urodyn. 1984, 3, 7. [Google Scholar] [CrossRef]
- Burleigh, D.E. Selectivity of bethanechol on muscarinic receptors. J. Pharm. Pharmacol. 1978, 30, 398–399. [Google Scholar] [CrossRef]
- Simonart, A.; Simonart, E.F. Le carbaminoyl-beta-methylcholine. Arch. Int. Pharmacodyn. Ther. 1935, 51, 7. [Google Scholar]
- Farber, S. On the pharmacology of carbaminoyl-betamethylcholine. Arch. Int. Pharmacodyn. Ther. 1936, 53, 11. [Google Scholar]
- Khanna, O.P.; Heber, D.; Gonick, P. Cholinergic and adrenergic neuroreceptors in urinary tract of female dogs. Evaluation of function with pharmacodynamics. Urology 1975, 5, 616–623. [Google Scholar] [CrossRef]
- Khanna, O.P. Disorders of micturition: Neuropharmacologic basis and results of drug therapy. Urology 1976, 8, 316–328. [Google Scholar] [CrossRef]
- Khanna, O.P.; Gonick, P. Effects of phenoxybenzamine hydrochloride on canine lower urinary tract: Clinical implications. Urology 1975, 6, 323–330. [Google Scholar] [CrossRef]
- Rohner, T.J., Jr.; Bolton, T.C.; Berkich, K.A.; Sanford, E.J. In vitro dog detrusor strip contractile responses and calcium ion activity. Investig. Urol. 1976, 14, 76–78. [Google Scholar]
- Tulloch, A.G.; Rossier, A.B. The action of neuropharmacologic agents on the bladder and urethra during experimental spinal shock. Investig. Urol. 1977, 14, 312–316. [Google Scholar]
- Buranakarl, C.; Kijtawornrat, A.; Angkanaporn, K.; Komolvanich, S.; Bovee, K.C. Effects of bethanechol on canine urinary bladder smooth muscle function. Res. Vet. Sci. 2001, 71, 175–181. [Google Scholar] [CrossRef]
- el-Salmy, S.; Downie, J.W.; Awad, S.A. Bladder and urethral function and supersensitivity to subcutaneously administered bethanechol in cats with chronic cauda equina lesions. J. Urol. 1985, 134, 1011–1018. [Google Scholar] [CrossRef]
- Girado, J.M.; Campbell, J.B. The innervation of the urethra of the female cat. Exp. Neurol. 1959, 1, 44–64. [Google Scholar] [CrossRef] [PubMed]
- Creed, K.E.; Tulloch, A.G. The effect of pelvic nerve stimulation and some drugs on the urethra and bladder of the dog. Br. J. Urol. 1978, 50, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Slack, B.E.; Downie, J.W. Pharmacological analysis of the responses of the feline urethra to autonomic nerve stimulation. J. Auton. Nerv. Syst. 1983, 8, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.A.; Chancellor, M.B.; Blaivas, J.G. Bladder and sphincter behavior in patients with spinal cord lesions. J. Urol. 1991, 146, 113–117. [Google Scholar] [CrossRef]
- Wyndaele, J.J. Correlation between clinical neurological data and urodynamic function in spinal cord injured patients. Spinal Cord 1997, 35, 213–216. [Google Scholar] [CrossRef]
- Weld, K.J.; Dmochowski, R.R. Association of level of injury and bladder behavior in patients with post-traumatic spinal cord injury. Urology 2000, 55, 490–494. [Google Scholar] [CrossRef]
- Agrawal, M.; Joshi, M. Urodynamic patterns after traumatic spinal cord injury. J. Spinal Cord Med. 2015, 38, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Z.; Granger, N.; Jeffery, N.D. Pathophysiology, Clinical Importance, and Management of Neurogenic Lower Urinary Tract Dysfunction Caused by Suprasacral Spinal Cord Injury. J. Vet. Intern. Med. 2016, 30, 1575–1588. [Google Scholar] [CrossRef]
- Lane, I.F. Disorders of micturition. In Canine and Feline Nephrology and Urology; Osborne, C.A.F.D.R., Ed.; Williams & Wilkins: Philadelphia, PA, USA, 1995; pp. 693–717. [Google Scholar]
- Dewey, C.W. Neurology and Neuropharmacology of Normal and Abnormal Urination. In Practical Guide to Canine and Feline Neurology; Dewey, C.W., da Costa, R.C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 419–426. [Google Scholar]
- Diokno, A.C.; Koppenhoefer, R. Bethanechol chloride in neurogenic bladder dysfunction. Urology 1976, 8, 455–458. [Google Scholar] [CrossRef]
- Granger, N.; Olby, N.J.; Nout-Lomas, Y.S.; the Canine Spinal Cord Injury Consortium (CANSORT-SCI). Bladder and Bowel Management in Dogs With Spinal Cord Injury. Front. Vet. Sci. 2020, 7, 583342. [Google Scholar] [CrossRef]
- Fisher, J.; Lane, I.F. Micturition disorders. In Nephrology and Urology of Small Animals; Polzin, J.B.a.D.J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 765–777. [Google Scholar]
- Byron, J.K. Micturition and Associated Disorders. In Clinical Small Animal Internal Medicine; Bruyetta, D., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2020; pp. 1181–1188. [Google Scholar]
- Diaz Espineira, M.M.; Viehoff, F.W.; Nickel, R.F. Idiopathic detrusor-urethral dyssynergia in dogs: A retrospective analysis of 22 cases. J. Small Anim. Pract. 1998, 39, 264–270. [Google Scholar] [CrossRef]
- Lorenz, M.D.; Coates, J.R.; Kent, M. Disorders of micturition. In Handbook of Veterinary Neurology, 5th ed.; Lorenz, M.D., Coates, J.R., Kent, M., Eds.; Elsevier Saunders: Saint-Louis, Senegal, 2011; pp. 58–74. [Google Scholar]
- Finkbeiner, A.E. Is bethanechol chloride clinically effective in promoting bladder emptying? A literature review. J. Urol. 1985, 134, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Zhu, Q.; Irwin, B.; Maghaydah, Y.; Tsimikas, J.; Pilbeam, C.; Leng, L.; Bucala, R.; Kuchel, G.A. Null mutation in macrophage migration inhibitory factor prevents muscle cell loss and fibrosis in partial bladder outlet obstruction. Am. J. Physiol. Renal Physiol. 2006, 291, F1343–F1353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rahardjo, H.E.; Marker, V.; Tsikas, D.; Kuczyk, M.A.; Uckert, S.; Bannowsky, A. Fibrotic Diseases of the Human Urinary and Genital Tract: Current Understanding and Potential Strategies for Treatment. J. Clin. Med. 2023, 12, 4770. [Google Scholar] [CrossRef]
- Diokno, A.C.; Lapides, J. Action of oral and parenteral bethanechol on decompensated bladder. Urology 1977, 10, 23–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stuart, M.; Applebaum, M.D. Pharmacologic agents in micturitional disorders. Urology 1980, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.R. Tail, anal and bladder dysfunction. In BSAVA Manual of Canine and Feline Neurology, 4th ed.; Olby, S.P.a.N., Ed.; Wiley: New York, NY, USA, 2013; pp. 368–387. [Google Scholar]
- Lapides, J.; Hodgson, N.B.; Boyd, R.E.; Shook, E.L.; Lichtwardt, J.R. Further observations on pharmacologic reactions of the bladder. J. Urol. 1958, 79, 707–713. [Google Scholar] [CrossRef]
- Barendrecht, M.M.; Oelke, M.; Laguna, M.P.; Michel, M.C. Is the use of parasympathomimetics for treating an underactive urinary bladder evidence-based? BJU Int. 2007, 99, 749–752. [Google Scholar] [CrossRef]
- Gaitonde, S.; Malik, R.D.; Christie, A.L.; Zimmern, P.E. Bethanechol: Is it still being prescribed for bladder dysfunction in women? Int. J. Clin. Pract. 2019, 73, e13248. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluzzi, F.; Menozzi, A.; Saleri, R.; De Rensis, F.; Spattini, G. Rational Use of Bethanechol in Dogs and Cats with Bladder Dysfunction. Vet. Sci. 2025, 12, 918. https://doi.org/10.3390/vetsci12090918
Galluzzi F, Menozzi A, Saleri R, De Rensis F, Spattini G. Rational Use of Bethanechol in Dogs and Cats with Bladder Dysfunction. Veterinary Sciences. 2025; 12(9):918. https://doi.org/10.3390/vetsci12090918
Chicago/Turabian StyleGalluzzi, Franco, Alessandro Menozzi, Roberta Saleri, Fabio De Rensis, and Giliola Spattini. 2025. "Rational Use of Bethanechol in Dogs and Cats with Bladder Dysfunction" Veterinary Sciences 12, no. 9: 918. https://doi.org/10.3390/vetsci12090918
APA StyleGalluzzi, F., Menozzi, A., Saleri, R., De Rensis, F., & Spattini, G. (2025). Rational Use of Bethanechol in Dogs and Cats with Bladder Dysfunction. Veterinary Sciences, 12(9), 918. https://doi.org/10.3390/vetsci12090918





