Molecular Epidemiology and Genetic Evolution Analysis of Porcine Reproductive and Respiratory Syndrome Virus in Southern Xinjiang, China, from 2023 to 2025
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
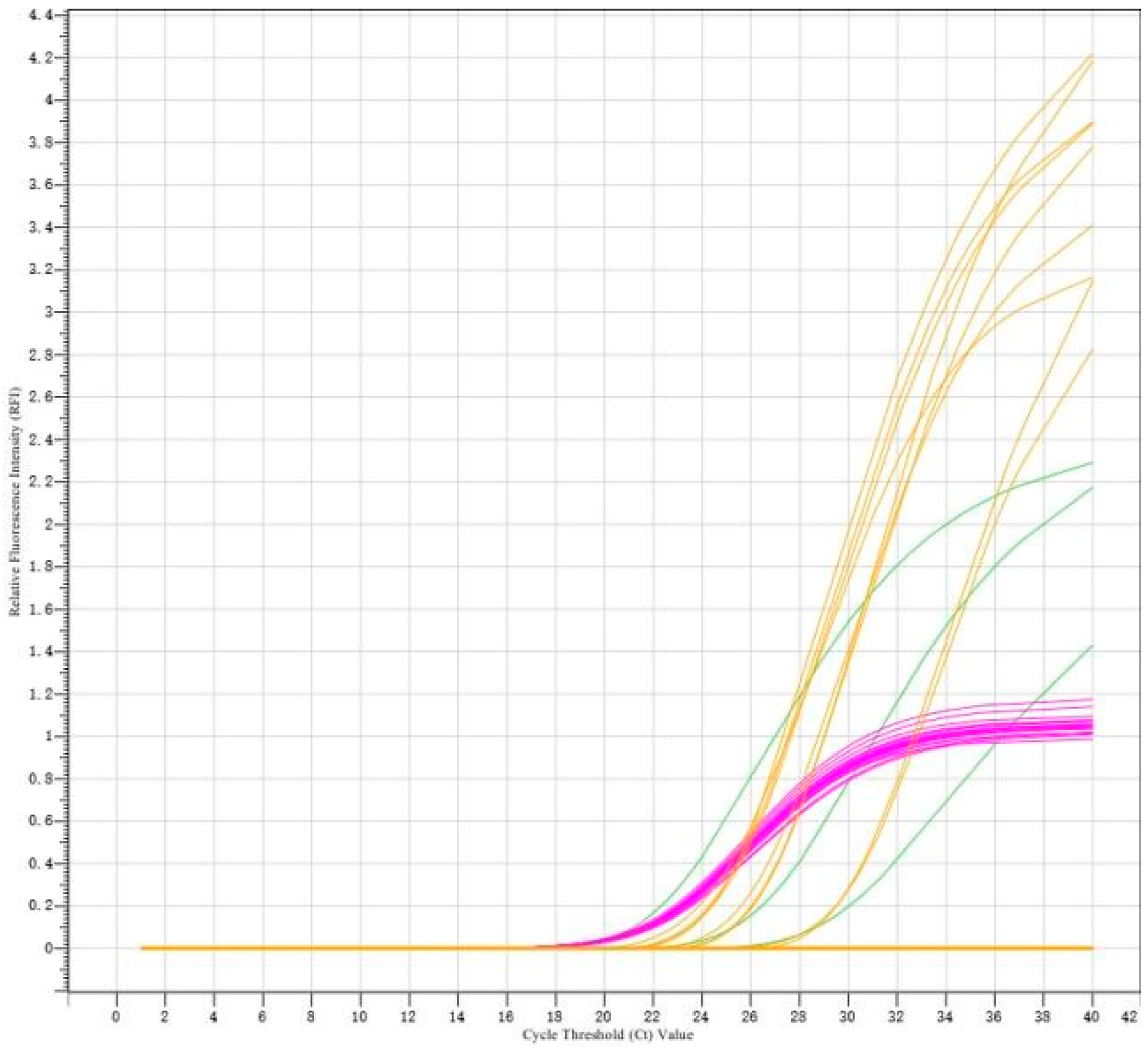
2.2. Fluorescence Quantitative PCR Detection for PRRSV Clinical Samples
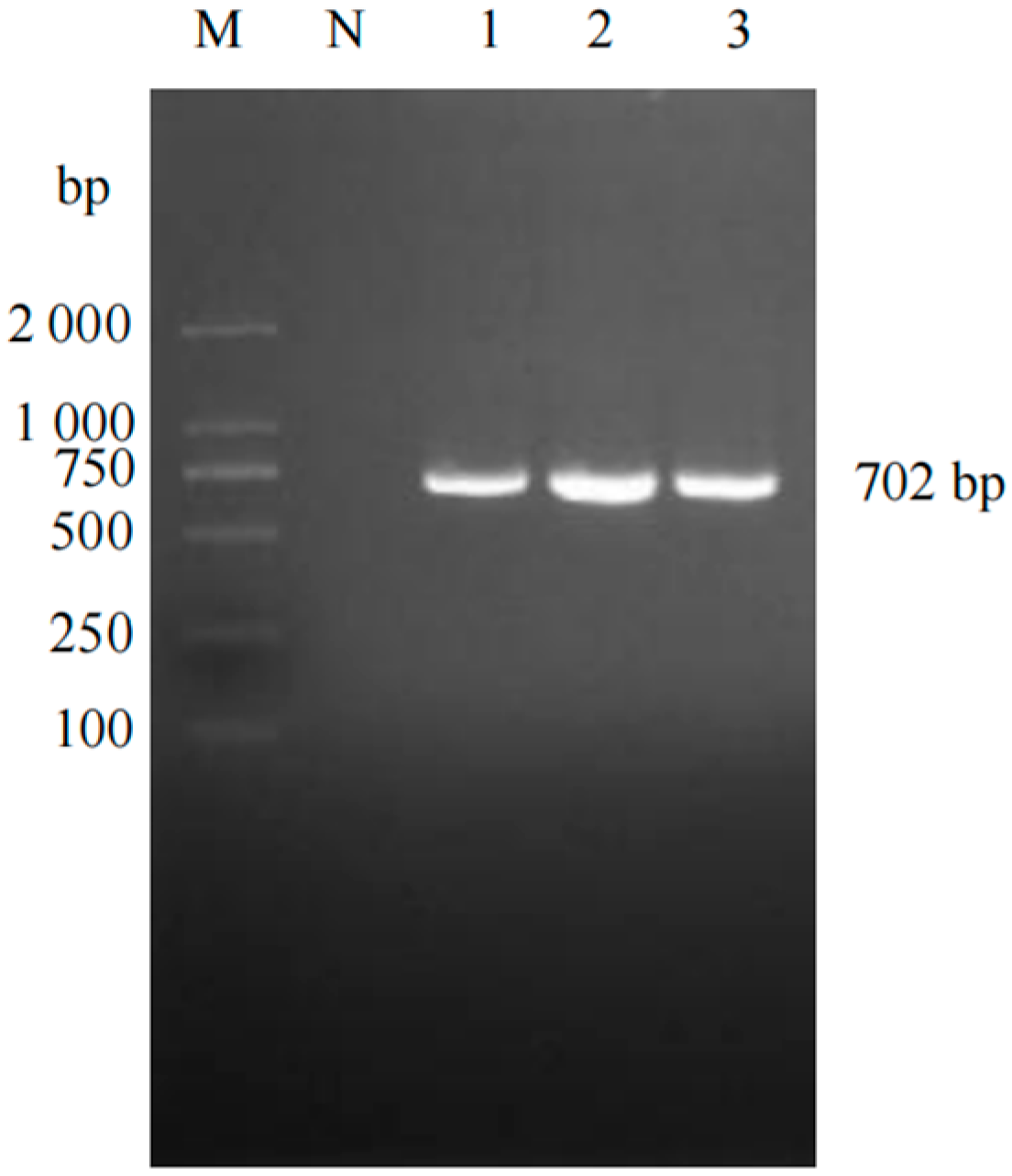
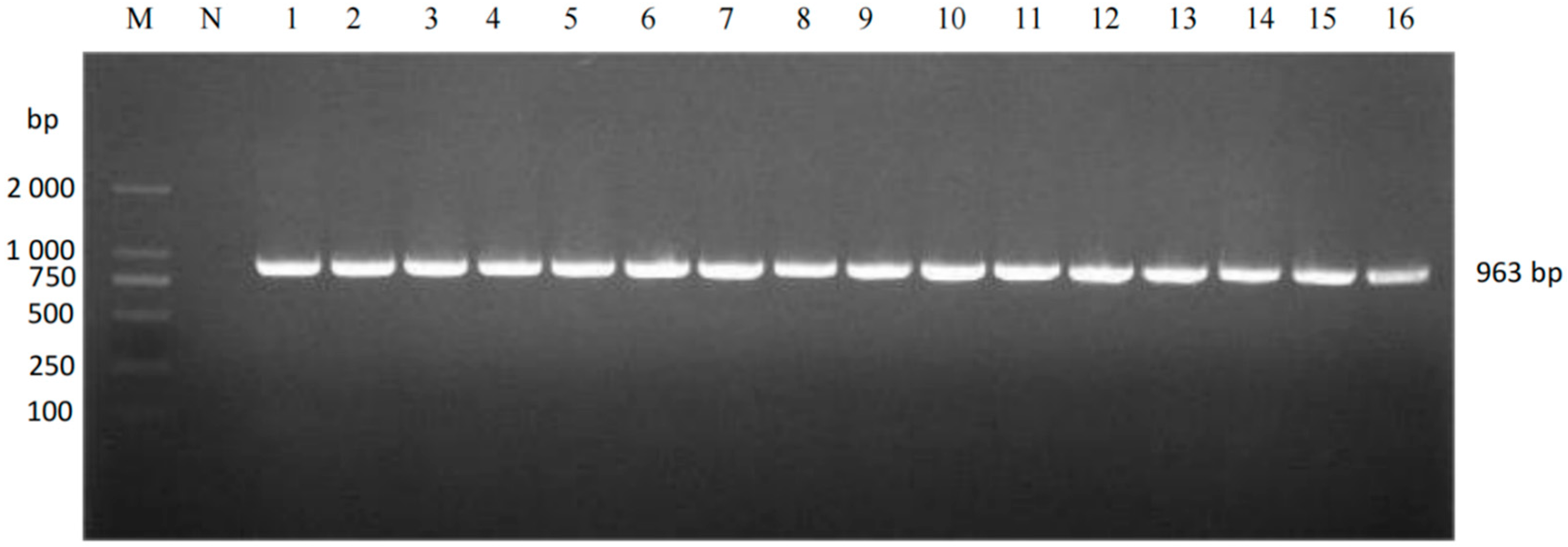
2.3. Amplification and Sequencing of ORF5 Gene from PRRSV-Positive Nucleic Acid
2.4. High-Throughput Sequencing (NGS) of the PRRSV Whole Genome
2.5. Sequence Alignment and Phylogenetic Analysis
2.6. PRRSV Antibody Monitoring
3. Results
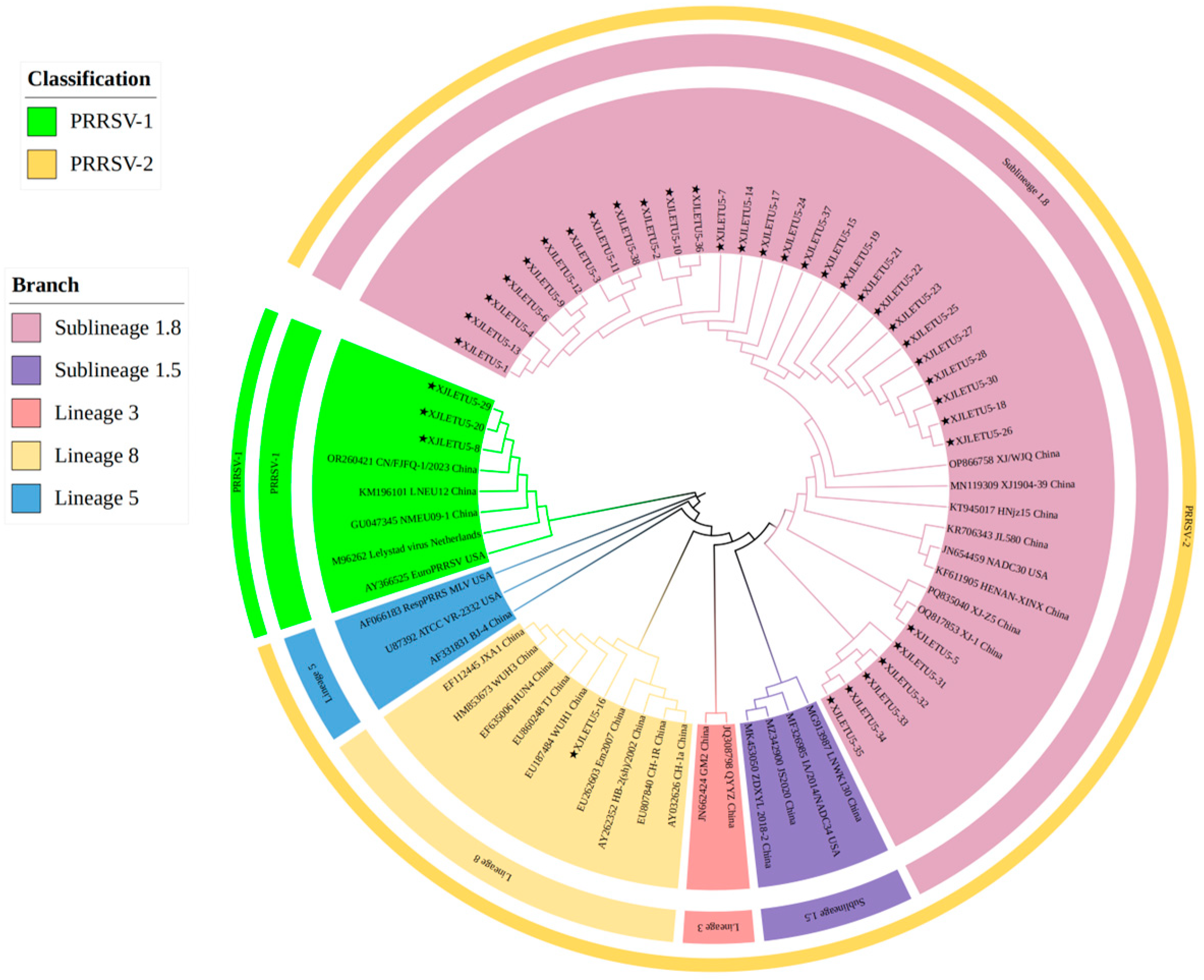
3.1. Virus Detection and Genetic Evolutionary Analysis of ORF5 Gene
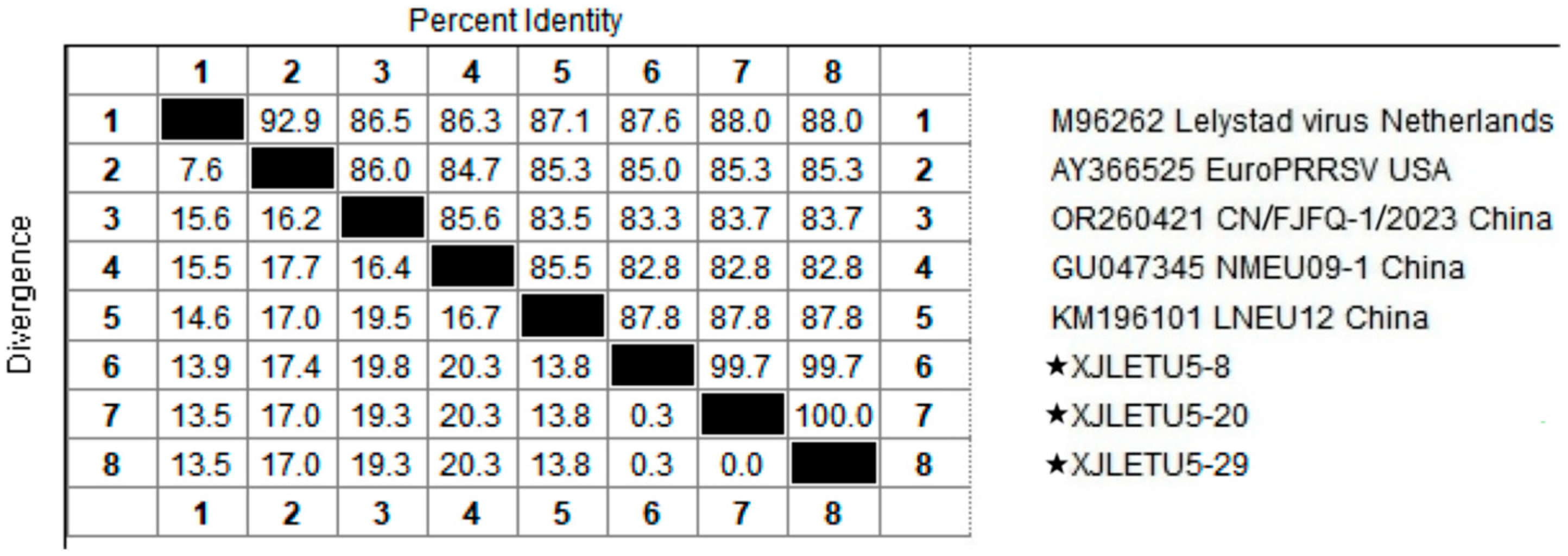
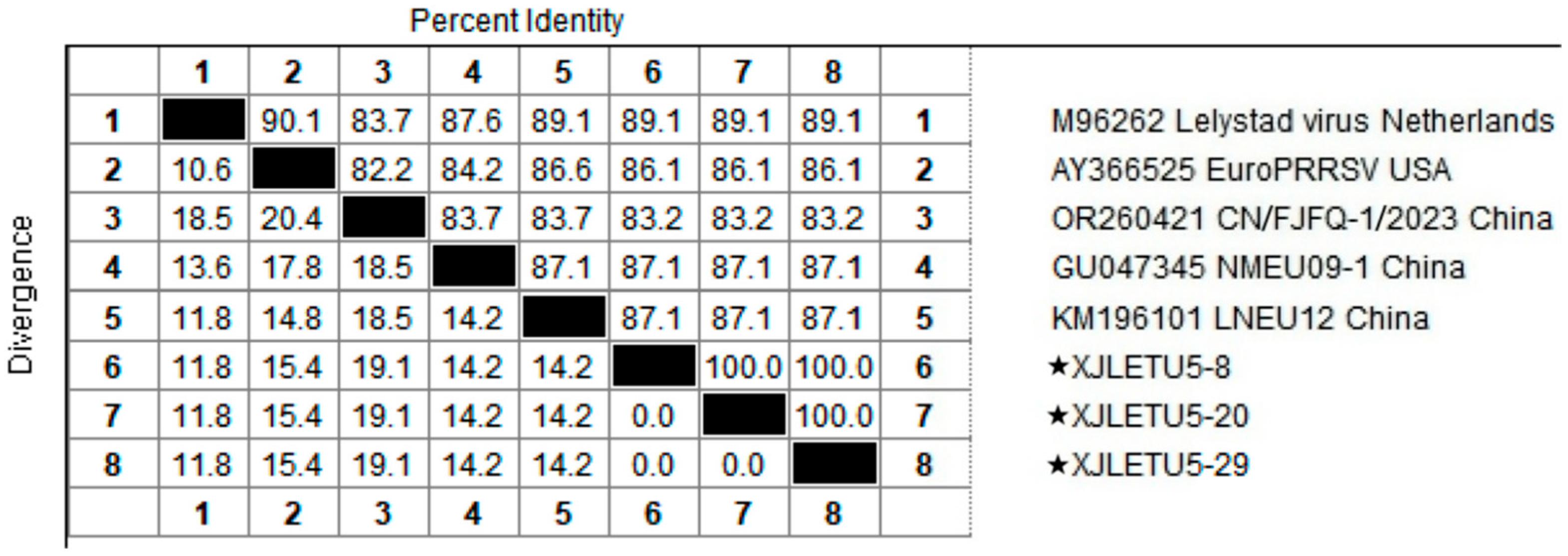
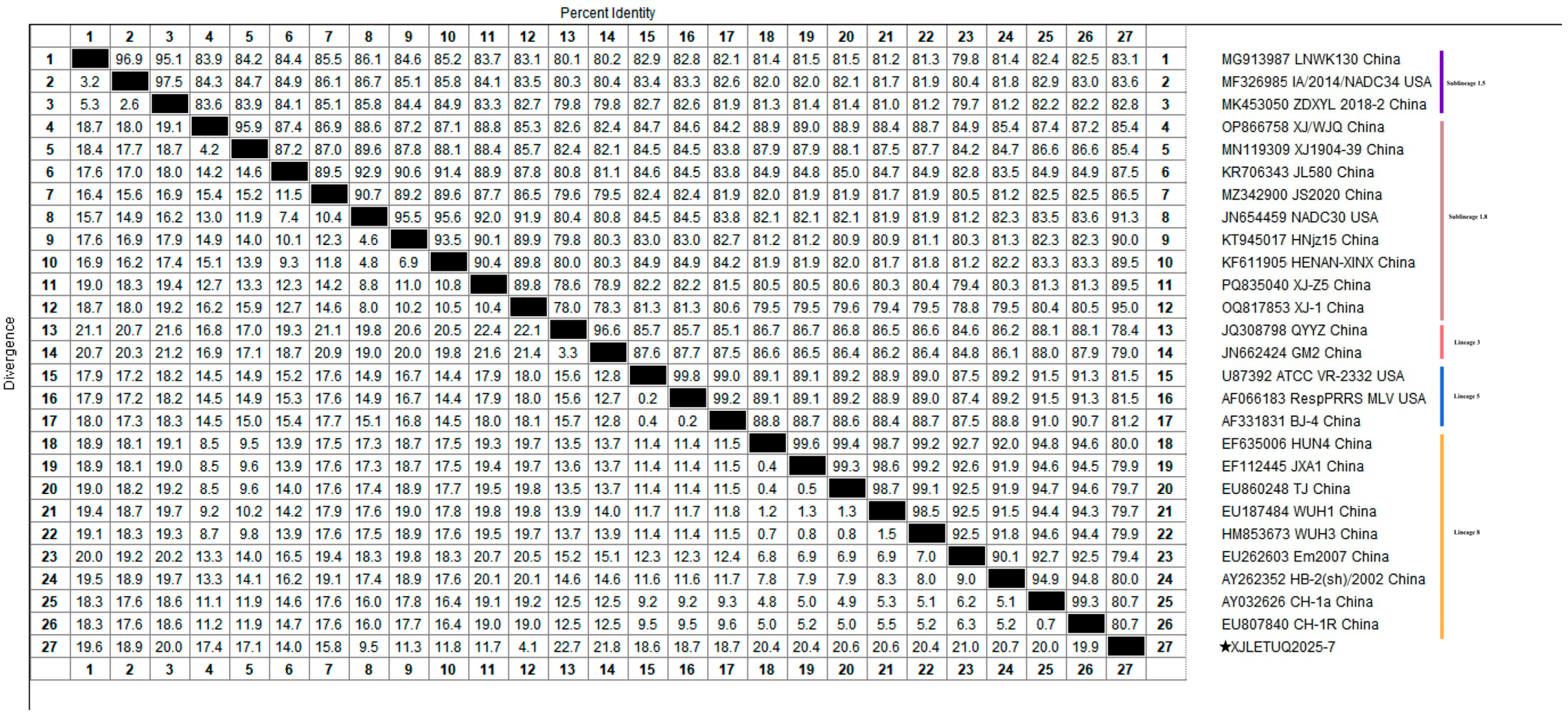
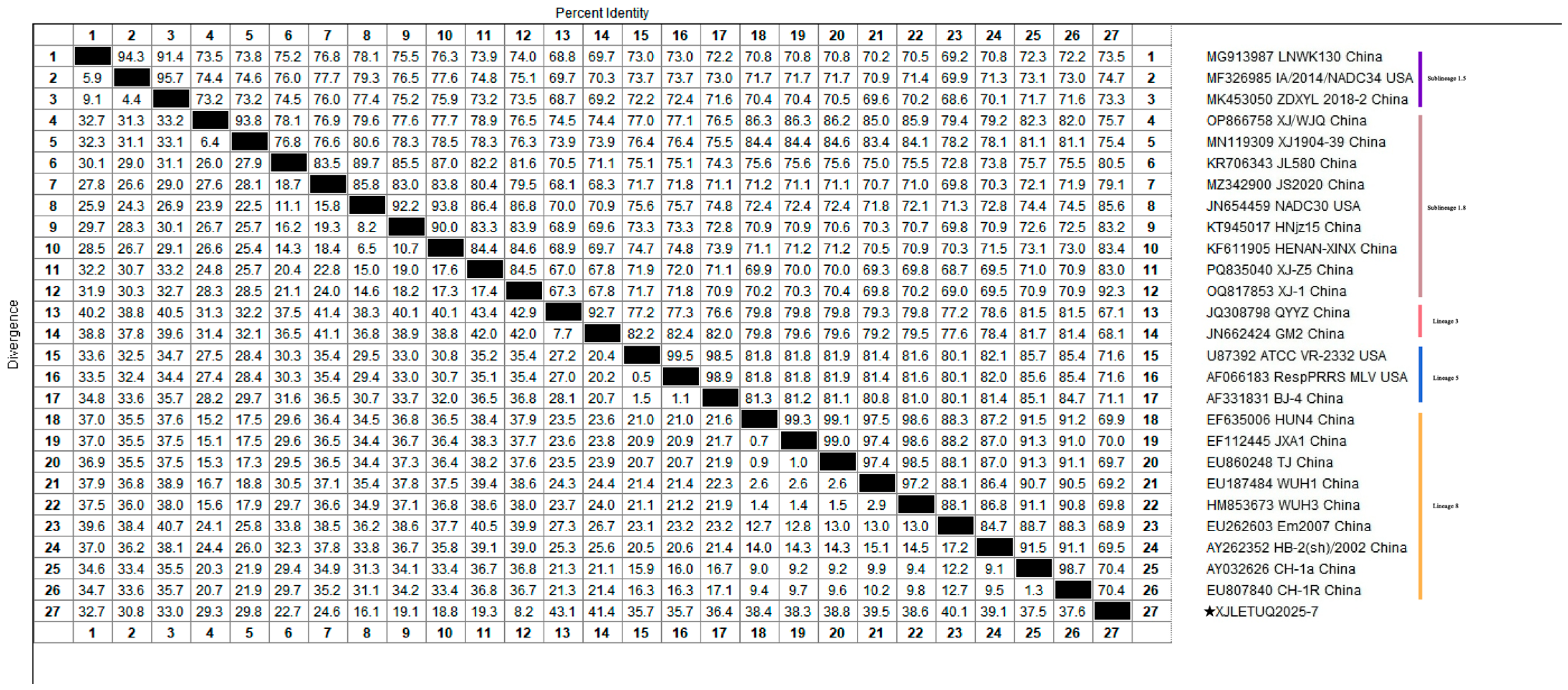
3.2. Homology Analysis of PRRSV ORF5 Gene
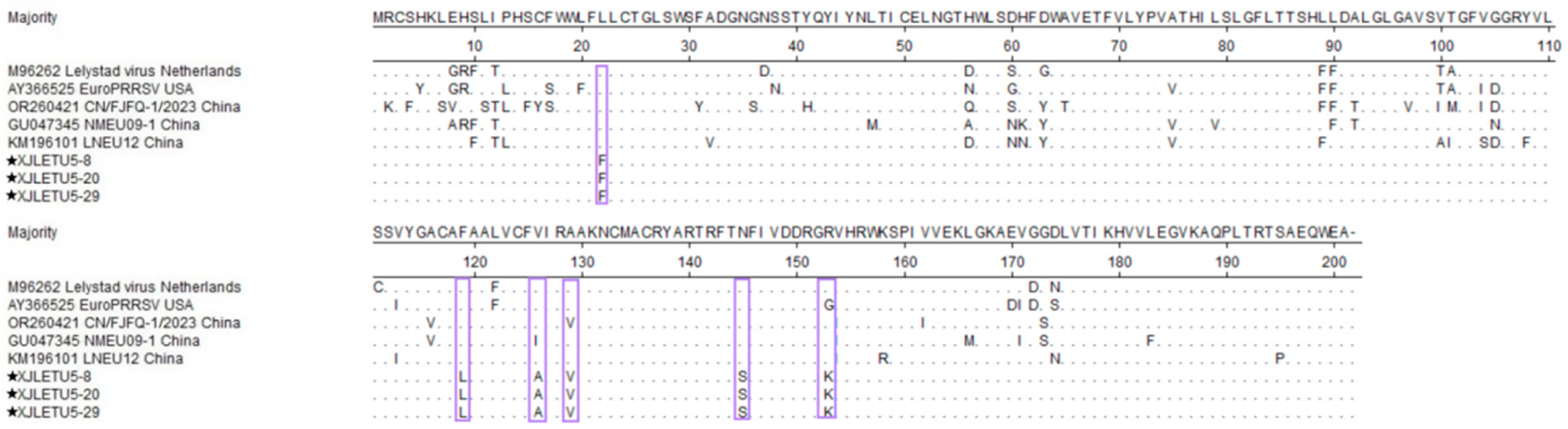
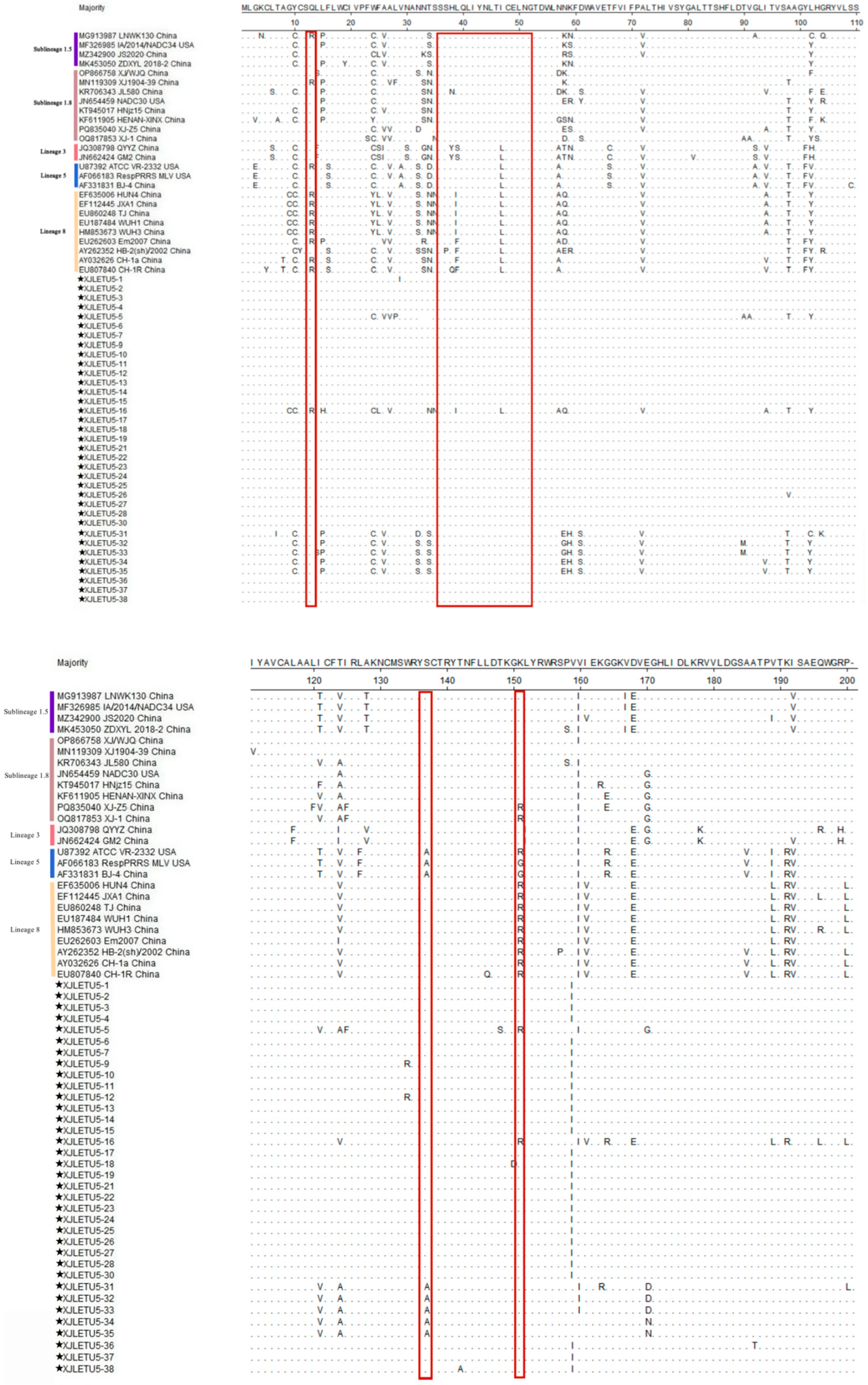
3.3. Mutation Analysis of ORF5 Gene-Encoded Protein
3.4. High-Throughput Sequencing (NGS) and Whole-Genome Sequence Analysis
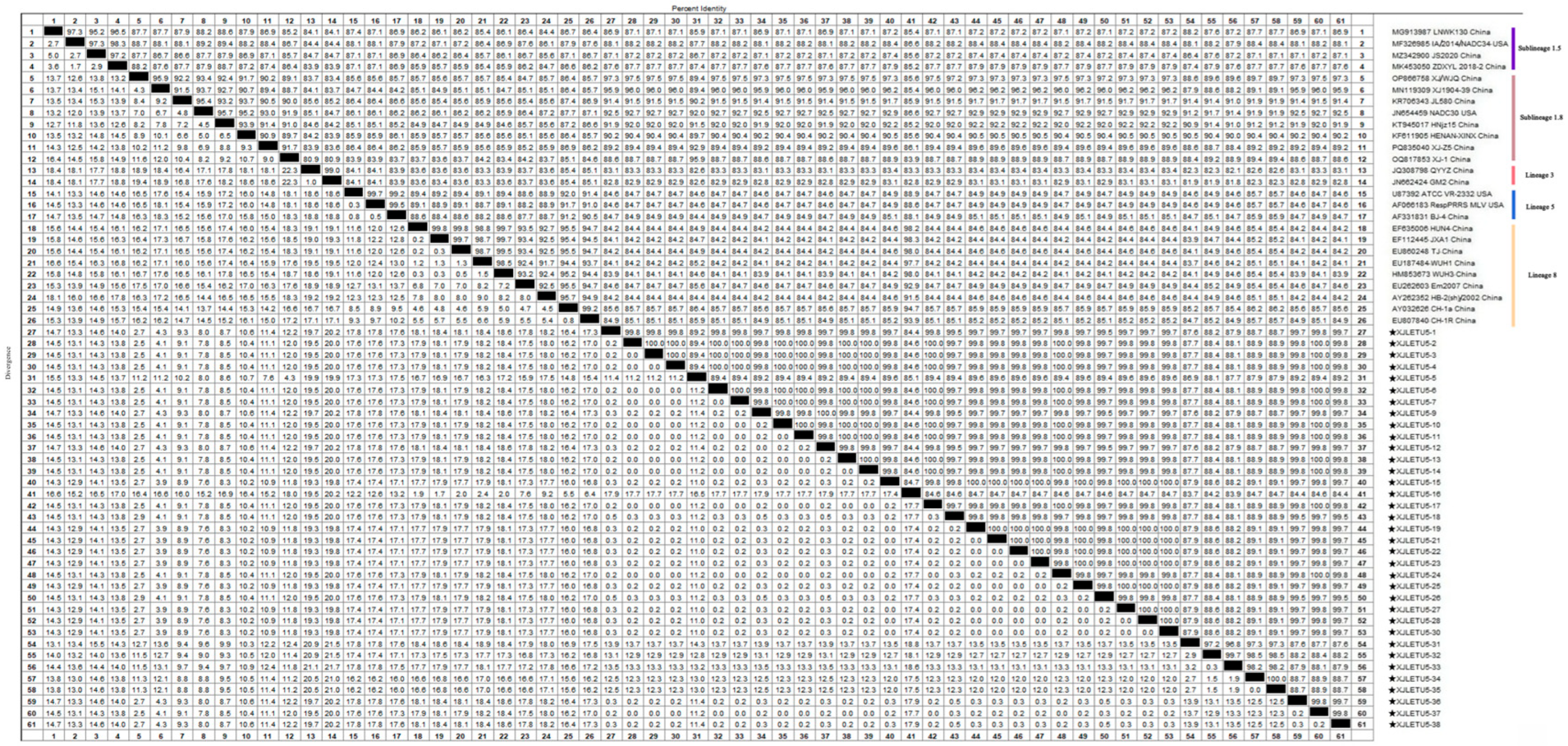
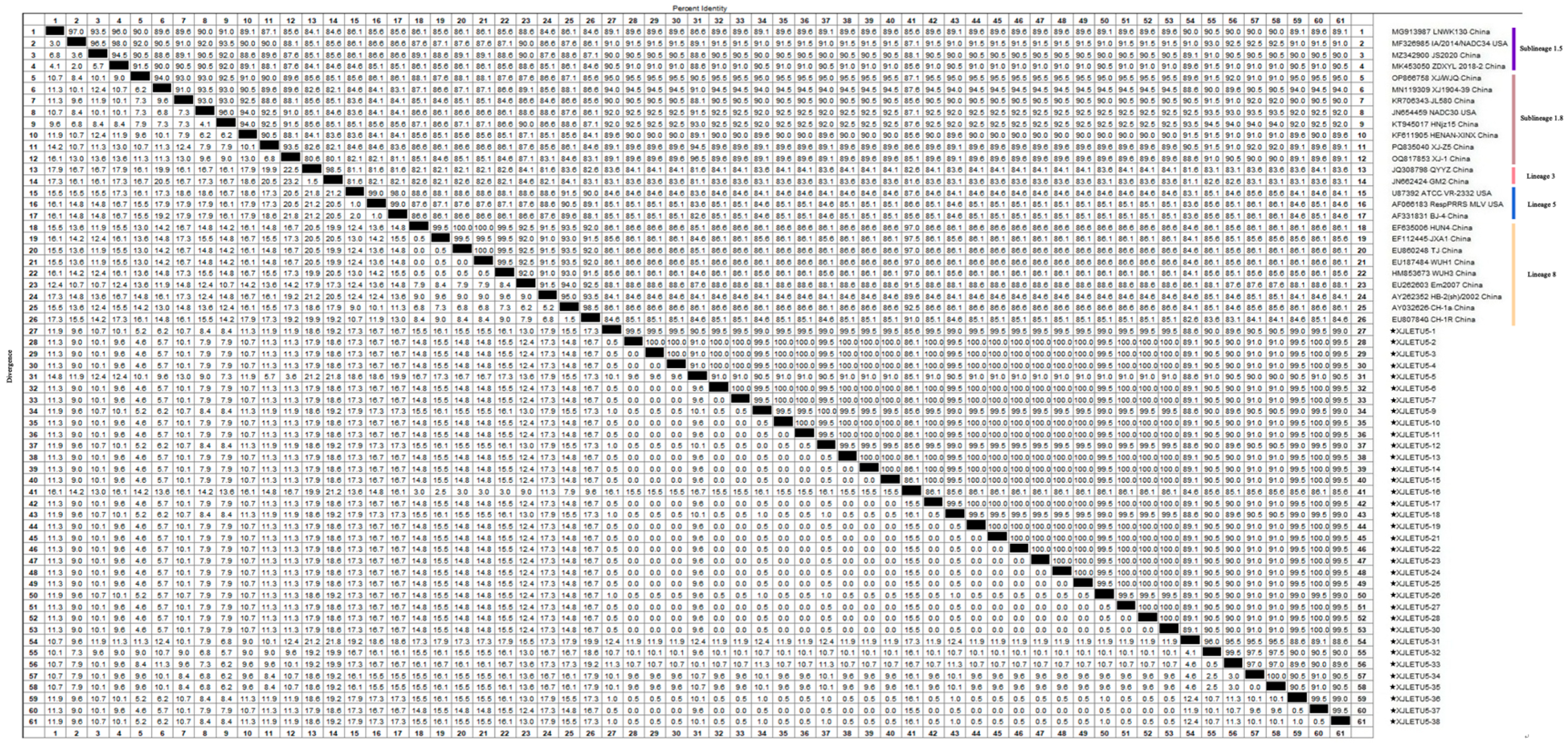
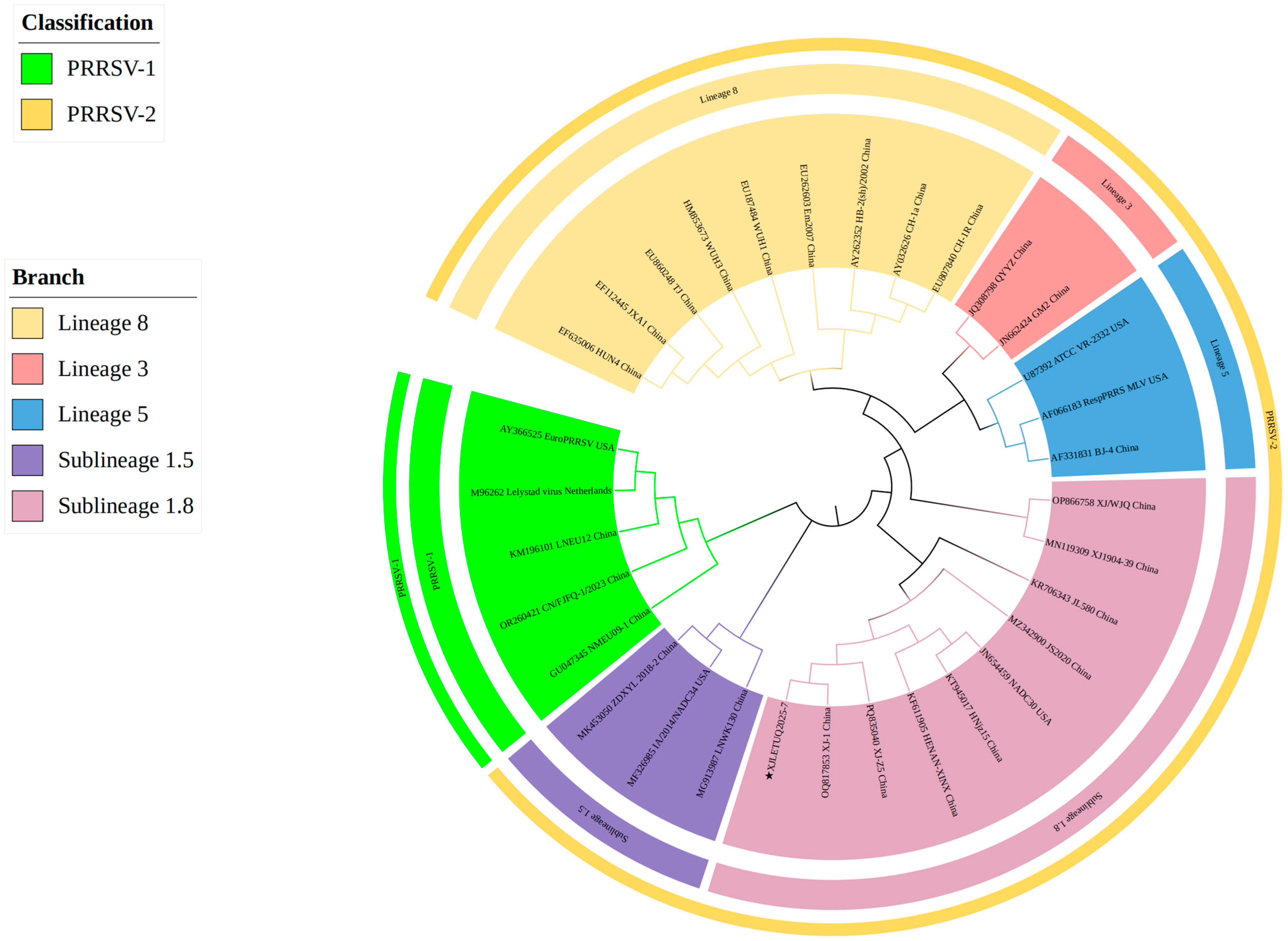
3.4.1. Whole-Genome Genetic Evolution and Homology Analysis
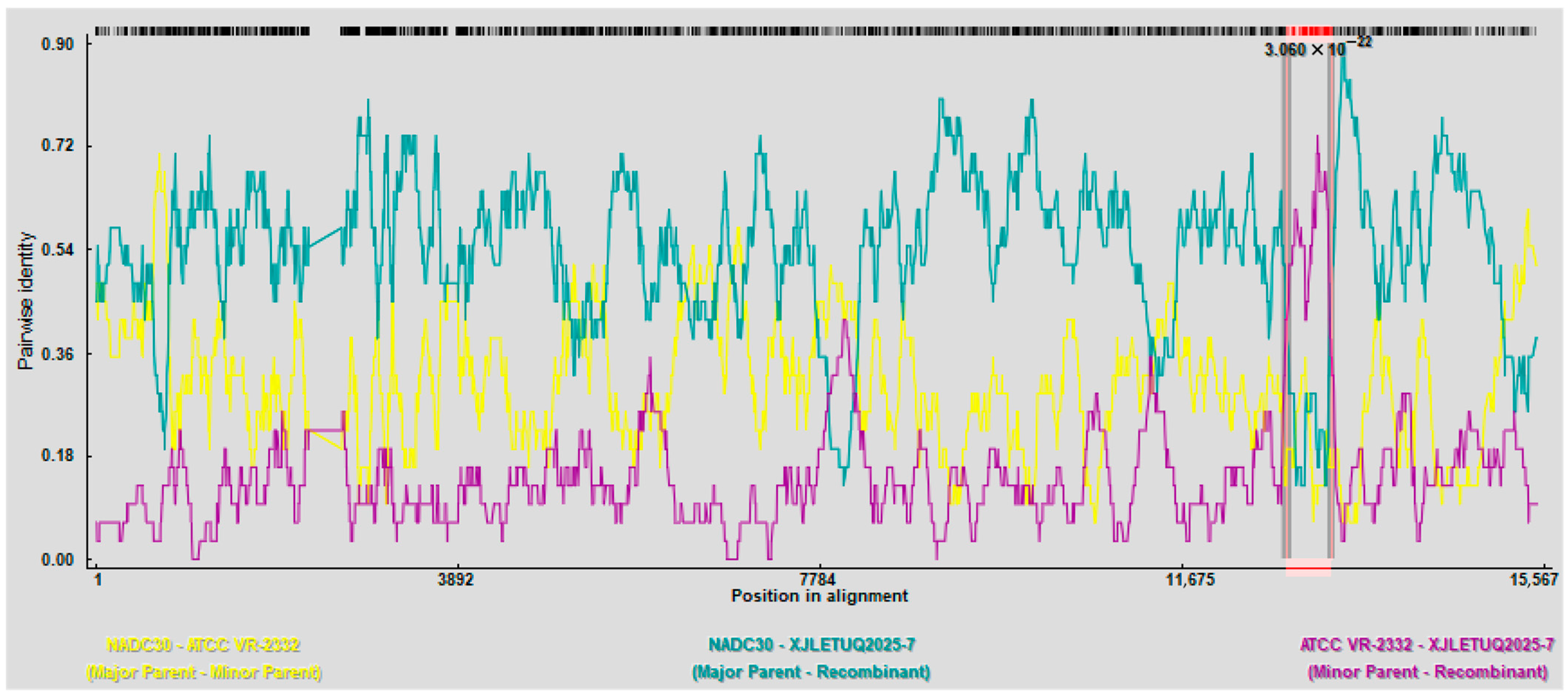
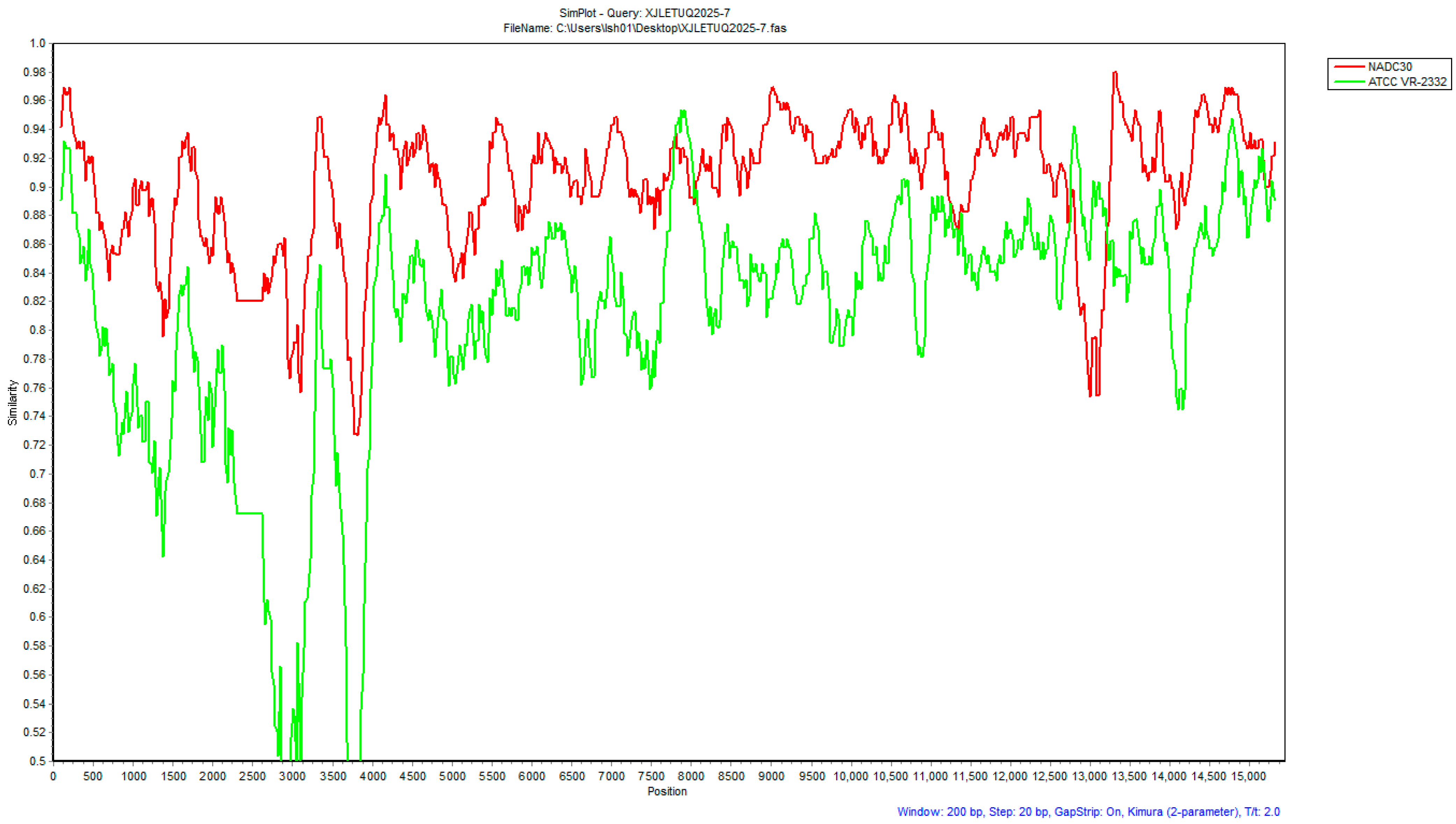
3.4.2. Recombinant Analysis
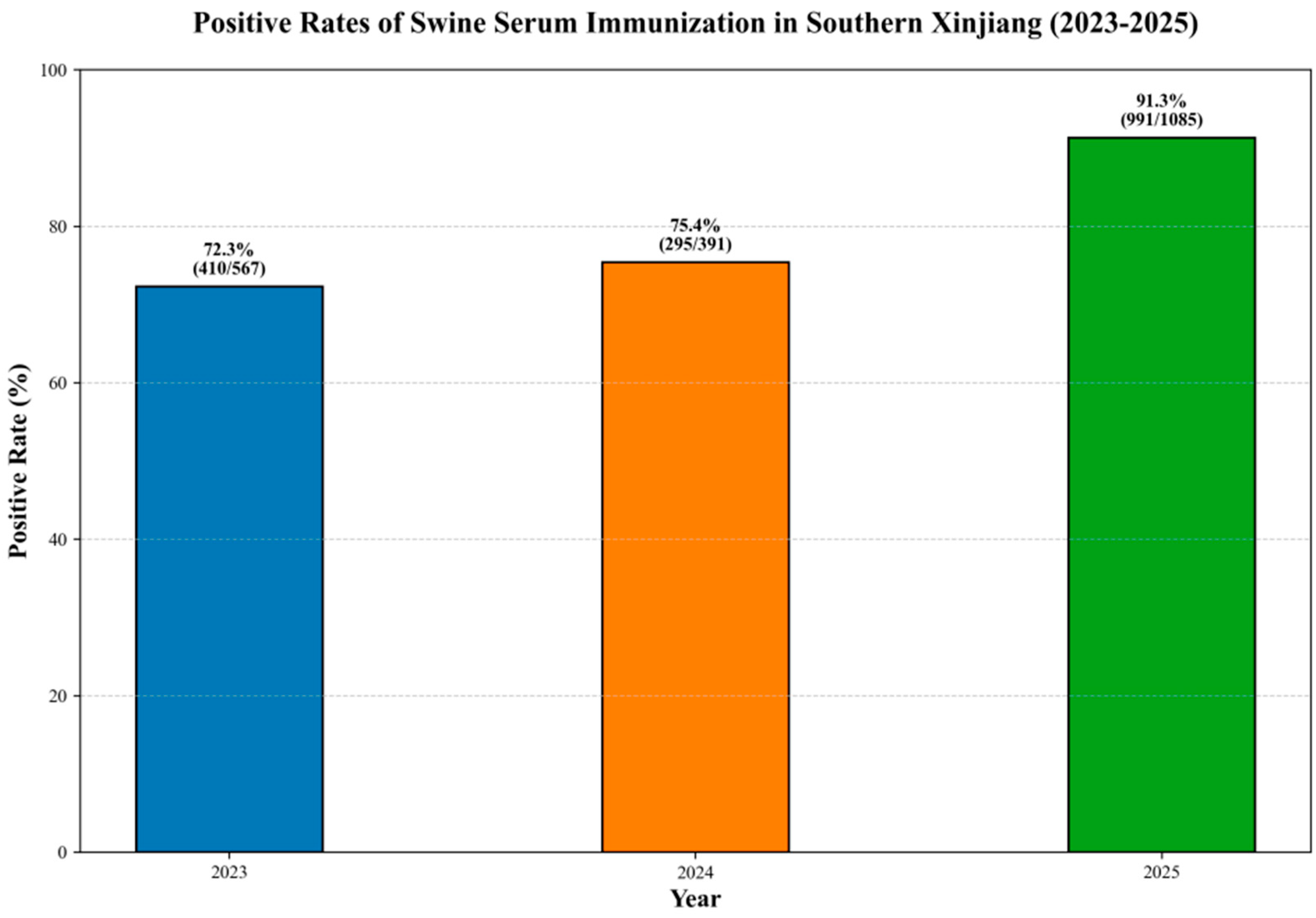
3.5. Antibody Level Monitoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Q.; Xu, H.; An, T.; Cai, X.; Tian, Z.; Zhang, H. Recent progress in studies of porcine reproductive and respiratory syndrome virus 1 in China. Viruses 2023, 15, 1528. [Google Scholar] [CrossRef]
- Liu, B.; Luo, L.; Shi, Z.; Ju, H.; Yu, L.; Li, G.; Cui, J. Research progress of porcine reproductive and respiratory syndrome virus NSP2 protein. Viruses 2023, 15, 2310. [Google Scholar] [CrossRef]
- Terpstra, C.; Wensvoort, G.; Pol, J.M. Experimental reproduction of porcine epidemic abortion and respiratory syndrome (mystery swine disease) by infection with Lelystad virus: Koch’s postulates fulfilled. Vet. Q. 1991, 13, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Wensvoort, G.; Terpstra, C.; Pol, J.M.; Ter Laak, E.A.; Bloemraad, M.; De Kluyver, E.P.; Kragten, C.; Van Buiten, L.; Den Besten, A.; Wagenaar, F.; et al. Mystery swine disease in The Netherlands: The isolation of Lelystad virus. Vet. Q. 1991, 13, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, H.; Cheng, H.; Liu, M.; Wen, S.; Ren, J. Progress in PRRSV infection and adaptive immune response mechanisms. Viruses 2023, 15, 1442. [Google Scholar] [CrossRef]
- Chen, X.; Pan, J.; Huang, L.; Zhao, M. Research progress on the E protein of porcine reproductive and respiratory syndrome virus. Front. Microbiol. 2023, 14, 1139628. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Z.; Yu, Y.; Huang, J.; Jiang, P.; Shan, H. Genetic analysis of a porcine reproductive and respiratory syndrome virus 1 strain in China with new patterns of amino acid deletions in nsp2, GP3 and GP4. Microb. Pathog. 2020, 149, 104531. [Google Scholar] [CrossRef]
- Yuan, S.; Wei, Z. Construction of infectious cDNA clones of PRRSV: Separation of coding regions for nonstructural and structural proteins. Sci. China Ser. C Life Sci. 2008, 51, 271–279. [Google Scholar] [CrossRef]
- Patton, J.B.; Rowland, R.R.; Yoo, D.; Chang, K.-O. Modulation of CD163 receptor expression and replication of porcine reproductive and respiratory syndrome virus in porcine macrophages. Virus Res. 2009, 140, 161–171. [Google Scholar] [CrossRef]
- Xiao, S.; Jia, J.; Mo, D.; Wang, Q.; Qin, L.; He, Z.; Zhao, X.; Huang, Y.; Li, A.; Yu, J. Understanding PRRSV infection in porcine lung based on genome—Wide transcriptome response identified by deep sequencing. PLoS ONE 2010, 5, e11377. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Li, X. Emergence and spread of NADC34-like PRRSV in China. Transbound. Emerg. Dis. 2021, 68, 3005–3008. [Google Scholar] [CrossRef]
- Shi, M.; Lam, T.T.-Y.; Hon, C.-C.; Hui, R.K.-H.; Faaberg, K.S.; Wennblom, T.; Murtaugh, M.P.; Stadejek, T.; Leung, F.C.-C. Molecular epidemiology of PRRSV: A phylogenetic perspective. Virus Res. 2010, 154, 7–17. [Google Scholar] [CrossRef]
- Shi, M.; Lam, T.T.-Y.; Hon, C.-C.; Murtaugh, M.P.; Davies, P.R.; Hui, R.K.-H.; Li, J.; Wong, L.T.-W.; Yip, C.-W.; Jiang, J.-W. Phylogeny—Based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. J. Virol. 2010, 84, 8700–8711. [Google Scholar] [CrossRef] [PubMed]
- Paploski, I.A.D.; Corzo, C.; Rovira, A.; Murtaugh, M.P.; Sanhueza, J.M.; Vilalta, C.; Schroeder, D.C.; VanderWaal, K. Temporal dynamics of co-circulating lineages of porcine reproductive and respiratory syndrome virus. Front. Microbiol. 2019, 10, 2486. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liang, W.; Wang, X.; Chen, H.; Fan, J.; Song, W.; Hua, L.; Tang, X.; Chen, H.; Peng, Z. Epidemiological and genetic characteristics of porcine reproduction and respiratory syndrome virus 2 in mainland China, 2017–2018. Arch. Virol. 2020, 165, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-C.; Xiong, J.-Y.; Ye, C.; Chang, X.-B.; Guo, J.-C.; Jiang, C.-G.; Zhang, G.-H.; Tian, Z.-J.; Cai, X.-H.; Tong, G.-Z. Genotypic and geographical distribution of porcine reproductive and respiratory syndrome viruses in mainland China in 1996–2016. Vet. Microbiol. 2017, 208, 164–172. [Google Scholar]
- Normile, D. China, Vietnam grapple with′rapidly evolving′pig virus. Science 2007, 317, 1017. [Google Scholar]
- Zhao, K.; Ye, C.; Chang, X.-B.; Jiang, C.-G.; Wang, S.-J.; Cai, X.-H.; Tong, G.-Z.; Tian, Z.-J.; Shi, M.; An, T.-Q. Importation and recombination are responsible for the latest emergence of highly pathogenic porcine reproductive and respiratory syndrome virus in China. J. Virol. 2015, 89, 10712–10716. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, P.; Song, Z.; Lv, L.; Li, L.; Bai, J. Pathogenicity and antigenicity of a novel NADC30-like strain of porcine reproductive and respiratory syndrome virus emerged in China. Vet. Microbiol. 2016, 197, 93–101. [Google Scholar]
- Chen, N.; Cao, Z.; Yu, X.; Deng, X.; Zhao, T.; Wang, L.; Liu, Q.; Li, X.; Tian, K. Emergence of novel European genotype porcine reproductive and respiratory syndrome virus in mainland China. J. Gen. Virol. 2011, 92, 880–892. [Google Scholar] [CrossRef]
- Li, C.; Qiu, M.; Li, S.; Sun, Z.; Huang, Z.; Qi, W.; Qiu, Y.; Li, J.; Feng, B.; Zhao, D. Metagenomic and pathogenic assessments identify a pathogenic porcine reproductive and respiratory syndrome virus 1 with new deletions from adult slaughter pig in 2022. Transbound. Emerg. Dis. 2023, 2023, 1975039. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cui, M.; Li, C.; Qiu, M.; Zhu, X.; Lin, Y.; Meng, Y.; Qiu, Y.; Qi, W.; Lin, H. Isolation and Genomic Characterization of a Novel Porcine Reproductive and Respiratory Syndrome Virus 1 from Severely Diseased Piglets in China in 2024. Vet. Sci. 2025, 12, 61. [Google Scholar] [CrossRef]
- Luo, Q.; Zheng, Y.; He, Y.; Li, G.; Zhang, H.; Sha, H.; Zhang, Z.; Huang, L.; Zhao, M. Genetic variation and recombination analysis of the GP5 (GP5a) gene of PRRSV-2 strains in China from 1996 to 2022. Front. Microbiol. 2023, 14, 1238766. [Google Scholar] [CrossRef]
- An, T.-Q.; Tian, Z.-J.; Leng, C.-L.; Peng, J.-M.; Tong, G.-Z. Highly pathogenic porcine reproductive and respiratory syndrome virus, Asia. Emerg. Infect. Dis. 2011, 17, 1782. [Google Scholar] [CrossRef]
- Mateu, E.; Martín, M.; Vidal, D. Genetic diversity and phylogenetic analysis of glycoprotein 5 of European-type porcine reproductive and respiratory virus strains in Spain. J. Gen. Virol. 2003, 84, 529–534. [Google Scholar] [CrossRef]
- Fang, K.; Liu, S.; Li, X.; Chen, H.; Qian, P. Epidemiological and genetic characteristics of porcine reproductive and respiratory syndrome virus in South China between 2017 and 2021. Front. Vet. Sci. 2022, 9, 853044. [Google Scholar] [PubMed]
- Yin, B.; Qi, S.; Sha, W.; Qin, H.; Liu, L.; Yun, J.; Zhu, J.; Li, G.; Sun, D. Molecular characterization of the Nsp2 and ORF5 (ORF5a) genes of PRRSV strains in nine provinces of China during 2016–2018. Front. Vet. Sci. 2021, 8, 605832. [Google Scholar] [CrossRef] [PubMed]
- Do, D.T.; Park, C.; Choi, K.; Jeong, J.; Nguyen, T.T.; Le, D.T.H.; Vo, K.M.; Chae, C. Nucleotide sequence analysis of Vietnamese highly pathogenic porcine reproductive and respiratory syndrome virus from 2013 to 2014 based on the NSP2 and ORF5 coding regions. Arch. Virol. 2016, 161, 669–675. [Google Scholar]
- Li, P.; Shen, Y.; Wang, T.; Li, J.; Li, Y.; Zhao, Y.; Liu, S.; Li, B.; Liu, M.; Meng, F. Epidemiological survey of PRRS and genetic variation analysis of the ORF5 gene in Shandong Province, 2020–2021. Front. Vet. Sci. 2022, 9, 987667. [Google Scholar]
- Wesley, R.D.; Mengeling, W.L.; Lager, K.M.; Clouser, D.F.; Landgraf, J.G.; Frey, M.L. Differentiation of a porcine reproductive and respiratory syndrome virus vaccine strain from North American field strains by restriction fragment length polymorphism analysis of ORF 5. J. Vet. Diagn. Investig. 1998, 10, 140–144. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, S.; Zhang, J.; Zeng, J.; Guo, X.; Ge, X.; Zhang, D.; Yang, H. Molecular variation analysis of porcine reproductive and respiratory syndrome virus in China. Virus Res. 2009, 145, 97–105. [Google Scholar] [CrossRef]
- Zhou, L.; Han, J.; Yang, H. The evolution and diversity of porcine reproductive and respiratory syndrome virus in China. Vet. Microbiol. 2024, 298, 110252. [Google Scholar] [CrossRef]
- Li, C.; Zhuang, J.; Wang, J.; Han, L.; Sun, Z.; Xiao, Y.; Ji, G.; Li, Y.; Tan, F.; Li, X. Outbreak Investigation of NADC 30-Like PRRSV in South-East China. Transbound. Emerg. Dis. 2016, 63, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, W.; Mao, L.; Pan, X.; Wu, Q.; Guo, Y.; Jiang, J.; Tang, H.; Zhao, Y.; Cheng, L. Molecular epizootiology of porcine reproductive and respiratory syndrome virus in the Xinjiang Uygur Autonomous Region of China. Front. Microbiol. 2024, 15, 1419499. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zheng, Y.; Zhang, H.; Yang, Z.; Sha, H.; Kong, W.; Zhao, M.; Wang, N. Research progress on glycoprotein 5 of porcine reproductive and respiratory syndrome virus. Animals 2023, 13, 813. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Mo, Q.; Chen, G.; Lv, J.; Huang, J.; Pang, Y.; Wang, H.; Liu, W.; Huang, K. The emergence and pathogenesis of Recombinant viruses associated with NADC34-like strains and the predominant Circulating strains of Porcine reproductive and respiratory syndrome virus in Southern China. Viruses 2022, 14, 1695. [Google Scholar] [CrossRef]
- Huang, B.; Deng, L.; Xu, T.; Jian, Z.; Lai, S.; Ai, Y.; Xu, Z.; Zhu, L. Isolation and pathogenicity comparison of two novel natural recombinant porcine reproductive and respiratory syndrome viruses with different recombination patterns in Southwest China. Microbiol. Spectr. 2024, 12, e04071-23. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Li, Y.; Zhang, G.; Du, C.; Zhou, Y.; Jiang, D.; Luo, Y.; Yao, X.; Yang, Z.; Ren, M. Isolation, identification, recombination analysis and pathogenicity experiment of a PRRSV recombinant strain in Sichuan Province, China. Front. Microbiol. 2024, 15, 1362471. [Google Scholar] [CrossRef]
- Han, J.; Rutherford, M.S.; Faaberg, K.S. The Porcine Reproductive and Respiratory Syndrome Virus nsp2 Cysteine Protease Domain Possesses both trans-and cis-Cleavage Activities. J. Virol. 2009, 83, 9449–9463. [Google Scholar] [CrossRef]
- Music, N.; Gagnon, C.A. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non-structural proteins in virus pathogenesis. Anim. Health Res. Rev. 2010, 11, 135–163. [Google Scholar]
- Zhang, H.; Luo, Q.; He, Y.; Zheng, Y.; Sha, H.; Li, G.; Kong, W.; Liao, J.; Zhao, M. Research progress on the development of porcine reproductive and respiratory syndrome vaccines. Vet. Sci. 2023, 10, 491. [Google Scholar] [CrossRef] [PubMed]
| Target | Primer Sequence (5′ → 3′) | Size (bp) |
|---|---|---|
| PRRSV-1-ORF5 | F:TGAGGTGGGCTACAACCATT R:AGGCTAGCACGAGCTTTTGT | 702 |
| PRRSV-2-ORF5 | F:ACCTGAGACCATGAGGTGGGCAA R:CAAACGGCATCTGGAGGTGATGAAT | 963 |
| Reference Strains | Time | Country/Province | GenBank Accession Number | Reference Strains | Time | Country/Province | GenBank Accession Number |
|---|---|---|---|---|---|---|---|
| Lelystad virus | 1993 | Netherlands | M96262 | CH-1R | 2008 | Heilongjiang, China | EU807840 |
| EuroPRRSV | 2003 | USA | AY366525 | LNWK130 | 2017 | Heilongjiang, China | MG913987 |
| RespPRRS MLV | 1998 | USA | AF066183 | JL580 | 2014 | Heilongjiang, China | KR706343 |
| ATCC VR-2332 | 1992 | USA | U87392 | HENAN-XINX | 2013 | Henan, China | KF611905 |
| NADC30 | 2008 | USA | JN654459 | HNjz15 | 2015 | Henan, China | KT945017 |
| IA/2014/NADC34 | 2014 | USA | MF326985 | QYYZ | 2011 | Guangdong, China | JQ308798 |
| BJ-4 | 1996 | Beijing, China | AF331831 | GM2 | 2011 | Guangdong, China | JN662424 |
| NMEU09-1 | 2009 | Beijing, China | GU047345 | JS2020 | 2020 | Jiangsu, China | MZ342900 |
| TJ | 2008 | Tianjin, China | EU860248 | ZDXYL 2018-2 | 2018 | Jilin, China | MK453050 |
| HB-2(sh)/2002 | 2002 | Hebei, China | AY262352 | LNEU12 | 2014 | Liaoning, China | KM196101 |
| Em2007 | 2007 | Hubei, China | EU262603 | CN/FJFQ-1/2023 | 2023 | Fujian, China | OR260421 |
| WUH1 | 2006 | Hubei, China | EU187484 | XJ/WJQ | 2021 | Xinjiang, China | OP866758 |
| WUH3 | 2008 | Hubei, China | HM853673 | XJ1904-39 | 2019 | Xinjiang, China | MN119309 |
| HUN4 | 2007 | Hunan, China | EF635006 | XJ-Z5 | 2022 | Xinjiang, China | PQ835040 |
| JXA1 | 2006 | Jiangxi, China | EF112445 | XJ-1 | 2021 | Xinjiang, China | OQ817853 |
| CH-1a | 1996 | Heilongjiang, China | AY032626 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Hou, M.; Chen, X.; Ma, B.; Zhang, Z.; Guo, M.; Chen, Y.; Li, L. Molecular Epidemiology and Genetic Evolution Analysis of Porcine Reproductive and Respiratory Syndrome Virus in Southern Xinjiang, China, from 2023 to 2025. Vet. Sci. 2025, 12, 874. https://doi.org/10.3390/vetsci12090874
Liu S, Hou M, Chen X, Ma B, Zhang Z, Guo M, Chen Y, Li L. Molecular Epidemiology and Genetic Evolution Analysis of Porcine Reproductive and Respiratory Syndrome Virus in Southern Xinjiang, China, from 2023 to 2025. Veterinary Sciences. 2025; 12(9):874. https://doi.org/10.3390/vetsci12090874
Chicago/Turabian StyleLiu, Shuhua, Mengzhe Hou, Xin Chen, Baihe Ma, Zhen Zhang, Meiliang Guo, Yunlai Chen, and Lianrui Li. 2025. "Molecular Epidemiology and Genetic Evolution Analysis of Porcine Reproductive and Respiratory Syndrome Virus in Southern Xinjiang, China, from 2023 to 2025" Veterinary Sciences 12, no. 9: 874. https://doi.org/10.3390/vetsci12090874
APA StyleLiu, S., Hou, M., Chen, X., Ma, B., Zhang, Z., Guo, M., Chen, Y., & Li, L. (2025). Molecular Epidemiology and Genetic Evolution Analysis of Porcine Reproductive and Respiratory Syndrome Virus in Southern Xinjiang, China, from 2023 to 2025. Veterinary Sciences, 12(9), 874. https://doi.org/10.3390/vetsci12090874





