Factors Affecting the Applicability of Infrared Thermography as a Measure of Temperament in Cattle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
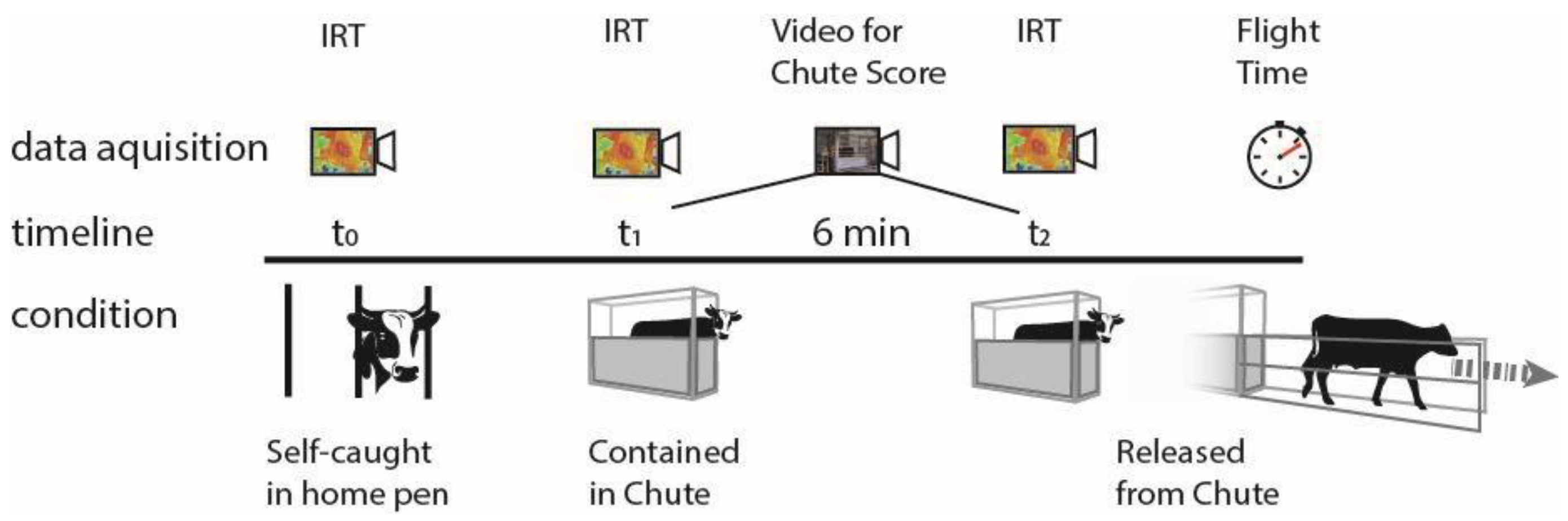
2.1. Experiment 1
2.1.1. Farm and Management
2.1.2. Animals and Experimental Design
2.1.3. Superficial Temperature
2.1.4. Chute Score
2.1.5. Flight Time
2.1.6. Temperature and Humidity Index
2.1.7. Statistical Analysis
2.2. Experiment 2
3. Results
3.1. Experiment 1
3.1.1. Environmental Conditions
3.1.2. Flight Time and Chute Score
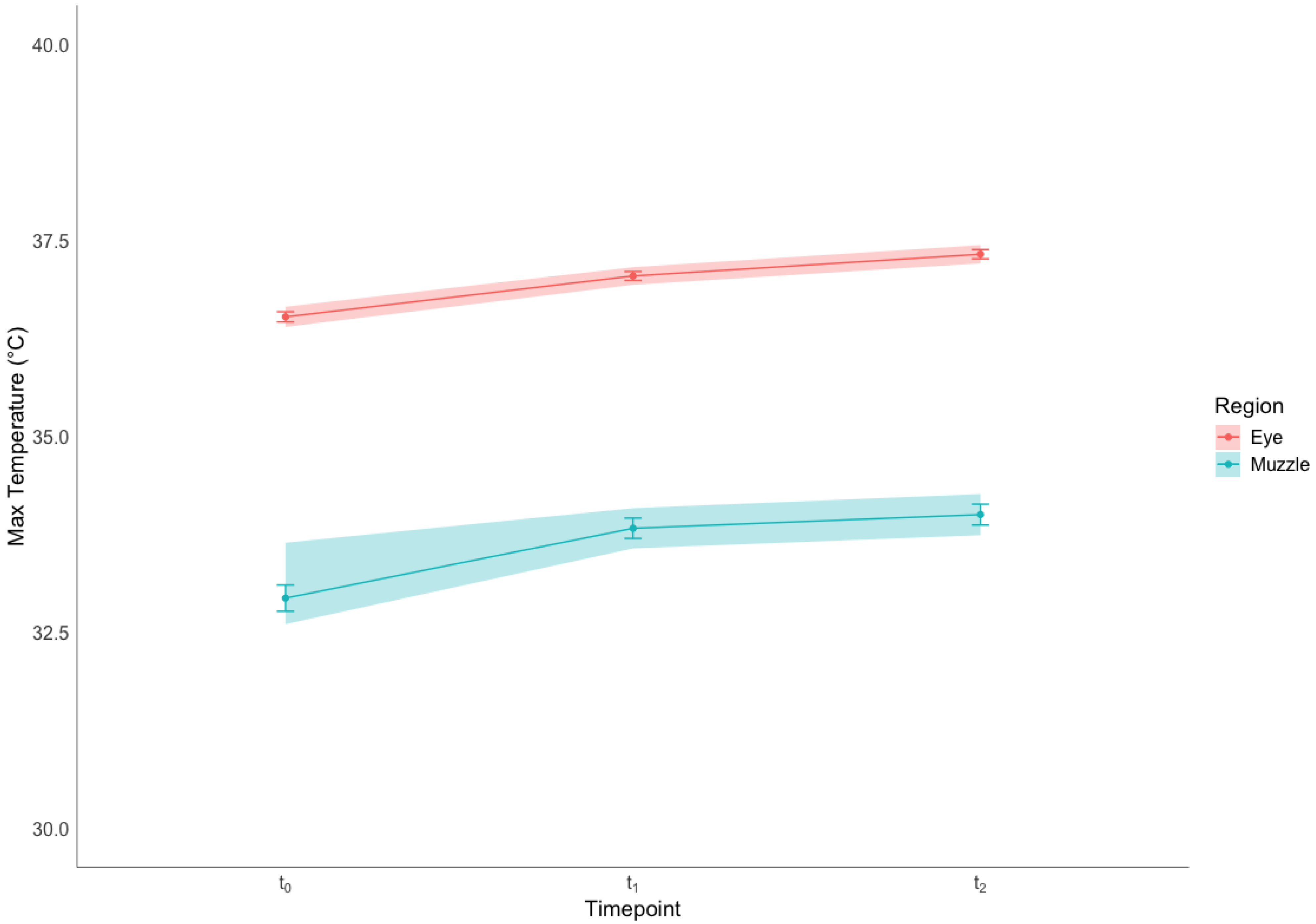
3.1.3. Superficial Temperature
3.1.4. Effect of Temperature, Humidity and Temperament on Increment in Eye and Muzzle Temperatures
3.2. Experiment 2
4. Discussion
4.1. Summary of Main Results
4.2. Baseline Temperatures in the Eye and Muzzle Regions
4.3. Effect of Restraint on IRT
4.4. Effect of THI and Temperament on Variations in Eye Temperature
4.5. Effect of THI and Temperament on Variations in Muzzle Temperature
4.6. Relationship Between Chute Score, Flight Time and Temperature Changes
4.7. Effect of THI and Temperament on IRT After Three Months
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutherland, M.A.; Rogers, A.R.; Verkerk, G.A. The Effect of Temperament and Responsiveness towards Humans on the Behavior, Physiology and Milk Production of Multi-Parous Dairy Cows in a Familiar and Novel Milking Environment. Physiol. Behav. 2012, 107, 329–337. [Google Scholar] [CrossRef]
- Smolinger, J.; Škorjanc, D. Methods of Assessing Cattle Temperament and Factors Affecting It: A Review. Agric. Sci. 2021, 18, 23–37. [Google Scholar] [CrossRef]
- Le Neindre, P.; Boivin, X.; Boissy, A. Handling of Extensively Kept Animals. Appl. Anim. Behav. Sci. 1996, 49, 73–81. [Google Scholar] [CrossRef]
- Cafe, L.M.; Robinson, D.L.; Ferguson, D.M.; Geesink, G.H.; Greenwood, P.L. Temperament and Hypothalamic-Pituitary-Adrenal Axis Function Are Related and Combine to Affect Growth, Efficiency, Carcass, and Meat Quality Traits in Brahman Steers. Domest. Anim. Endocrinol. 2011, 40, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Curley, K.O.J.; Neuendorff, D.A.; Lewis, A.W.; Rouquette, F.M.J.; Randel, R.D.; Welsh, T.H.J. The Effectiveness of Vasopressin as an ACTH Secretagogue in Cattle Differs with Temperament. Physiol. Behav. 2010, 101, 699–704. [Google Scholar] [CrossRef]
- O’Connor, T.G.; Scheible, K.; Sefair, A.V.; Gilchrist, M.; Blackmore, E.R.; Winter, M.A.; Gunnar, M.R.; Wyman, C.; Carnahan, J.; Moynihan, J.A.; et al. Immune and Neuroendocrine Correlates of Temperament in Infancy. Dev. Psychopathol. 2017, 29, 1589–1600. [Google Scholar] [CrossRef]
- Sebastian, T.; Watts, J.; Stookey, J.; Buchanan, F.; Waldner, C. Temperament in Beef Cattle: Methods of Measurement and Their Relationship to Production. Can. J. Anim. Sci. 2011, 91, 557–565. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping Styles in Animals: Current Status in Behavior and Stress-Physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Boivin, X.; Le Neindre, P.; Garel, J.P.; Chupin, J.M. Influence of Breed and Rearing Management on Cattle Reactions during Human Handling. Appl. Anim. Behav. Sci. 1994, 39, 115–122. [Google Scholar] [CrossRef]
- Grandin, T. Behavioral Agitation during Handling of Cattle Is Persistent over Time. Appl. Anim. Behav. Sci. 1993, 36, 1–9. [Google Scholar] [CrossRef]
- Cafe, L.M.; Robinson, D.L.; Ferguson, D.M.; McIntyre, B.L.; Geesink, G.H.; Greenwood, P.L. Cattle Temperament: Persistence of Assessments and Associations with Productivity, Efficiency, Carcass and Meat Quality Traits1. J. Anim. Sci. 2011, 89, 1452–1465. [Google Scholar] [CrossRef]
- Fell, L.R.; Colditz, I.G.; Walker, K.H.; Watson, D.L. Associations between Temperament, Performance and Immune Function in Cattle Entering a Commercial Feedlot. Aust. J. Exp. Agric. 1999, 39, 795. [Google Scholar] [CrossRef]
- Curley, K.O.; Paschal, J.C.; Welsh, T.H.; Randel, R.D. Technical Note: Exit Velocity as a Measure of Cattle Temperament Is Repeatable and Associated with Serum Concentration of Cortisol in Brahman Bulls1. J. Anim. Sci. 2006, 84, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.F.B.; Medrado, B.D.; Pedrosa, V.B.; Brito, L.F. A Systematic Review with Meta-analysis of Heritability Estimates for Temperament-related Traits in Beef and Dairy Cattle Populations. J. Anim. Breed. Genet. 2025, 142, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.E.; Neuendorff, D.A.; Riley, D.G.; Vann, R.C.; Willard, S.T.; Welsh, T.H.J.; Randel, R.D. Genetic Parameters of Three Methods of Temperament Evaluation of Brahman Calves. J. Anim. Sci. 2014, 92, 3082–3087. [Google Scholar] [CrossRef]
- Littlejohn, B.P.; Riley, D.G.; Welsh, T.H.J.; Randel, R.D.; Willard, S.T.; Vann, R.C. Use of Random Regression to Estimate Genetic Parameters of Temperament across an Age Continuum in a Crossbred Cattle Population. J. Anim. Sci. 2018, 96, 2607–2621. [Google Scholar] [CrossRef]
- Gibbons, J.M.; Lawrence, A.B.; Haskell, M.J. Consistency of Flight Speed and Response to Restraint in a Crush in Dairy Cattle. Appl. Anim. Behav. Sci. 2011, 131, 15–20. [Google Scholar] [CrossRef]
- Colditz, I.G. Adrenergic Tone as an Intermediary in the Temperament Syndrome Associated with Flight Speed in Beef Cattle. Front. Anim. Sci. 2021, 2, 652306. [Google Scholar] [CrossRef]
- Lees, A.M.; Salvin, H.E.; Colditz, I.G.; Lee, C. The Influence of Temperament on Body Temperature Response to Handling in Angus Cattle. Animals 2020, 10, 172. [Google Scholar] [CrossRef]
- Kovács, L.; Kézér, F.L.; Tőzsér, J.; Szenci, O.; Póti, P.; Pajor, F. Heart Rate and Heart Rate Variability in Dairy Cows with Different Temperament and Behavioural Reactivity to Humans. PLoS ONE 2015, 10, e0136294. [Google Scholar] [CrossRef]
- Chen, X.; Ogdahl, W.; Hulsman Hanna, L.L.; Dahlen, C.R.; Riley, D.G.; Wagner, S.A.; Berg, E.P.; Sun, X. Evaluation of Beef Cattle Temperament by Eye Temperature Using Infrared Thermography Technology. Comput. Electron. Agric. 2021, 188, 106321. [Google Scholar] [CrossRef]
- Ouyang, J.Q.; Macaballug, P.; Chen, H.; Hodach, K.; Tang, S.; Francis, J.S. Infrared Thermography Is an Effective, Noninvasive Measure of HPA Activation. Stress 2021, 24, 584–589. [Google Scholar] [CrossRef]
- Silva, J.E. Thermogenic Mechanisms and Their Hormonal Regulation. Physiol. Rev. 2006, 86, 435–464. [Google Scholar] [CrossRef]
- Adriaan Bouwknecht, J.; Olivier, B.; Paylor, R.E. The Stress-Induced Hyperthermia Paradigm as a Physiological Animal Model for Anxiety: A Review of Pharmacological and Genetic Studies in the Mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef]
- Chen, A.; Zhu, J.; Lin, Q.; Liu, W. A Comparative Study of Forehead Temperature and Core Body Temperature under Varying Ambient Temperature Conditions. Int. J. Environ. Res. Public Health 2022, 19, 15883. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Stafford, K.J.; Dowling, S.K.; Schaefer, A.L.; Webster, J.R. Eye Temperature and Heart Rate Variability of Calves Disbudded with or without Local Anaesthetic. Physiol. Behav. 2008, 93, 789–797. [Google Scholar] [CrossRef]
- Jerem, P.; Jenni-Eiermann, S.; McKeegan, D.; McCafferty, D.J.; Nager, R.G. Eye Region Surface Temperature Dynamics during Acute Stress Relate to Baseline Glucocorticoids Independently of Environmental Conditions. Physiol. Behav. 2019, 210, 112627. [Google Scholar] [CrossRef]
- Wongsaengchan, C.; McCafferty, D.J.; Evans, N.P.; McKeegan, D.E.F.; Nager, R.G. Body Surface Temperature of Rats Reveals Both Magnitude and Sex Differences in the Acute Stress Response. Physiol. Behav. 2023, 264, 114138. [Google Scholar] [CrossRef] [PubMed]
- Church, J.S.; Hegadoren, P.R.; Paetkau, M.J.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K.S. Influence of Environmental Factors on Infrared Eye Temperature Measurements in Cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.; Sullivan, M.; Gaughan, J.B.; Phillips, C.J.C. The Relationship between the Infrared Eye Temperature of Beef Cattle and Associated Biological Responses at High Environmental Temperatures. Animals 2024, 14, 2898. [Google Scholar] [CrossRef]
- Vieira, R.A.; Dias, E.A.; Stumpf, M.T.; Pereira, G.R.; Barcellos, J.O.J.; Kolling, G.J.; McManus, C. Use of Thermography and Physiological Rate to Assess Heat Tolerance in Cattle Breeds. Trop. Anim. Health Prod. 2023, 55, 223. [Google Scholar] [CrossRef]
- Proctor, H.; Carder, G. Can Changes in Nasal Temperature Be Used as an Indicator of Emotional State in Cows? Appl. Anim. Behav. Sci. 2016, 184, 1–6. [Google Scholar] [CrossRef]
- Shephard, R.; Maloney, S. A Review of Thermal Stress in Cattle. Aust. Vet. J. 2023, 101, 417–429. [Google Scholar] [CrossRef]
- Bagath, M.; Krishnan, G.; Devaraj, C.; Rashamol, V.P.; Pragna, P.; Lees, A.M.; Sejian, V. The Impact of Heat Stress on the Immune System in Dairy Cattle: A Review. Res. Vet. Sci. 2019, 126, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.R. Behavioural, Physiological, Neuro-Endocrine and Molecular Responses of Cattle against Heat Stress: An Updated Review. Trop. Anim. Health Prod. 2021, 53, 400. [Google Scholar] [CrossRef]
- Lemal, P.; May, K.; König, S.; Schroyen, M.; Gengler, N. Invited Review: From Heat Stress to Disease-Immune Response and Candidate Genes Involved in Cattle Thermotolerance. J. Dairy Sci. 2023, 106, 4471–4488. [Google Scholar] [CrossRef]
- Giaretta, E.; Mongillo, P.; Da Dalt, L.; Gianesella, M.; Bortoletti, M.; Degano, L.; Vicario, D.; Gabai, G. Temperature and Humidity Index (THI) Affects Salivary Cortisol (HC) and Dehydroepiandrosterone (DHEA) Concentrations in Growing Bulls Following Stress Generated by Performance Test Procedures. Front. Vet. Sci. 2023, 10, 1237634. [Google Scholar] [CrossRef]
- Mincu, M.; Nicolae, I.; Gavojdian, D. Infrared Thermography as a Non-Invasive Method for Evaluating Stress in Lactating Dairy Cows during Isolation Challenges. Front. Vet. Sci. 2023, 10, 1236668. [Google Scholar] [CrossRef]
- Salles, M.S.V.; Da Silva, S.C.; Salles, F.A.; Roma, L.C.; El Faro, L.; Bustos Mac Lean, P.A.; Lins De Oliveira, C.E.; Martello, L.S. Mapping the Body Surface Temperature of Cattle by Infrared Thermography. J. Therm. Biol. 2016, 62, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, S.; Vannetti, F.; Sodi, A.; Corvi, A. Infrared Thermographic Investigation on the Ocular Surface Temperature of Normal Subjects. Physiol. Meas. 2020, 41, 045003. [Google Scholar] [CrossRef] [PubMed]
- Wanner, S.P.; Prímola-Gomes, T.N.; Pires, W.; Guimarães, J.B.; Hudson, A.S.R.; Kunstetter, A.C.; Fonseca, C.G.; Drummond, L.R.; Damasceno, W.C.; Teixeira-Coelho, F. Thermoregulatory Responses in Exercising Rats: Methodological Aspects and Relevance to Human Physiology. Temp. Austin Tex 2015, 2, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, R.G.; Coyle, E.F. Cutaneous Blood Flow during Exercise Is Higher in Endurance-Trained Humans. J. Appl. Physiol. 2000, 88, 738–744. [Google Scholar] [CrossRef]
- Formenti, D.; Ludwig, N.; Gargano, M.; Gondola, M.; Dellerma, N.; Caumo, A.; Alberti, G. Thermal Imaging of Exercise-Associated Skin Temperature Changes in Trained and Untrained Female Subjects. Ann. Biomed. Eng. 2013, 41, 863–871. [Google Scholar] [CrossRef]
- Lei, M.C.; Félix, L.; Cardoso, R.; Monteiro, S.M.; Silva, S.; Venâncio, C. Non-Invasive Biomarkers in Saliva and Eye Infrared Thermography to Assess the Stress Response of Calves during Transport. Animals 2023, 13, 2311. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-Invasive Measurement of Stress in Dairy Cows Using Infrared Thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef]
- Kuraoka, K.; Nakamura, K. The Use of Nasal Skin Temperature Measurements in Studying Emotion in Macaque Monkeys. Physiol. Behav. 2011, 102, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Burdick Sanchez, N.C.; Carroll, J.A.; Broadway, P.R.; Hughes, H.D.; Roberts, S.L.; Richeson, J.T.; Schmidt, T.B.; Vann, R.C. Cattle Temperament Influences Metabolism: Metabolic Response to Glucose Tolerance and Insulin Sensitivity Tests in Beef Steers. Domest. Anim. Endocrinol. 2016, 56, 85–95. [Google Scholar] [CrossRef]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Willard, S.T.; Vann, R.C.; Welsh, T.H.; Randel, R.D. Relationships between Temperament and Transportation with Rectal Temperature and Serum Concentrations of Cortisol and Epinephrine in Bulls. Livest. Sci. 2010, 129, 166–172. [Google Scholar] [CrossRef]
- Burdick, N.C.; Carroll, J.A.; Randel, R.D.; Willard, S.T.; Vann, R.C.; Chase, C.C.; Lawhon, S.D.; Hulbert, L.E.; Welsh, T.H. Influence of Temperament and Transportation on Physiological and Endocrinological Parameters in Bulls. Livest. Sci. 2011, 139, 213–221. [Google Scholar] [CrossRef]
- Geburt, K.; Piechotta, M.; König Von Borstel, U.; Gauly, M. Influence of Testosterone on the Docility of German Simmental and Charolais Heifers during Behavior Tests. Physiol. Behav. 2015, 141, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.; Silpa, M.V.; Sejian, V. Environmental Physiology and Thermoregulation in Farm Animals. In Textbook of Veterinary Physiology; Springer Nature: Singapore, 2023; pp. 723–749. ISBN 978-981-19940-9-8. [Google Scholar]
- Parham, J.T.; Tanner, A.E.; Barkley, K.; Pullen, L.; Wahlberg, M.L.; Swecker, W.S.; Lewis, R.M. Temperamental Cattle Acclimate More Substantially to Repeated Handling. Appl. Anim. Behav. Sci. 2019, 212, 36–43. [Google Scholar] [CrossRef]
- Parham, J.T.; Blevins, S.R.; Tanner, A.E.; Wahlberg, M.L.; Swecker, W.S.; Lewis, R.M. Subjective Methods of Quantifying Temperament in Heifers Are Indicative of Physiological Stress. Appl. Anim. Behav. Sci. 2021, 234, 105197. [Google Scholar] [CrossRef]
| Month | N | THI0 | THI1 |
|---|---|---|---|
| 1 | 25 | 51.2 ± 5.3 | 52.7 ± 6.2 |
| 2 | 29 | 57.3 ± 2.0 | 56.0 ± 5.3 |
| 3 | 18 | 57.4 ± 0.0 | 59.7 ± 2.6 |
| 5 | 17 | 74.5 ± 0.0 | 75.0 ± 0.0 |
| 6 | 18 | 77.6 ± 0.0 | 74.2 ± 4.8 |
| 7 | 18 | 80.1 ± 1.1 | 79.0 ± 1.8 |
| 8 | 18 | 82.9 ± 0.0 | 83.6 ± 0.0 |
| 9 | 17 | 67.9 ± 0.0 | 64.7 ± 2.1 |
| 10 | 18 | 68.1 ± 0.0 | 69.7 ± 0.0 |
| 11 | 18 | 61.1 ± 1.3 | 62.7 ± 1.6 |
| 12 | 27 | 50.0 ± 0.0 | 50.0 ± 2.2 |
| Score | N | % |
|---|---|---|
| 1 | 6 | 3 |
| 2 | 35 | 17 |
| 3 | 98 | 48 |
| 4 | 58 | 28 |
| 5 | 8 | 4 |
| Between-Subjects Effects | |||||
|---|---|---|---|---|---|
| Dependent Variable | Model R2 | Factor | F | p | Partial Eta Squared |
| ΔMT1-0 | 0.652 | THI0 | 7.78 | 0.006 | 0.064 |
| ΔTHI1-0 | 12.69 | <0.001 | 0.101 | ||
| Chute Score | 2.32 | 0.102 | 0.039 | ||
| Flight Time | 9.65 | 0.002 | 0.079 | ||
| ΔMT2-1 | 0.406 | THI0 | 0.52 | 0.471 | 0.003 |
| ΔTHI1-0 | 2.28 | 0.132 | 0.017 | ||
| Chute Score | 1.43 | 0.242 | 0.021 | ||
| Flight Time | 5.22 | 0.023 | 0.038 | ||
| ΔET1-0 | 0.490 | THI0 | 1.23 | 0.269 | 0.009 |
| ΔTHI1-0 | 3.62 | 0.059 | 0.027 | ||
| Chute Score | 1.06 | 0.347 | 0.016 | ||
| Flight Time | 6.15 | 0.014 | 0.045 | ||
| ΔET2-1 | 0.375 | THI0 | 1.93 | 0.167 | 0.014 |
| ΔTHI1-0 | 0.31 | 0.576 | 0.002 | ||
| Chute Score | 1.16 | 0.316 | 0.018 | ||
| Flight Time | 0.01 | 0.969 | 0.000 | ||
| R | p | |
|---|---|---|
| Flight Time | 0.325 | <0.001 |
| Chute Score | 0.083 1 | 0.442 |
| MT0 | 0.459 | <0.001 |
| ET0 | 0.785 | <0.001 |
| ΔMT1-0 | −0.180 | 0.093 |
| ΔMT2-1 | 0.218 | 0.027 |
| ΔET1-0 | 0.245 | 0.015 |
| ΔET2-1 | −0.082 | 0.411 |
| Dependent Variable | Marginal Pseudo-R2 | Factor | F | p |
|---|---|---|---|---|
| ΔMT1-0 | 0.038 | THI0 | 0.66 | 0.417 |
| ΔTHI1-0 | 5.07 | 0.026 | ||
| Flight Time | 0.63 | 0.426 | ||
| Age | 3.18 | 0.076 | ||
| ΔMT2-1 | 0.101 | THI0 | 10.35 | 0.002 |
| ΔTHI1-0 | 2.05 | 0.153 | ||
| Flight Time | 5.69 | 0.018 | ||
| Age | 0.00 | 0.970 | ||
| ΔET1-0 | 0.033 | THI0 | 0.30 | 0.583 |
| ΔTHI1-0 | 4.71 | 0.031 | ||
| Flight Time | 1.36 | 0.245 | ||
| Age | 1.35 | 0.245 | ||
| ΔET2-1 | 0.027 | THI0 | 3.54 | 0.061 |
| ΔTHI1-0 | 1.90 | 0.170 | ||
| Flight Time | 0.46 | 0.498 | ||
| Age | 1.48 | 0.224 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongillo, P.; Giaretta, E.; Fiore, E.; Fabbri, G.; Stefanon, B.; Degano, L.; Vicario, D.; Gabai, G. Factors Affecting the Applicability of Infrared Thermography as a Measure of Temperament in Cattle. Vet. Sci. 2025, 12, 913. https://doi.org/10.3390/vetsci12090913
Mongillo P, Giaretta E, Fiore E, Fabbri G, Stefanon B, Degano L, Vicario D, Gabai G. Factors Affecting the Applicability of Infrared Thermography as a Measure of Temperament in Cattle. Veterinary Sciences. 2025; 12(9):913. https://doi.org/10.3390/vetsci12090913
Chicago/Turabian StyleMongillo, Paolo, Elisa Giaretta, Enrico Fiore, Giorgia Fabbri, Bruno Stefanon, Lorenzo Degano, Daniele Vicario, and Gianfranco Gabai. 2025. "Factors Affecting the Applicability of Infrared Thermography as a Measure of Temperament in Cattle" Veterinary Sciences 12, no. 9: 913. https://doi.org/10.3390/vetsci12090913
APA StyleMongillo, P., Giaretta, E., Fiore, E., Fabbri, G., Stefanon, B., Degano, L., Vicario, D., & Gabai, G. (2025). Factors Affecting the Applicability of Infrared Thermography as a Measure of Temperament in Cattle. Veterinary Sciences, 12(9), 913. https://doi.org/10.3390/vetsci12090913







