Synthesis of Giardia Species and Genotypes in Wild Birds: A Review
Simple Summary
Abstract
1. Introduction
- Life Cycle
- Diagnosis of Giardia
- Importance of giardiasis in human, animal, and ecosystem health
- Effects of Climate Change on the Distribution of Giardia and its Transmission
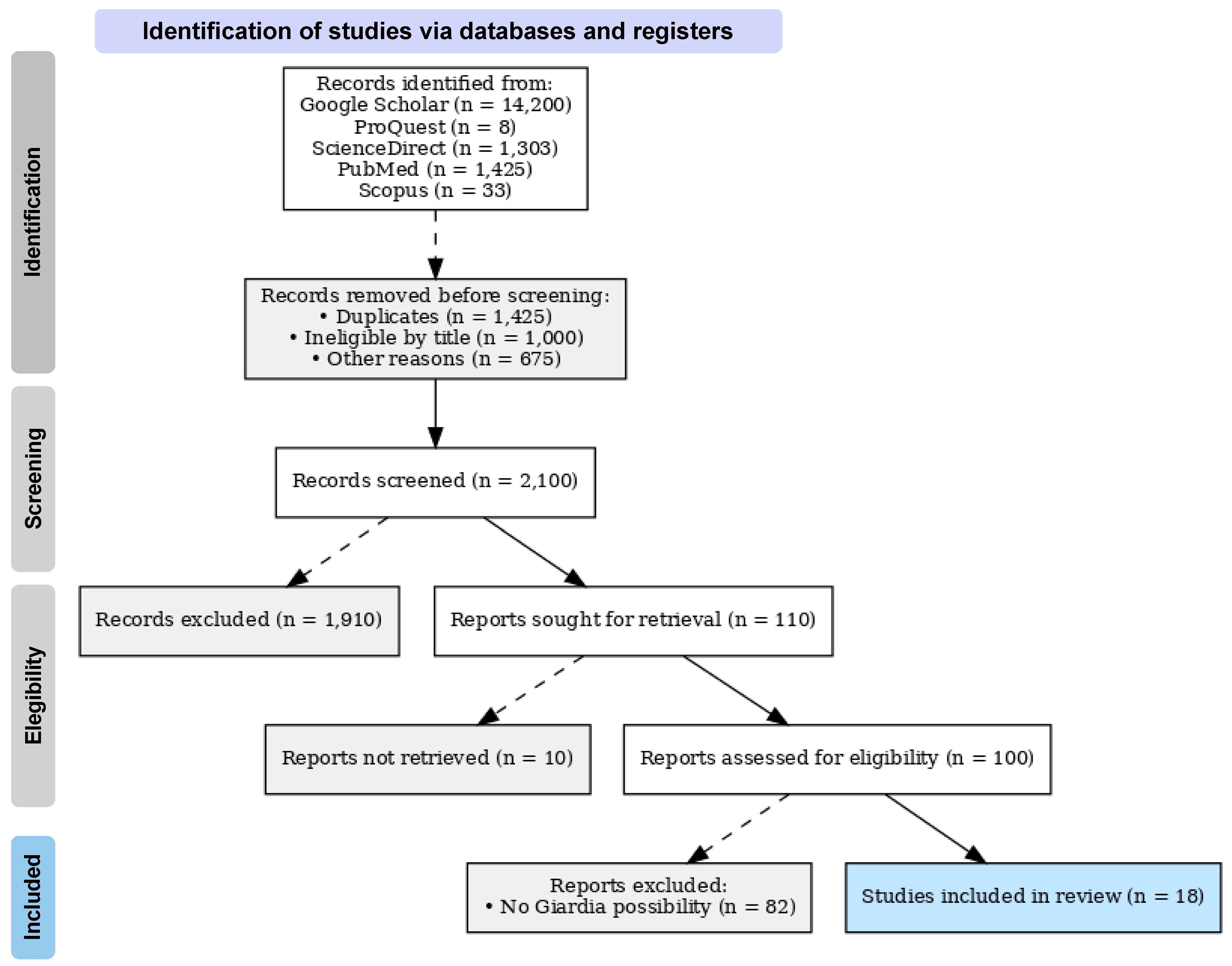
2. Materials and Methods
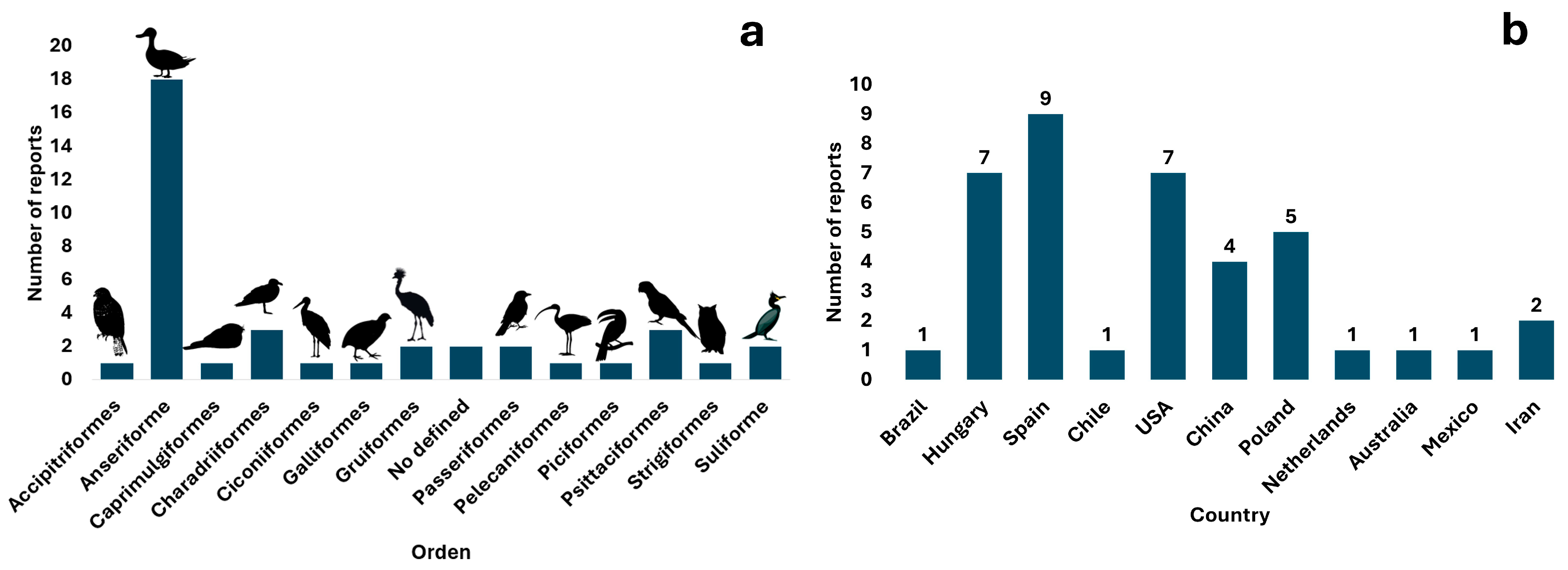
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wielinga, C.; Williams, A.; Monis, P.; Thompson, R.C.A. Proposed Taxonomic Revision of Giardia duodenalis. Infect. Genet. Evol. 2023, 111, 105430. [Google Scholar] [CrossRef]
- Ortega, Y.R.; Adam, R.D. Giardia: Overview and Update. Clin. Infect. Dis. 1997, 25, 545–549. [Google Scholar] [CrossRef]
- Ryan, U.M.; Feng, Y.; Fayer, R.; Xiao, L. Taxonomy and Molecular Epidemiology of Cryptosporidium and Giardia—A 50 Year Perspective (1971–2021). Int. J. Parasitol. 2021, 51, 1099–1119. [Google Scholar] [CrossRef]
- Adam, R.D. Giardia duodenalis: Biology and Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00024-19. [Google Scholar] [CrossRef]
- Ryan, U.; Zahedi, A. Molecular Epidemiology of Giardiasis from a Veterinary Perspective. In Advances in Parasitology; Academic Press: Cambridge, MA, USA, 2019; Volume 106, pp. 209–254. ISBN 9780128177204. [Google Scholar]
- Feng, Y.; Xiao, L. Zoonotic Potential and Molecular Epidemiology of Giardia Species and Giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Morrison, H.G.; McArthur, A.G.; Gillin, F.D.; Aley, S.B.; Adam, R.D.; Olsen, G.J.; Best, A.A.; Cande, W.Z.; Chen, F.; Cipriano, M.J.; et al. Genomic Minimalism in the Early Diverging Intestinal Parasite Giardia lamblia. Science 2007, 317, 1921–1926. [Google Scholar] [CrossRef]
- Kooyman, F.N.J.; Wagenaar, J.A.; Zomer, A. Whole-Genome Sequencing of Dog-Specific Assemblages C and D of Giardia duodenalis from Single and Pooled Cysts Indicates Host-Associated Genes. Microb. Genom. 2019, 5, e000302. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.D.; Dahlstrom, E.W.; Martens, C.A.; Bruno, D.P.; Barbian, K.D.; Ricklefs, S.M.; Hernandez, M.M.; Narla, N.P.; Patel, R.B.; Porcella, S.F.; et al. Genome Sequencing of Giardia lamblia Genotypes A2 and B Isolates (DH and GS) and Comparative Analysis with the Genomes of Genotypes A1 and E (WB and Pig). Genome Biol. Evol. 2013, 5, 2498–2511. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Cacciò, S.M. Zoonotic Potential of Giardia. Int. J. Parasitol. 2013, 43, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Procesi, I.G.; Di Filippo, M.M.; De Liberato, C.; Lombardo, A.; Brocherel, G.; Perrucci, S.; Di Cave, D.; Berrilli, F. Giardia duodenalis in Wildlife: Exploring Genotype Diversity in Italy and across Europe. Pathogens 2022, 11, 105. [Google Scholar] [CrossRef]
- Jian, Y.; Zhang, X.; Li, X.; Schou, C.; Charalambidou, I.; Ma, L.; Karanis, P. Occurrence of Cryptosporidium and Giardia in Wild Birds from Qinghai Lake on the Qinghai-Tibetan Plateau, China. Parasitol. Res. 2021, 120, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Invasive Species Challenge the Global Response to Emerging Diseases. Trends Parasitol. 2014, 30, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef]
- Barash, N.R.; Maloney, J.G.; Singer, S.M.; Dawson, S.C. Giardia Alters Commensal Microbial Diversity throughout the Murine Gut. Infect. Immun. 2017, 85, 10–1128. [Google Scholar] [CrossRef]
- Einarsson, E.; Ma’ayeh, S.; Svärd, S.G. An Up-Date on Giardia and Giardiasis. Curr. Opin. Microbiol. 2016, 34, 47–52. [Google Scholar] [CrossRef]
- Olson, M.E.; Goh, J.; Phillips, M.; Guselle, N.; McAllister, T.A. Giardia Cyst and Cryptosporidium Oocyst Survival in Water, Soil, and Cattle Feces. J. Environ. Qual. 1999, 28, 1991–1996. [Google Scholar] [CrossRef]
- Coffey, C.M.; Collier, S.A.; Gleason, M.E.; Yoder, J.S.; Kirk, M.D.; Richardson, A.M.; Fullerton, K.E.; Benedict, K.M. Evolving Epidemiology of Reported Giardiasis Cases in the United States, 1995–2016. Clin. Infect. Dis. 2021, 72, 764–770. [Google Scholar] [CrossRef]
- McDonnell, P.A.; Upcroft, J.; Upcroft, P.; Buret, A. Morphological identification markers for distinguishing avian from mammalian Giardia species—Do they need reconsideration? Eur. J. Protistol. 2001, 37, 273–280. [Google Scholar] [CrossRef]
- Ballweber, L.R.; Xiao, L.; Bowman, D.D.; Kahn, G.; Cama, V.A. Giardiasis in Dogs and Cats: Update on Epidemiology and Public Health Significance. Trends Parasitol. 2010, 26, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Cacciò, S.M.; Beck, R.; Lalle, M.; Marinculic, A.; Pozio, E. Multilocus Genotyping of Giardia duodenalis Reveals Striking Differences between Assemblages A and B. Int. J. Parasitol. 2008, 38, 1523–1531. [Google Scholar] [CrossRef]
- Hanevik, K.; Bakken, R.; Brattbakk, H.R.; Saghaug, C.S.; Langeland, N. Whole Genome Sequencing of Clinical Isolates of Giardia lamblia. Clin. Microbiol. Infect. 2015, 21, 192.e1–192.e3. [Google Scholar] [CrossRef]
- Yason, J.A.D.L.; Rivera, W.L. Genotyping of Giardia duodenalis Isolates among Residents of Slum Area in Manila, Philippines. Parasitol. Res. 2007, 101, 681–687. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites. Microbiological Risk Assessment Series No. 23. Food and Agriculture Organization of the United Nations/World Health Organization. FAO Headquarters, Rome, Italy. 2014. Available online: http://apps.who.int/iris/bitstream/10665/112672/1/9789241564700_eng.pdf (accessed on 9 September 2025).
- Horton, B.; Bridle, H.; Alexander, C.L.; Katzer, F. Giardia duodenalis in the UK: Current Knowledge of Risk Factors and Public Health Implications. Parasitology 2019, 146, 413–424. [Google Scholar] [CrossRef]
- Jenkins, E.J.; Simon, A.; Bachand, N.; Stephen, C. Wildlife parasites in a One Health world. Trends Parasitol. 2015, 31, 174–180. [Google Scholar] [CrossRef]
- Ebani, V.V.; Guardone, L.; Bertelloni, F.; Perrucci, S.; Poli, A.; Mancianti, F. Survey on the Presence of Bacterial and Parasitic Zoonotic Agents in the Feces of Wild Birds. Vet. Sci. 2021, 8, 171. [Google Scholar] [CrossRef]
- Reboredo-Fernandez, A.; Ares-Mazás, E.; Caccio, S.M.; Gómez-Couso, H. Occurrence of Giardia and Cryptosporidium in Wild Birds in Galicia (Northwest Spain). Parasitology 2015, 142, 917–925. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, M.J.R.; Cury, M.C.; Santín, M. Molecular Identification of Enterocytozoon Bieneusi, Cryptosporidium, and Giardia in Brazilian Captive Birds. Parasitol. Res. 2017, 116, 487–493. [Google Scholar] [CrossRef]
- Cano, L.; de Lucio, A.; Bailo, B.; Cardona, G.A.; Muadica, A.S.O.; Lobo, L.; Carmena, D. Identification and Genotyping of Giardia spp. and Cryptosporidium spp. Isolates in Aquatic Birds in the Salburua Wetlands, Álava, Northern Spain. Vet. Parasitol. 2016, 221, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Van Hemert, C.; Ballweber, L.R.; Sinnett, D.R.; Atwood, T.C.; Fischbach, A.; Gustine, D.D.; Pabilonia, K.L. Giardia and Cryptosporidium in resident wildlife species in Arctic Alaska. Food Waterborne Parasitol. 2023, 32, e00206. [Google Scholar] [CrossRef]
- Salubi, E.A.; Gizaw, Z.; Schuster-Wallace, C.J.; Pietroniro, A. Climate change and waterborne diseases in temperate regions: A systematic review. J. Water Health 2025, 23, 58–78. [Google Scholar] [CrossRef]
- Dietrich, J.; Hammerl, J.A.; Johne, A.; Kappenstein, O.; Loeffler, C.; Nöckler, K.; Rosner, B.; Spielmeyer, A.; Szabo, I.; Richter, M.H. Impact of climate change on foodborne infections and intoxications. J. Health Monit. 2023, 8 (Suppl. 3), 78. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.A. Parasite zoonoses and wildlife: One health, spillover and human activity. Int. J. Parasitol. 2013, 43, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.; Simard, M.; Kutz, S.J.; Kapel, C.M.; Hamnes, I.S.; Robertson, L.J. Arctic parasitology: Why should we care? Trends Parasitol. 2011, 27, 239–245. [Google Scholar] [CrossRef]
- Robertson, L.J.; Debenham, J.J. Cryptosporidiosis and Giardiosis in the Arctic: Increasing Threats in a Warmer World? In Arctic One Health: Challenges for Northern Animals and People; Springer International Publishing: Cham, Switzerland, 2022; pp. 339–362. [Google Scholar] [CrossRef]
- Tape, K.D.; Clark, J.A.; Jones, B.M.; Kantner, S.; Gaglioti, B.V.; Grosse, G.; Nitze, I. Expanding beaver pond distribution in Arctic Alaska, 1949 to 2019. Sci. Rep. 2022, 12, 7123. [Google Scholar] [CrossRef]
- Britton, E.; Hales, S.; Venugopal, K.; Baker, M.G. The impact of climate variability and change on cryptosporidiosis and giardiasis rates in New Zealand. J. Water Health 2010, 8, 561–571. [Google Scholar] [CrossRef]
- Lal, A.; Baker, M.G.; Hales, S.; French, N.P. Potential effects of global environmental changes on cryptosporidiosis and giardiasis transmission. Trends Parasitol. 2013, 29, 83–90. [Google Scholar] [CrossRef]
- Jenkins, E.J.; Castrodale, L.J.; de Rosemond, S.J.; Dixon, B.R.; Elmore, S.A.; Gesy, K.M.; Hoberg, E.P.; Polley, L.; Schurer, J.M.; Simard, M.; et al. Tradition and transition: Parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv. Parasitol. 2013, 82, 33–204. [Google Scholar] [CrossRef]
- Talazadeh, F.; Razijalali, M.H.; Roshanzadeh, N.; Davoodi, P. Survey on the gastrointestinal parasites in Passeriformes and Psittaciformes with a focus on zoonotic parasites: Survey on the gastrointestinal parasites in Passeriformes and Psittaciformes with a focus on zoonotic parasites. J. Hell. Vet. Med. Soc. 2023, 74, 6237–6245. [Google Scholar] [CrossRef]
- McRoberts, K.M.; Meloni, B.P.; Morgan, U.M.; Marano, R.; Binz, N.; Erlandsen, S.L.; Halse, S.A.; Thompson, R.C.A. Morphological and Molecular Characterization of Giardia Isolated from the Straw-Necked Ibis (Threskiornis spinicollis) in Western Australia. J. Parasitol. 1996, 82, 711. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Zapata, A.M.; Romero-Salas, D.; Chaparro-Gutiérrez, J.J.; González-Hernández, M.; Ojeda-Chi, M.; Serrano-Solís, A. Frequency of Giardia spp. and Cryptosporidium spp. in domestic and captive wild animals in the north of Veracruz, Mexico. Pak. Vet. J. 2023, 43, 814–818. [Google Scholar] [CrossRef]
- Kuhn, R.C.; Rock, C.M.; Oshima, K.H. Occurrence of Cryptosporidium and Giardia in Wild Ducks along the Rio Grande River Valley in Southern New Mexico. Appl. Env. Microbiol. 2002, 68, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Erlandsen, S.L.; Bemrick, W.J.; Wells, C.L.; Feely, D.E.; Knudson, L.; Campbell, S.R.; van Keulen, H.; Jarroll, E.L. Axenic culture and characterization of Giardia ardeae from the great blue heron (Ardea herodias). J. Parasitol. 1990, 76, 717–724. [Google Scholar] [CrossRef]
- Oates, S.C.; Miller, M.A.; Hardin, D.; Conrad, P.A.; Melli, A.; Jessup, D.A.; Dominik, C.; Roug, A.; Tinker, M.T.; Miller, W.A. Prevalence, environmental loading, and molecular characterization of Cryptosporidium and Giardia isolates from domestic and wild animals along the Central California Coast. Appl. Environ. Microbiol. 2012, 78, 8762–8772. [Google Scholar] [CrossRef]
- Sandoval-Rodríguez, A.; Marcone, D.; Alegría-Morán, R.; Larraechea, M.; Yévenes, K.; Fredes, F.; Briceño, C. Cryptosporidium spp. and Giardia spp. in Free-Ranging Introduced Monk Parakeets from Santiago, Chile. Animals 2021, 11, 801. [Google Scholar] [CrossRef]
- Plutzer, J.; Tomor, B. The Role of Aquatic Birds in the Environmental Dissemination of Human Pathogenic Giardia duodenalis Cysts and Cryptosporidium Oocysts in Hungary. Parasitol. Int. 2009, 58, 227–231. [Google Scholar] [CrossRef]
- Franssen, F.F.J.; Hooimeijer, J.; Blankenstein, B.; Houwers, D.J. Giardiasis in a white stork in The Netherlands. J. Wildl. Dis. 2000, 36, 764–766. [Google Scholar] [CrossRef][Green Version]
- Majewska, A.C.; Graczyk, T.K.; Słodkowicz-Kowalska, A.; Tamang, L.; Jędrzejewski, S.; Zduniak, P.; Solarczyk, P.; Nowosad, A.; Nowosad, P. The Role of Free-Ranging, Captive, and Domestic Birds of Western Poland in Environmental Contamination with Cryptosporidium parvum Oocysts and Giardia lamblia Cysts. Parasitol. Res. 2009, 104, 1093–1099. [Google Scholar] [CrossRef]
- Shemshadi, B.; Ranjbar-Bahadori, S.; Faghihzadeh-Gorgi, S. Occurrence of parasitic protozoa in wild waterfowl in southern coastal Caspian Sea lagoons. Iran. J. Vet. Med. 2014, 8, 261–267. [Google Scholar]
- Papini, R.; Girivetto, M.; Marangi, M.; Mancianti, F.; Giangaspero, A. Endoparasite Infections in Pet and Zoo Birds in Italy. Sci. World J. 2012, 2012, 253127. [Google Scholar] [CrossRef]
- Abe, N.; Makino, I.; Kojima, A. Molecular Characterization of Giardia psittaci by Multilocus Sequence Analysis. Infect. Genet. Evol. 2012, 12, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Boehm, A.B.; Soller, J.A. Refined Ambient Water Quality Thresholds for Human-Associated Fecal Indicator HF183 for Recreational Waters with and without Co-Occurring Gull Fecal Contamination. Microb. Risk Anal. 2020, 16, 100139. [Google Scholar] [CrossRef]
- Appelbee, A.J.; Frederick, L.M.; Heitman, T.L.; Olson, M.E. Prevalence and Genotyping of Giardia duodenalis from Beef Calves in Alberta, Canada. Vet. Parasitol. 2003, 112, 289–294. [Google Scholar] [CrossRef]
- Li, J.; Qin, H.; Li, X.; Zhang, L. Role of Rodents in the Zoonotic Transmission of Giardiasis. One Health 2023, 16, 100500. [Google Scholar] [CrossRef]
- Durrani, A.Z.; Saleem, M.H.; Ahmad, H.A.; Ali, M.H.; Chaudhary, M.; Usman, M. Bacterial and Parasitic Profiling of Native Pigeons in District Lahore-Pakistan. In Proceedings of the 2020 17th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 14–18 January 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 191–194. [Google Scholar]
- Dong, H.; Cheng, R.; Li, X.; Li, J.; Chen, Y.; Ban, C.; Zhang, X.; Liu, F.; Zhang, L. Molecular Identification of Cryptosporidium spp., Enterocytozoon bieneusi, and Giardia duodenalis in Captive Pet Birds in Henan Province, Central China. J. Eukaryot. Microbiol. 2021, 68, e12839. [Google Scholar] [CrossRef]
- Zahedi, A.; Field, D.; Ryan, U. Molecular Typing of Giardia duodenalis in Humans in Queensland—First Report of Assemblage E. Parasitology 2017, 144, 1154–1161. [Google Scholar] [CrossRef]
- Egan, S.; Barbosa, A.D.; Feng, Y.; Xiao, L.; Ryan, U. The Risk of Wild Birds Contaminating Source Water with Zoonotic Cryptosporidium and Giardia Is Probably Overestimated. Sci. Total Environ. 2024, 912, 169032. [Google Scholar] [CrossRef]
- Sarria-Guzmán, Y.; Chávez-Romero, Y.; Bernal, J.E.; González-Jiménez, F.E.; Serrano-Silva, N.; Fusaro, C. Molecular Identification of Giardia spp. in Latin America: An Updated Systematic Review on Reports from 2017 to 2021. J. Infect. Dev. Ctries. 2022, 16, 392–401. [Google Scholar] [CrossRef]
- Bomfim, T.C.B.; Gomes, R.S.; Huber, F.; Couto, M.C.M. The importance of poultry in environmental dissemination of Cryptosporidium spp. Open Vet. Sci. J. 2013, 7, 12–17. [Google Scholar] [CrossRef][Green Version]
- Qi, M.; Wang, R.; Ning, C.; Li, X.; Zhang, L.; Jian, F.; Sun, Y.; Xiao, L. Cryptosporidium spp. in pet birds: Genetic diversity and potential public health significance. Exp. Parasitol. 2011, 128, 336–340. [Google Scholar] [CrossRef]
- Wang, R.; Wang, F.; Zhao, J.; Qi, M.; Ning, C.; Zhang, L.; Xiao, L. Cryptosporidium spp. in quails (Coturnix coturnix japonica) in Henan, China: Molecular characterization and public health significance. Vet. Parasitol. 2012, 187, 534–537. [Google Scholar] [CrossRef]
- Nakamura, A.A.; Simões, D.; Antunes, R.; Silva, D.C.D.; Meireles, M. Molecular characterization of Cryptosporidium spp. from fecal samples of birds kept in captivity in Brazil. Vet. Parasitol. 2009, 166, 47–51. [Google Scholar] [CrossRef]
- Antunes, R.; Simões, D.; Nakamura, A.A.; Meireles, M. Natural Infection with Cryptosporidium galli in Canaries (Serinus canaria), in a Cockatiel (Nymphicus hollandicus), and in Lesser Seed-Finches (Oryzoborus angolensis) from Brazil. Avian Dis. 2008, 52, 702–705. [Google Scholar] [CrossRef]
- Nakamura, A.A.; Homem, C.G.; Silva, A.J.D.D.; Meireles, M. Diagnosis of gastric cryptosporidiosis in birds using a duplex real-time PCR assay. Vet. Parasitol. 2014, 205, 7–13. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Zhang, L.; Qi, N.; Liao, S.; Lv, M.; Wu, C.; Sun, M. Molecular characterization of Cryptosporidium spp. in domestic pigeons (Columba livia domestica) in Guangdong Province, Southern China. Parasitol. Res. 2015, 114, 2237–2241. [Google Scholar] [CrossRef]
- Nakamura, A.A.; Meireles, M. Cryptosporidium infections in birds-a review Infecção por Cryptosporidium em aves-uma revisão. Rev. Bras. Parasitol. Vet. 2015, 24, 253–267. [Google Scholar] [CrossRef]
- Santana, B.N.; Kurahara, B.; Nakamura, A.A.; Camargo, V.D.S.; Ferrari, E.D.; Silva, G.S.D.; Nagata, W.; Meireles, M. Detection and characterization of Cryptosporidium species and genotypes in three chicken production systems in Brazil using different molecular diagnosis protocols. Prev. Vet. Med. 2018, 151, 73–78. [Google Scholar] [CrossRef]
- Sevá, A.P.; Funada, M.R.; Richtzenhain, L.; Guimarães, M.B.; Souza, S.; Allegretti, L.; Sinhorini, J.; Duarte, V.V.; Soares, R. Genotyping of Cryptosporidium spp. from free-living wild birds from Brazil. Vet. Parasitol. 2011, 175, 27–32. [Google Scholar] [CrossRef]
- Baroudi, D.; Khelef, D.; Goucem, R.; Adjou, K.; Adamu, H.; Zhang, H.W.; Xiao, L. Common occurrence of zoonotic pathogen Cryptosporidium meleagridis in broiler chickens and turkeys in Algeria. Vet. Parasitol. 2013, 196, 334–340. [Google Scholar] [CrossRef]
- Ryan, U. Cryptosporidium in birds, fish and amphibians. Exp. Parasitol. 2010, 124, 113–120. [Google Scholar] [CrossRef]
- Cunha, M.J.D.D.; Cury, M.; Santín, M. Widespread presence of human-pathogenic Enterocytozoon bieneusi genotypes in chickens. Vet. Parasitol. 2016, 217, 108–112. [Google Scholar] [CrossRef]
- Makino, I.; Abe, N.; Reavill, D. Cryptosporidium Avian Genotype III as a Possible Causative Agent of Chronic Vomiting in Peach-Faced Lovebirds (Agapornis roseicollis). Avian Dis. 2010, 54, 1102–1107. [Google Scholar] [CrossRef]
- Abe, N.; Makino, I. Multilocus genotypic analysis of Cryptosporidium isolates from cockatiels, Japan. Parasitol. Res. 2010, 106, 1491–1497. [Google Scholar] [CrossRef]
- Ryan, U.; Xiao, L.; Read, C.; Sulaiman, I.; Monis, P.; Lal, A.; Fayer, R.; Pavlásek, I. A Redescription of Cryptosporidium galli pavlasek, 1999 (apicomplexa: Cryptosporidiidae) from birds. J. Parasitol. 2003, 89, 809–813. [Google Scholar] [CrossRef]
- Holubová, N.; Sak, B.; Hořčičková, M.; Hlásková, L.; Květoňová, D.; Menchaca, S.; Mcevoy, J.; Kváč, M. Cryptosporidium avium n. sp. (Apicomplexa: Cryptosporidiidae) in birds. Parasitol. Res. 2016, 115, 2243–2251. [Google Scholar] [CrossRef]
- Graczyk, T.; Majewska, A.; Schwab, K. The role of birds in dissemination of human waterborne enteropathogens. Trends Parasitol. 2008, 24, 55–59. [Google Scholar] [CrossRef]
- Wang, L.; Xue, X.; Li, J.Q.; Zhou, Q.J.; Yu, Y.; Du, A. Cryptosporidiosis in broiler chickens in Zhejiang Province, China: Molecular characterization of oocysts detected in fecal samples. Parasite 2014, 21, 36. [Google Scholar] [CrossRef]
- Wang, R.; Jian, F.; Sun, Y.; Hu, Q.; Zhu, J.; Wang, F.; Ning, C.; Zhang, L.; Xiao, L. Large-scale survey of Cryptosporidium spp. in chickens and Pekin ducks (Anas platyrhynchos) in Henan, China: Prevalence and molecular characterization. Avian Pathol. 2010, 39, 447–451. [Google Scholar] [CrossRef]
- Chelladurai, J.J.; Clark, M.; Kváč, M.; Holubová, N.; Khan, E.; Stenger, B.L.S.; Giddings, C.; Mcevoy, J. Cryptosporidium galli and novel Cryptosporidium avian genotype VI in North American red-winged blackbirds (Agelaius phoeniceus). Parasitol. Res. 2016, 115, 1901–1906. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, N.; Zhao, G.H.; Zhao, Q.; Zhu, X.Q. Prevalence and Genotyping of Cryptosporidium Infection in Pet Parrots in North China. BioMed Res. Int. 2015, 2015, 549798. [Google Scholar] [CrossRef]
- Silva, D.C. Avaliação fÍsica, Epidemiológica e Molecular da Infecção por Cryptosporidium spp. em passeriformes. Master’s Thesis, Universidade Estadual Paulista, Sao Paulo, Brazil, 2009. [Google Scholar]
- El-Ghany, W.A.A. Avian Cryptosporidiosis: A significant parasitic disease of public health hazard. Slov. Vet. Res. 2022, 59, 5. [Google Scholar] [CrossRef]
- Meireles, M.; Soares, R.; Santos, M.M.A.B.D.; Gennari, S. Biological studies and molecular characterization of a Cryptosporidium isolate from ostriches (Struthio camelus). J. Parasitol. 2006, 92, 623–626. [Google Scholar] [CrossRef]
- Wang, K.; Gazizova, A.; Wang, Y.; Zhang, K.; Zhang, Y.; Chang, Y.; Cui, Y.; Zhang, Y.; Zhang, S.M.; Zhang, L. First Detection of Cryptosporidium spp. in Migratory Whooper Swans (Cygnus cygnus) in China. Microorganisms 2019, 8, 6. [Google Scholar] [CrossRef]
- Paulo, S. Determinação da Ocorrência de Cryptosporidium galli em Amostras Fecais de Aves por meio da PCR em Tempo Real. Doctoral Dissertation, Universidade de São Paulo, São Paulo, Brazil, 2013. [Google Scholar]
- Laatamna, A.E.; Holubová, N.; Sak, B.; Kváč, M. Cryptosporidium meleagridis and C. baileyi (Apicomplexa) in domestic and wild birds in Algeria. Folia Parasitol. 2017, 64, 018. [Google Scholar] [CrossRef]
- Silva, D.C.; Homem, C.G.; Nakamura, A.A.; Teixeira, W.; Perri, S.; Meireles, M. Physical, epidemiological, and molecular evaluation of infection by Cryptosporidium galli in Passeriformes. Parasitol. Res. 2010, 107, 271–277. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Krücken, J.; Abdelwhab, E.S.M.; Samson-Himmelstjerna, G.V.; Hafez, H. Molecular diagnosis and characterization of Cryptosporidium spp. in turkeys and chickens in Germany reveals evidence for previously undetected parasite species. PLoS ONE 2017, 12, e0177150. [Google Scholar] [CrossRef]
- Ng, J.; Pavlásek, I.; Ryan, U. Identification of Novel Cryptosporidium Genotypes from Avian Hosts. Appl. Environ. Microbiol. 2006, 72, 7548–7553. [Google Scholar] [CrossRef]
- Máca, O.; Pavlásek, I. First finding of spontaneous infections with Cryptosporidium baileyi and C. meleagridis in the red-legged partridge Alectoris rufa from an aviary in the Czech Republic. Vet. Parasitol. 2015, 209, 164–168. [Google Scholar] [CrossRef]
- Gomes, R.S.; Huber, F.; Silva, S.; Bomfim, T.C.B. Cryptosporidium spp. parasitize exotic birds that are commercialized in markets, commercial aviaries, and pet shops. Parasitol. Res. 2011, 110, 1363–1370. [Google Scholar] [CrossRef]
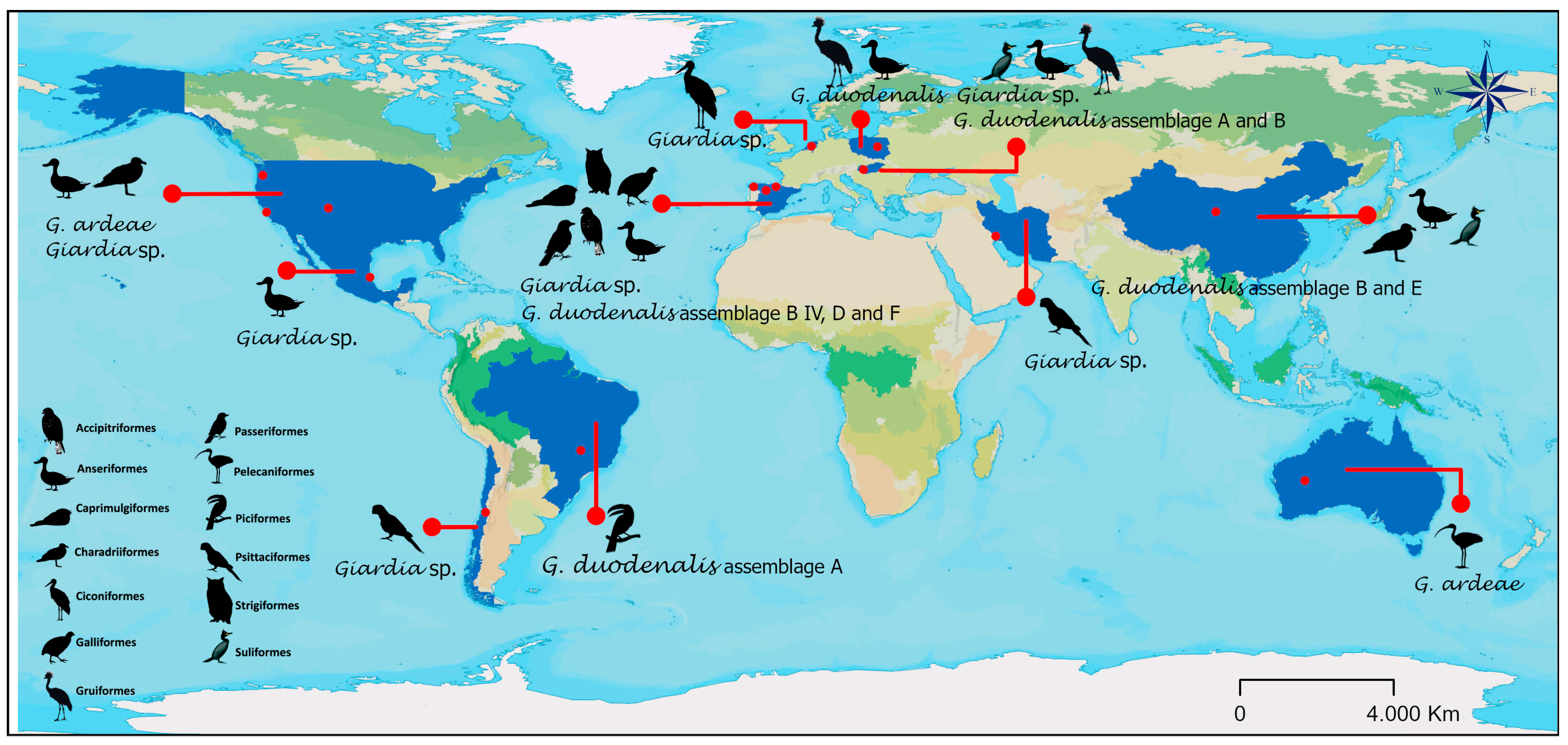
| Continent | Country | Order | Bird Species | Common Name | Giardia Assemblage | Year | Ref. | Frequency (%) | Prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Asia | China | Anseriformes | Anser indicus | Bar-headed goose | G. duodenalis assemblages B and E | 2021 | [12] | 3.39 (23/679) * | 4.4% |
| China | Charadriiformes | Chroicocephalus brunnicephalus | Brown-headed Gull | G. duodenalis assemblages B and E | 2021 | [12] | 3.39 (23/679) * | ||
| China | Charadriiformes | Larus ichthyaetus | Great black-headed gull | G. duodenalis assemblages B and E | 2021 | [12] | 3.39 (23/679) * | ||
| China | Suliformes | Phalacrocorax carbo | Great Cormorant | G. duodenalis assemblages B and E | 2021 | [12] | 3.39 (23/679) * | ||
| Iran | Psittaciformes | Melopsittacus undulatus | Common parakeet | Giardia sp. | 2023 | [41] | 21.73 (5/23) | ||
| Iran | Psittaciformes | Nymphicus hollandicus | Cockatiel | Giardia sp. | 2023 | [41] | 10 (3/30) | ||
| Oceania | Australia | Pelecaniformes | Threskiornis spinicollis | Straw-necked ibis | G. ardeae | 1996 | [42] | 70 (44/63) | 70% |
| North America | Mexico | Anseriformes | Anser anser | Greylag goose | Giardia spp. | 2023 | [43] | 100 (2/2) | 10% |
| USA | Anseriformes | Ana america | American wigeon | Giardia sp. | 2002 | [44] | 66.7 (2/3) | ||
| USA | Anseriformes | Anas acuta | Northern pintail | Giardia sp. | 2002 | [44] | 100 (1/1) | ||
| USA | Anseriformes | Anas discors | Blue-winged teal | Giardia sp. | 2002 | [44] | 25 (1/4) | ||
| USA | Anseriformes | Anas platyrhynchos | Mallard | Giardia sp. | 2002 | [44] | 25.5 (13/51) | ||
| USA | Anseriformes | Ardea herodias | Great blue heron | G. ardeae | 1990 | [45] | 12.5 (1/8) | ||
| USA | Anseriformes | Mergus merganser | Common merganser | Giardia sp. | 2002 | [44] | 33.3 (1/3) | ||
| USA | Charadriiformes | Larus spp. | Gulls | Giardia sp. | 2012 | [46] | 2.1 (1/145) | ||
| South America | Brazil | Piciformes | Ramphastos toco | Giant toucan | G. duodenalis assemblage A | 2017 | [29] | 100 (1/1) | 12.4% |
| Chile | Psittaciformes | Myopsitta monachus | Monk parakeet | Giardia sp. | 2021 | [47] | 12 (25/207) | ||
| Europe | Hungary | Anseriformes | Anas strepera | Gadwall | G. duodenalis assemblage A | 2009 | [48] | 25 (1/4) | 13.7% |
| Hungary | Anseriformes | Anser anser | Greylag goose | G. duodenalis assemblage B | 2009 | [48] | 2 (1/48) | ||
| Hungary | Anseriformes | Anser fabalis | Taiga bean | Giardia sp. | 2009 | [48] | 12.5 (1/8) | ||
| Hungary | Anseriformes | Mareca strepera | The gadwall | G. duodenalis assemblage A | 2009 | [48] | 25 (1/4) | ||
| Hungary | Gruiformes | Fulica atra | Eurasian coot | Giardia sp. | 2009 | [48] | 25 (1/4) | ||
| Hungary | Suliformes | Phalacrocorax carbo | Great Cormorant | Giardia sp. | 2009 | [48] | 100 (1/1) | ||
| Netherlands | Ciconiiformes | Ciconia ciconia | White stork | Giardia sp. | 2000 | [49] | 100 (1/1) | ||
| Poland | Anseriformes | Anas platyrhynchos | Mallard | G. duodenalis | 2009 | [50] | 21.9 (7/32) | ||
| Poland | Anseriformes | Anser anser | Greylag goose | G. duodenalis | 2009 | [50] | 29.4 (10/34) | ||
| Poland | Anseriformes | Cygnus olor | Mute swan | G. duodenalis | 2009 | [50] | 12.5 (4/33) | ||
| Poland | Anseriformes | Mergus merganser | Common merganser | G. duodenalis | 2009 | [50] | 1.4 (1/72) | ||
| Poland | Gruiformes | Balearica pavonina | Black crowned crane | G. duodenalis | 2009 | [50] | 25 (1/4) | ||
| Spain | Accipitriformes | Buteo buteo | Common buzzard | G. duodenalis assemblage B | 2015 | [28] | 1.2 (1/84) | ||
| Spain | Anseriformes | Anas platyrhynchos | Mallard | G. duodenalis Assemblage F | 2015 | [28] | 50 (2/4) | ||
| Spain | Caprimulgiformes | Caprimulgus europaeus | European nightjar | Giardia sp. | 2015 | [28] | 16.7(1/6) | ||
| Spain | Galliformes | Coturnix coturnix | Common quail | G. duodenalis assemblage B | 2015 | [28] | 100 (1/1) | ||
| Spain | No defined | Waterfowl | Not defined | G. duodenalis | 2014 | [51] | 24.2 (71/293) | ||
| Spain | No defined | Waterfowl | Not defined | G. duodenalis assemblage B IV | 2016 | [30] | 8.3 (22/265) | ||
| Spain | Passeriformes | Garrulus glandarius | Eurasian jay | G. duodenalis assemblage D | 2015 | [28] | 100 (1/1) | ||
| Spain | Passeriformes | Pica pica | Eurasian magpie | G. duodenalis assemblage B | 2015 | [28] | 20 (1/5) | ||
| Spain | Strigiformes | Tyto alba | Barn Owl | Giardia sp. | 2015 | [28] | 2 (1/48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverry, D.; Oyarzún-Ruiz, P.; Landaeta-Aqueveque, C. Synthesis of Giardia Species and Genotypes in Wild Birds: A Review. Vet. Sci. 2025, 12, 911. https://doi.org/10.3390/vetsci12090911
Echeverry D, Oyarzún-Ruiz P, Landaeta-Aqueveque C. Synthesis of Giardia Species and Genotypes in Wild Birds: A Review. Veterinary Sciences. 2025; 12(9):911. https://doi.org/10.3390/vetsci12090911
Chicago/Turabian StyleEcheverry, Diana, Pablo Oyarzún-Ruiz, and Carlos Landaeta-Aqueveque. 2025. "Synthesis of Giardia Species and Genotypes in Wild Birds: A Review" Veterinary Sciences 12, no. 9: 911. https://doi.org/10.3390/vetsci12090911
APA StyleEcheverry, D., Oyarzún-Ruiz, P., & Landaeta-Aqueveque, C. (2025). Synthesis of Giardia Species and Genotypes in Wild Birds: A Review. Veterinary Sciences, 12(9), 911. https://doi.org/10.3390/vetsci12090911







