Cases of Interspecies Transmission of Influenza A Virus from Swine to Humans
Simple Summary
Abstract
1. Introduction
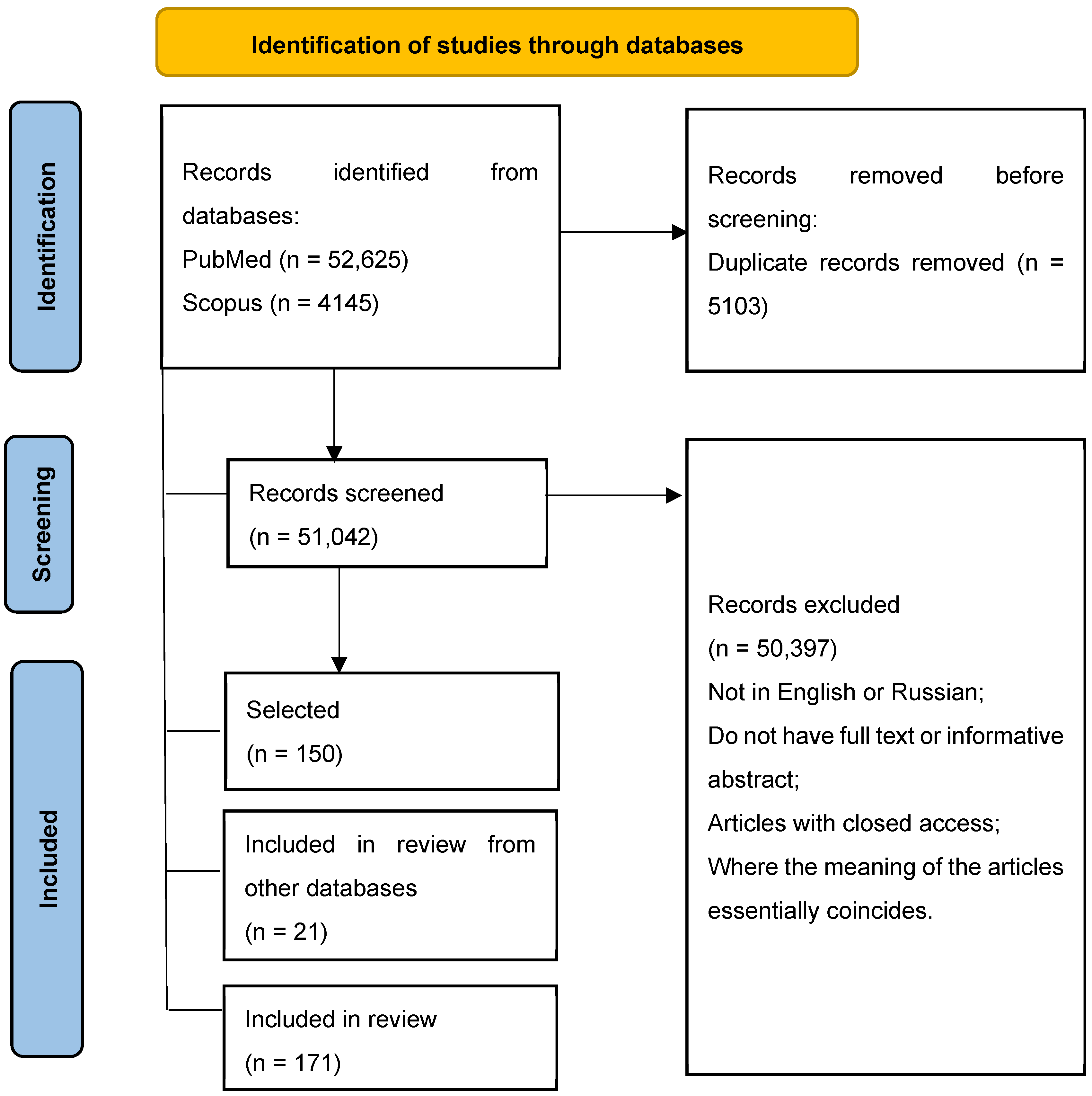
2. Materials and Methods
3. Results
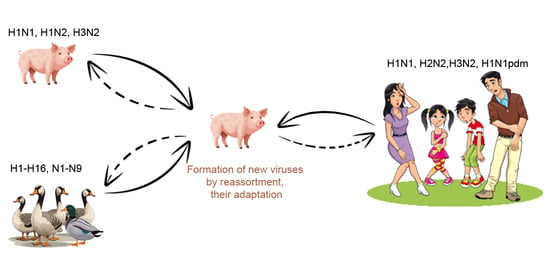
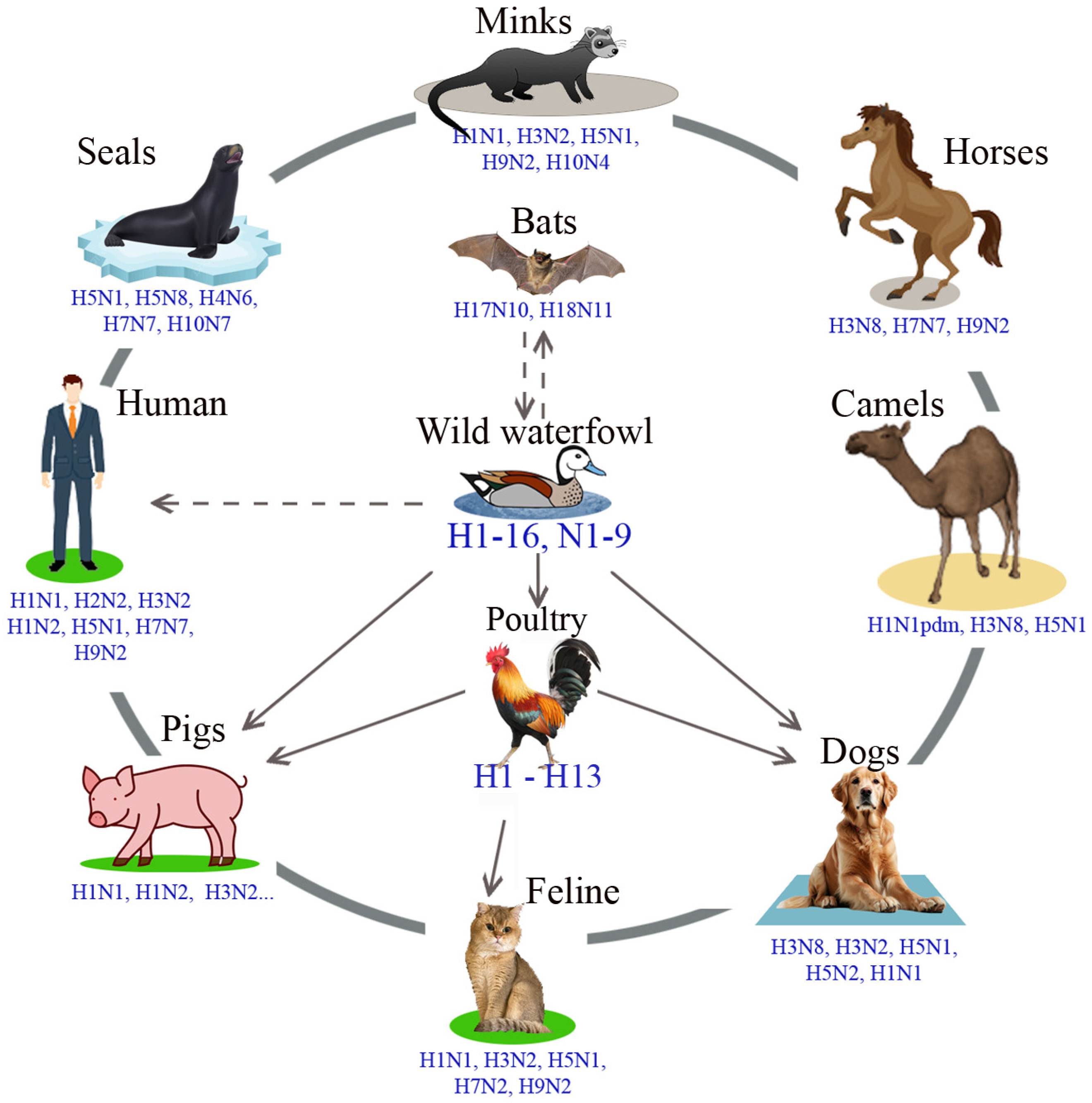
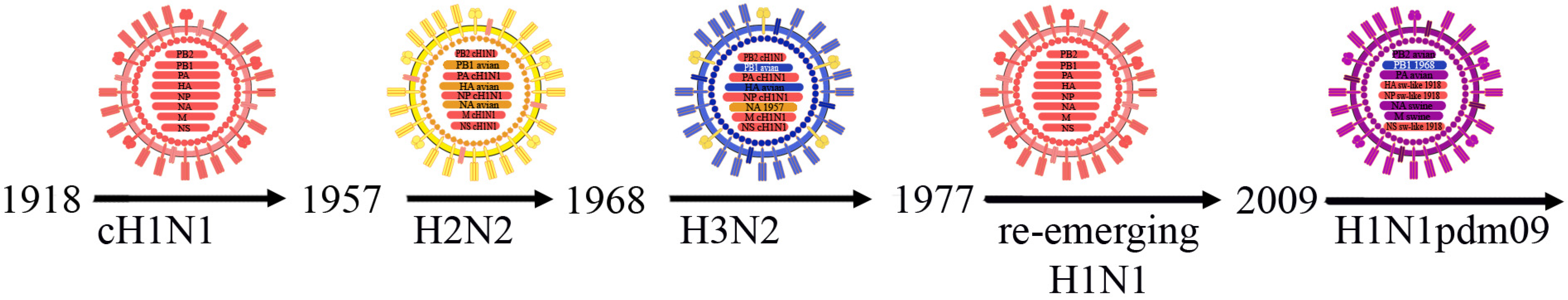
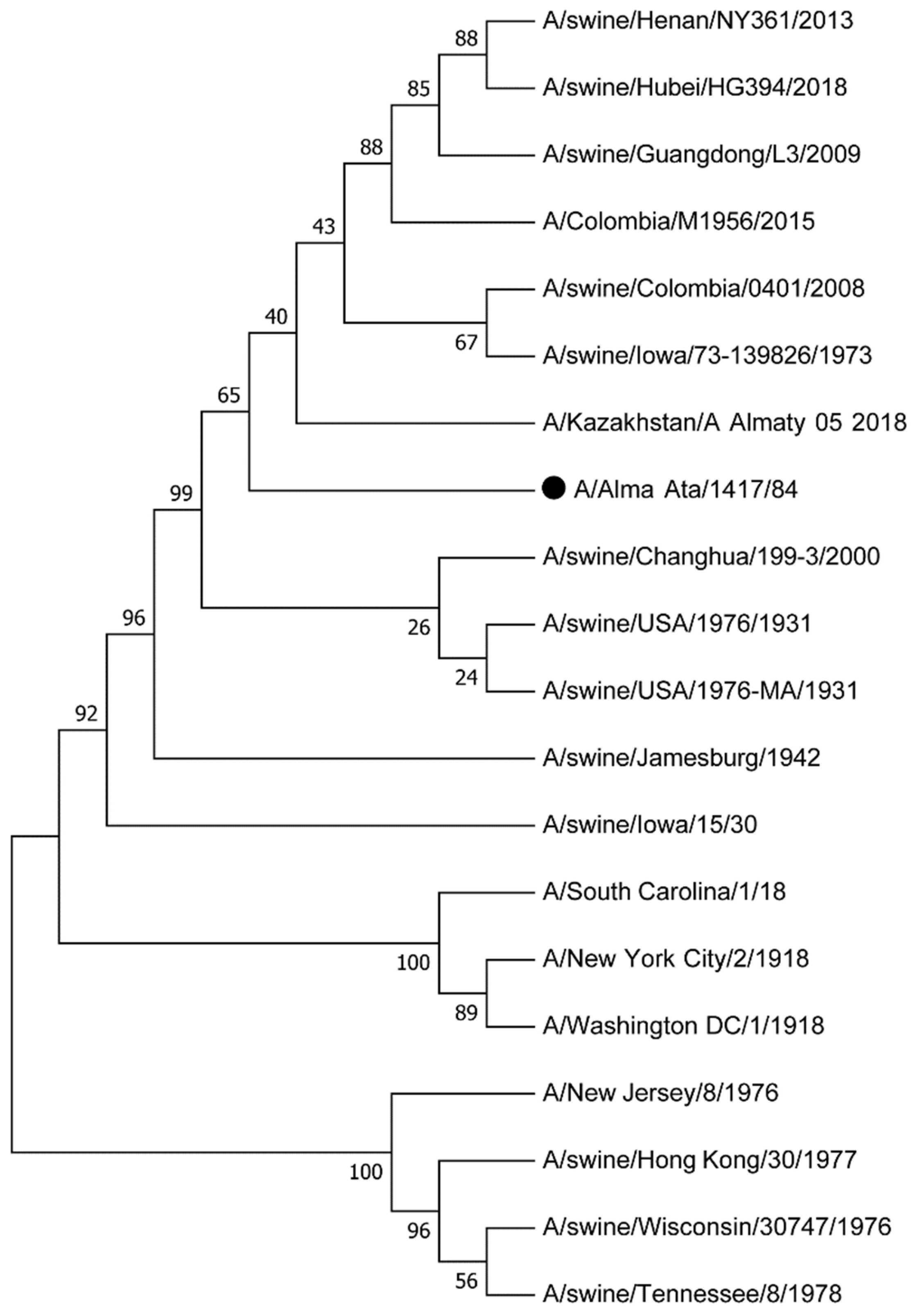
3.1. Influenza A Viruses, Their Evolution and Interspecies Transmission
3.2. Transmission of the Swine Influenza Virus to Humans
3.2.1. Transmission of the Swine Influenza Virus to Humans in America
3.2.2. Transmission of the Swine Influenza Virus to Humans in Europe
3.2.3. Transmission of Swine Influenza Virus to Humans in Asia
3.2.4. Transmission of the Swine Influenza Virus to Humans in Australia
3.2.5. Transmission of the Influenza Virus A/H1N1pdm to Humans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wright, P.F.; Neumann, G.; Kawaoka, Y. Orthomyxoviruses. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincot Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1691–1740. [Google Scholar]
- Webster, R.; Bean, W.; Gorman, O.; Chambers, T.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taubenberger, J.K.; Morens, D.M. Pandemic influenza—Including a risk assessment of H5N1. Rev. Sci. Tech. 2009, 28, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y. Exploring Potential Intermediates in the Cross-Species Transmission of Influenza A Virus to Humans. Viruses 2024, 16, 1129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, W.; Kahn, R.; Richt, J. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. J. Mol. Genet. Med. 2008, 3, 158–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelwhab, E.M.; Mettenleiter, T.C. Zoonotic Animal Influenza Virus and Potential Mixing Vessel Hosts. Viruses 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taubenberger, J.K.; Kash, J.C. Influenza Virus Evolution, Host Adaptation, and Pandemic Formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palese, P.; Shaw, M.L. Chapter 47. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 1647–1690. [Google Scholar]
- Nakatsu, S.; Murakami, S.; Shindo, K.; Horimoto, T.; Sagara, H.; Noda, T.; Kawaoka, Y. Influenza C and D Viruses Package Eight Organized Ribonucleoprotein Complexes. J. Virol. 2018, 92, e02084-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fouchier, R.; Munster, V.; Wallensten, A.; Bestebroer, T.; Herfst, S.; Smith, D.; Rimmelzwaan, G.; Olsen, B.; Osterhaus, A. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.; Chen, L.; Recuenco, S.; Ellison, J.; Davis, C.; York, I.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Wu, Y.; Tefsen, B.; Shi, Y.; Gao, G.F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol. 2014, 22, 183–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Shen, H.; McDowell, C.; Liu, Q.; Duff, M.; Lee, J.; Lang, Y.; Hesse, D.; Richt, J.A.; Ma, W. Virus survival and fitness when multiple genotypes and subtypes of influenza A viruses exist and circulate in swine. J. Virol. 2019, 532, 30–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bunpapong, N.; Nonthabenjawan, N.; Chaiwong, S.; Tangwangvivat, R.; Boonyapisitsopa, S.; Jairak, W.; Tuanudom, R.; Prakairungnamthip, D.; Suradhat, S.; Thanawongnuwech, R.; et al. Genetic characterization of canine influenza A virus (H3N2) in Thailand. Virus Genes 2014, 48, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Donatelli, I.; Castrucci, M.R.; De Marco, M.A.; Delogu, M.; Webster, R.G. Human-Animal Interface: The Case for Influenza Interspecies Transmission. Adv. Exp. Med. Biol. 2017, 972, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.F.; Webster, R.G. Orthomyxoviruses. In Fields Virology, 4th ed.; Fields, B.N., Knipe, D.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 1533–1579. [Google Scholar]
- Subbarao, K. The Critical Interspecies Transmission Barrier at the Animal-Human Interface. Trop. Med. Infect. Dis. 2019, 4, 72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peacock, T.P.; Moncla, L.; Dudas, G.; VanInsberghe, D.; Sukhova, K.; Lloyd-Smith, J.O.; Worobey, M.; Lowen, A.C.; Nelson, M.I. The global H5N1 influenza panzootic in mammals. Nature 2025, 637, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Borkenhagen, L.K.; Salman, M.D.; Ma, M.J.; Gray, G.C. Animal influenza virus infections in humans: A commentary. Int. J. Infect. Dis. 2019, 88, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Husain, M. Host factors involved in influenza virus infection. Emerg. Top. Life Sci. 2020, 4, 389–398. [Google Scholar] [CrossRef]
- Russell, C.J. Hemagglutinin Stability and Its Impact on Influenza A Virus Infectivity, Pathogenicity, and Transmissibility in Avians, Mice, Swine, Seals, Ferrets, and Humans. Viruses 2021, 13, 746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glebova, T.I.; Klivleyeva, N.G.; Baimukhametova, A.M.; Saktaganov, N.T.; Lukmanova, G.V.; Ongarbayeva, N.S.; Shamenova, M.G.; Baimakhanova, B.B. Circulation of influenza viruses in the epidemic season of 2018–2019 among people residing in Northern and Western Kazakhstan. Infekc. Bolezn. (Infect. Dis.) 2021, 19, 70–75. (In Russian) [Google Scholar] [CrossRef]
- Klivleyeva, N.; Lukmanova, G.; Glebova, T.; Shamenova, M.; Ongarbayeva, N.; Saktaganov, N.; Baimukhametova, A.; Baiseiit, S.; Ismagulova, D.; Kassymova, G.; et al. Spread of Pathogens Causing Respiratory Viral Diseases Before and During CoVID-19 Pandemic in Kazakhstan. Indian J. Microbiol. 2023, 63, 129–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shamenova, M.G.; Glebova, T.I.; Klivleyeva, N.G.; Baiseiit, S.B.; Baimukhametova, A.M.; Saktaganov, N.T.; Ongarbayeva, N.S.; Ismagulova, D.A. Serological studies of influenza infection among population in southern region of Kazakhstan during the 2018-2021 epidemic season. J. Pak. Med. Assoc. 2023, 73, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Ma, W. Swine influenza virus: Current status and challenge. Virus Res. 2020, 288, 198118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chauhan, R.P.; Gordon, M.L. Deciphering transmission dynamics and spillover of avian influenza viruses from avian species to swine populations globally. Virus Genes 2021, 57, 541–555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saktaganov, N.; Klivleyeva, N.; Ongarbayeva, N.; Glebova, T.; Lukmanova, G.; Baimukhametova, A. Study on antigenic relationships and biological properties of swine influenza A/H1N1 virus strains isolated in Northern Kazakhstan in 2018. Sel’skokhozyaistvennaya Biol. (Agric. Biol.) 2020, 55, 355–363. [Google Scholar] [CrossRef]
- Klivleyeva, N.G.; Ongarbayeva, N.S.; Korotetskiy, I.S.; Glebova, T.I.; Saktaganov, N.T.; Shamenova, M.G.; Baimakhanova, B.B.; Shevtsov, A.B.; Amirgazin, A.; Berezin, V.E.; et al. Coding-Complete Genome Sequence of Swine Influenza Virus Isolate A/Swine/Karaganda/04/2020 (H1N1) from Kazakhstan. Microbiol. Resour. Announc. 2021, 30, e0078621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klivleyeva, N.; Saktaganov, N.; Glebova, T.; Lukmanova, G.; Ongarbayeva, N.; Webby, R. Influenza A Viruses in the Swine Population: Ecology and Geographical Distribution. Viruses 2024, 16, 1728. [Google Scholar] [CrossRef]
- Lukmanova, G.; Klivleyeva, N.; Glebova, T.; Ongarbayeva, N.; Shamenova, M.; Saktaganov, N.; Baimukhametova, A.; Baiseiit, S.; Ismagulova, D.; Ismailov, E.; et al. Influenza A virus surveillance in domestic pigs in Kazakhstan 2018-2021. Microbiolo. Cienc. Rural 2024, 54, e20230403. [Google Scholar] [CrossRef]
- Waddell, G.H.; Teigland, M.B.; Sigel, M.M. A new influenza virus associated with equine respiratory disease. J. Am. Vet. Med. Assoc. 1963, 143, 587–590. [Google Scholar] [PubMed]
- Guo, Y.; Wang, M.; Kawaoka, Y.; Gorman, O.; Ito, T.; Saito, T.; Webster, R.G. Characterization of a new avian-like influenza A virus from horses in China. Virology 1992, 188, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Oladunni, F.S.; Oseni, S.O.; Martinez-Sobrido, L.; Chambers, T.M. Equine Influenza Virus and Vaccines. Viruses 2021, 13, 1657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krog, J.S.; Hansen, M.S.; Holm, E.; Hjulsager, C.K.; Chriel, M.; Pedersen, K.; Andersen, L.O.; Abildstrom, M.; Jensen, T.H.; Larsen, L.E. Influenza A(H10N7) virus in dead harbir seals, Denmark. Emerg. Infect. Dis. 2015, 21, 684–687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puryear, W.; Sawatzki, K.; Hill, N.; Foss, A.; Stone, J.J.; Doughty, L.; Walk, D.; Gilbert, K.; Murray, M.; Cox, E.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in New England Seals, United States. Emerg. Infect. Dis. 2023, 29, 786–791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, P.; Sun, L.; Xiong, J.; Wang, C.; Chen, L.; Yang, P.; Yu, H.; Yan, Q.; Cheng, Y.; Jiang, L.; et al. Semiaquatic mammals might be intermediate hosts to spread avian influenza viruses from avian to human. Sci. Rep. 2019, 9, 11641. [Google Scholar] [CrossRef]
- Postel, A.; King, J.; Kaiser, F.K.; Kennedy, J.; Lombardo, M.S.; Reineking, W.; de le Roi, M.; Harder, T.; Pohlmann, A.; Gerlach, T.; et al. Infections with highly pathogenic avian influenza A virus (HPAIV) H5N8 in harbor seals at the German North Sea coast, 2021. Emerg. Microbes. Infect. 2022, 11, 725–729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parrish, C.R.; Voorhees, L.E. H3N8 and H3N2 Canine Influenza Viruses Understanding These New Viruses in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Glebova, T.I.; Klivleyeva, N.G.; Saktaganov, N.T.; Shamenova, M.G.; Lukmanova, G.V.; Baimukhametova, A.M.; Baiseiit, S.B.; Ongarbayeva, N.S.; Orynkhanov, K.A.; Ametova, A.V.; et al. Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024). Open Vet. J. 2024, 14, 1896–1904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, D.; Kang, B.; Lee, C.; Jung, K.; Ha, G.; Kang, D.; Park, S.; Park, B.; Oh, J. Transmission of avian influenza virus (H3N2) to dogs. Emerg. Infect. Dis. 2008, 14, 741–746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wille, M.; Holmes, E.C. The Ecology and Evolution of Influenza Viruses. Cold Spring Harb. Perspect. Med. 2020, 10, a038489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hale, B.G.; Albrecht, R.A.; Garcia-Sastre, A. Innate immune evasion strategies of influenza viruses. Future Microbiol. 2010, 5, 23–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26 (Suppl. S4), D49–D53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelly, H. The classical definition of a pandemic is not elusive. Bull. World Health Organ. 2011, 89, 540–541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mostafa, A.; Abdelwhab, E.; Mettenleiter, T.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. J. Viruses 2018, 10, 497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanjuan, R.; Nebot, M.R.; Chirico, N.; Mansky, L.M.; Belshaw, R. Viral mutation rates. J. Virol. 2010, 84, 9733–9748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dundon, W.G.; De Benedictis, P.; Viale, E.; Capua, I. Serologic evidence of pandemic (H1N1) 2009 infection in dogs, Italy. Emerg. Infect. Dis. 2010, 16, 2019–2021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, D.; Sun, S.; Du, L.; Ma, J.; Fan, L.; Pu, J.; Sun, Y.; Zhao, J.; Sun, H.; Liu, J. Natural and experimental infection of dogs with pandemic H1N1/2009 influenza virus. J. Gen. Virol. 2012, 93 Pt 1, 119–123. [Google Scholar] [CrossRef] [PubMed]
- White, M.C.; Lowen, A.C. Implications of segment mismatch for influenza A virus evolution. J. Gen. Virol. 2018, 99, 3–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wille, M.; Tolf, C.; Avril, A.; Latorre-Margalef, N.; Wallerstrom, S.; Olsen, B.; Waldenstrom, J. Frequency and patterns of reassortment in natural influenza A virus infection in a reservoir host. Virology 2013, 443, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Wille, M.; Latorre-Margalef, N.; Tolf, C.; Stallknecht, D.E.; Waldenström, J. No evidence for homosubtypic immunity of influenza H3 in mallards following vaccination in a natural experimental system. Mol. Ecol. 2017, 26, 1420–1431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, R.; Cao, B.; Hu, Y.; Feng, Z.; Wang, D.; Hu, W.; Chen, J.; Jie, Z.; Qiu, H.; Xu, K.; et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013, 368, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.N.; Paulsen, J.C. Receptor determinants of human and animal influenza virus isolates: Differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983, 127, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Couceiro, J.; Kelm, S.; Baum, L.; Krauss, S.; Castrucci, M.; Donatelli, I.; Kida, H.; Paulson, J.; Webster, R.; et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 1998, 72, 7367–7373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ito, T.; Kawaoka, Y. Host-range barrier of influenza A viruses. Vet. Microbiol. 2000, 74, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.K.; Chang, J.; Arendsee, Z.W.; Venkatesh, D.; Souza, C.K.; Kimble, J.B.; Lewis, N.S.; Davis, C.T.; Vincent, A.L. Swine influenza a viruses and the tangled relationship with humans. Cold Spring Harb. Perspect. Med. 2021, 11, a038737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steel, J.; Lowen, A.C. Influenza A virus reassortment. Curr. Top. Microbiol. Immunol. 2014, 385, 377–401. [Google Scholar] [CrossRef] [PubMed]
- Webby, R.J.; Swenson, S.L.; Krauss, S.L.; Gerrish, P.J.; Goyal, S.M.; Webster, R.G. Evolution of swine H3N2 influenza viruses in the United States. J. Virol. 2000, 74, 8243–8251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webby, R.J.; Rossow, K.; Erickson, G.; Sims, Y.; Webster, R. Multiple lineages of antigenically and genetically diverse influenza A virus cocirculate in the United States swine population. Virus Res. 2004, 103, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Vijaykrishna, D.; Smith, G.J.; Pybus, O.G.; Zhu, H.; Bhatt, S.; Poon, L.L.; Riley, S.; Bahl, J.; Ma, S.K.; Cheung, C.L.; et al. Long-term evolution and transmission dynamics of swine influenza A virus. Nature 2011, 473, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.I.; Gramer, M.R.; Vincent, A.L.; Holmes, E.C. Global transmission of influenza viruses from humans to swine. J. Gen. Virol. 2012, 93 Pt 10, 2195–2203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, M.I.; Viboud, C.; Vincent, A.L.; Culhane, M.R.; Detmer, S.E.; Wentworth, D.E.; Rambaut, A.; Suchard, M.A.; Holmes, E.C.; Lemey, P. Global migration of influenza A viruses in swine. Nat. Commun. 2015, 6, 6696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitikoon, P.; Nelson, M.I.; Killian, M.L.; Anderson, T.K.; Koster, L.; Culhane, M.R.; Vincent, A.L. Genotype patterns of contemporary reassorted H3N2 virus in US swine. J. Gen. Virol. 2013, 94 Pt 6, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Worobey, M. Origins of the 1918 pandemic: Revisiting the swine “mixing vessel” hypothesis. Am. J. Epidemiol. 2018, 187, 2498–2502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, J.; Lau, E.; Jin, Z.; Zhu, H.; Guan, Y.; Peiris, M. Influenza A virus transmission in swine farms and during transport in the swine supply chain. Transbound. Emerg. Dis. 2022, 69, e3101–e3110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Robertson, I. The epidemiology of swine influenza. Anim. Dis. 2021, 1, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goel, V.; Ding, J.; Hatuwal, B.; Giri, E.; Deliberto, T.; Lowe, J.; Webby, R.; Emch, M.; Wan, X. Ecological drivers of evolution of swine influenza in the United States: A review. Emerg. Microbes Infect. 2025, 14, 2455598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hause, B.M.; Ducatez, M.; Collin, E.A.; Ran, Z.; Liu, R.; Sheng, Z.; Armien, A.; Kaplan, B.; Chakravarty, S.; Hoppe, A.D.; et al. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog. 2013, 9, e1003176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borkenhagen, L.K.; Mallinson, K.A.; Tsao, R.W.; Ha, S.J.; Lim, W.H.; Toh, T.H.; Anderson, B.D.; Fieldhouse, J.K.; Philo, S.E.; Chong, K.S.; et al. Surveillance for respiratory and diarrheal pathogens at the human-pig interface in Sarawak, Malaysia. PLoS ONE 2018, 13, e0201295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trombetta, C.M.; Marchi, S.; Manini, I.; Kistner, O.; Li, F.; Piu, P.; Manenti, A.; Biuso, F.; Sreenivasan, C.; Druce, J.; et al. Influenza D Virus: Serological Evidence in the Italian Population from 2005 to 2017. Viruses 2020, 12, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trombetta, C.M.; Montomoli, E.; Di Bartolo, I.; Ostanello, F.; Chiapponi, C.; Marchi, S. Detection of antibodies against influenza D virus in swine veterinarians in Italy in 2004. J. Med. Virol. 2022, 94, 2855–2859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- White, S.K.; Ma, W.; McDaniel, C.J.; Gray, G.C.; Lednicky, J.A. Serologic evidence of exposure to influenza D virus among persons with occupational contact with cattle. J. Clin. Virol. 2016, 81, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Shope, R.E. Swine influenza: Iii. Filtration experiments and etiology. J. Exp. Med. 1931, 54, 373–385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shope, R.E. The incidence of neutralizing antibodies for swine influenza virus in the sera of human beings of different ages. J. Exp. Med. 1936, 63, 669–684. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Myers, K.P.; Olsen, C.W.; Gray, G.C. Cases of swine influenza in humans a review of the literature. Clin. Infect. Dis. 2007, 44, 1084–1088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kluska, V.; Macku, M.; Mensik, J. Demonstration of antibodies against swine influenza viruses in man. Cesk. Pediatr. 1961, 16, 408–414. (In Czech) [Google Scholar] [PubMed]
- de Jong, J.C.; de Ronde-Verloop, J.M.; Bangma, P.J.; van Kregten, E.; Kerckhaert, J.; Paccaud, M.F.; Wicki, F.; Wunderli, W. Isolation of swine-influenza-like A(H1N1) viruses from man in Europe, 1986. Lancet 1986, 2, 1329–1330. [Google Scholar] [CrossRef] [PubMed]
- Schnurrenberger, P.; Woods, G.; Martin, R. Serologic evidence of human infection with swine influenza virus. Am. Rev. Respir. Dis. 1970, 102, 356–361. [Google Scholar] [PubMed]
- Easterday, B. Swine influenza. In Diseases of Swine, 6th ed.; Leman, A.D., Straw, B., Glock, R., Mengeling, W., Penny, R., Scholl, E., Eds.; Iowa State University Press: Ames, IA, USA, 1986; pp. 244–255. [Google Scholar]
- Smith, T.F.; Burgert, E.O., Jr.; Dowdle, W.R.; Noble, G.R.; Campbell, R.J.; Van Scoy, R.E. Isolation of Swine Influenza virus from autopsy lung tissue of man. N. Engl. J. Med. 1976, 294, 708–710. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Noble, G.R.; Easterday, B.C.; Kendal, A.P.; Shasby, D.M.; Nelson, D.B.; Hattwick, M.A.; Dowdle, W.R. Swine-like influenza virus infection in a Wisconsin farm family. J. Infect. Dis. 1977, 136 (Suppl. S3), 90–96. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.L.; Sande, M.A.; Wenzel, R.P.; Hoke, C.H., Jr.; Gwaltney, J.M., Jr. Swine-influenza infection in civilians. Report. of two cases. N. Engl. J. Med. 1976, 295, 714–715. [Google Scholar] [CrossRef] [PubMed]
- McKinney, W.P.; Volkert, P.; Kaufman, J. Fatal Swine Influenza pneumonia during late pregnancy. Arch. Intern. Med. 1990, 150, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Top, F.H., Jr.; Russell, P.K. Swine Influenza A at fort dix, New Jersey (January–February 1976). IV. Summary and speculation. J. Infect. Dis. 1977, 136, 376–380. [Google Scholar] [CrossRef]
- Shortridge, K.; Webster, R. Geographical distribution of swine (Hsw1N1) and Hong Kong (H3N2) influenza virus variants in pigs in Southeast Asia. Intervirology 1979, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gaydos, J.C.; Top, F.H., Jr.; Hodder, R.A.; Russell, P.K. Swine influenza A outbreak, Fort Dix, New Jersey, 1976. Emerg. Infect. Dis. 2006, 12, 23–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Myers, K.P.; Olsen, C.W.; Setterquist, S.F.; Capuano, A.W.; Donham, K.J.; Thacker, E.L.; Merchant, J.A.; Gray, G.C. Are Swine workers in the United States at increased risk of infection with Zoonotic Influenza virus? Clin. Infect. Dis. 2006, 42, 14–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dowdle, W.R.; Hattwick, M.A. Swine Influenza virus infections in humans. J. Infect. Dis. 1977, 136 (Suppl. S3), 386–389. [Google Scholar] [CrossRef] [PubMed]
- Dacso, C.C.; Couch, R.B.; Six, H.R.; Young, J.F.; Quarles, J.M.; Kasel, J.A. Sporadic occurrence of Zoonotic Swine Influenza virus infections. J. Clin. Microbiol. 1984, 20, 833–835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patriarca, P.A.; Kendal, A.P.; Zakowski, P.C.; Cox, N.J.; Trautman, M.S.; Cherry, J.D.; Auerbach, D.M.; McCusker, J.; Belliveau, R.R.; Kappus, K.D. Lack of significant person-to-person spread of Swine Influenza-like virus following fatal infection in an immunocompromised child. Am. J. Epidemiol. 1984, 119, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L.; Hopfensperger, D.J.; Arden, N.H.; Harmon, M.W.; Davis, J.P.; Tipple, M.A.; Schonberger, L.B. Swine Influenza virus infections. Transmission from ill pigs to humans at a Wisconsin agricultural fair and subsequent probable person-to-person transmission. JAMA 1991, 265, 478–481. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.S.; Perofsky, A.C.; Nolting, J.M.; Nelson, M.I.; Bowman, A.S. Tracing the Source of Influenza A Virus Zoonoses in Interconnected Circuits of Swine Exhibitions. J. Infect. Dis. 2021, 224, 458–468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McBride, D.S.; Nolting, J.M.; Nelson, S.W.; Spurck, M.M.; Bliss, N.T.; Kenah, E.; Trock, S.C.; Bowman, A.S. Shortening duration of swine exhibitions to reduce risk for zoonotic transmission of influenza a virus. Emerg. Infect. Dis. 2022, 28, 2035–2042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Jong, J.C.; Paccaud, M.F.; de Ronde-Verloop, F.M.; Huffels, N.H.; Verwei, C.; Weijers, T.F.; Bangma, P.J.; van Kregten, E.; Kerckhaert, J.A.; Wicki, F.; et al. Isolation of Swine-like Influenza A (H1N1) viruses from man in Switzerland and the Netherlands. Ann. Inst. Pasteur Virol. 1988, 139, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Chuvakova, Z.K.; Rovnova, Z.I.; Isaeva, E.I.; Kim, E.V.; Ignat’eva, T.V. 3 cases of isolating the Influenza A virus with human hemagglutinin hsw1 in 1983 in alma-ata. Vopr. Virusol. 1985, 30, 530–536. [Google Scholar]
- Dem’yanenko, I.V.; Chuvakova, Z.K.; Isaeva, E.I. Immunological analysis of surface components of influenza A viruses similar to serovar Hsw1N1 isolated in Almaty in 1984–1985. Vopr Virusol 1987, 5, 533–538. [Google Scholar]
- Dem’yanenko, I.V.; Rovnova, Z.I.; Isaeva, E.I.; Chuvakova, Z.K. Antigenic structure of hemagglutinin of H1N1 influenza viruses (Hsw1N1) isolated from humans and ducks. Vopr Virusol. 1989, 6, 661–665. [Google Scholar]
- Wentworth, D.E.; Thompson, B.L.; Xu, X.; Regnery, H.L.; Cooley, A.J.; McGregor, M.W.; Cox, N.J.; Hinshaw, V.S. An Influenza A (H1N1) virus, closely related to Swine Influenza virus, responsible for a fatal case of human Influenza. J. Virol. 1994, 68, 2051–2058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wentworth, D.E.; McGregor, M.W.; Macklin, M.D.; Neumann, V.; Hinshaw, V.S. Transmission of Swine Influenza virus to humans after exposure to experimentally infected pigs. J. Infect. Dis. 1997, 175, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.W.; Brammer, L.; Easterday, B.C.; Arden, N.; Belay, E.; Baker, I.; Cox, N.J. Serologic evidence of H1 swine Influenza virus infection in swine farm residents and employees. Emerg. Infect. Dis. 2002, 8, 814–819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimura, K.; Adlakha, A.; Simon, P.M. Fatal case of Swine Influenza virus in an immunocompetent host. Mayo Clin. Proc. 1998, 73, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Claas, E.C.; Kawaoka, Y.; de Jong, J.C.; Masurel, N.; Webster, R.G. Infection of children with avian-human reassortant Influenza virus from pigs in Europe. Virology 1994, 204, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Rimmelzwaan, G.F.; de Jong, J.C.; Bestebroer, T.M.; van Loon, A.M.; Claas, E.C.; Fouchier, R.A.; Osterhaus, A.D. Antigenic and genetic characterization of Swine Influenza A (H1N1) viruses isolated from pneumonia patients in the Netherlands. Virology 2001, 282, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Gregory, V.; Lim, W.; Cameron, K.; Bennett, M.; Marozin, S.; Klimov, A.; Hall, H.; Cox, N.; Hay, A.; Lin, Y.P. Infection of a child in Hong Kong by an influenza A H3N2 virus closely related to viruses circulating in European pigs. J. Gen. Virol. 2001, 82 Pt 6, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Bastien, N.; Bowness, D.; Burton, L.; Bontovics, E.; Winter, A.L.; Tipples, G.; Minielly, D.; Gregg, B.; Cramer, C.; Schincariol, C.; et al. Parotitis in a child infected with triple-reassortant influenza A virus in Canada in 2007. J. Clin. Microbiol. 2009, 47, 1896–1898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, J.L.; Lee, B.E.; Patel, J.; Bastien, N.; Grimsrud, K.; Seal, R.F.; King, R.; Marshall, F.; Li, Y. Swine influenza (H3N2) infection in a child and possible community transmission, Canada. Emerg. Infect. Dis. 2007, 13, 1865–1870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cox, C.M.; Neises, D.; Garten, R.J.; Bryant, B.; Hesse, R.A.; Anderson, G.A.; Trevino-Garrison, I.; Shu, B.; Lindstrom, S.; Klimov, A.I.; et al. Swine influenza virus A (H3N2) infection in human, Kansas, USA, 2009. Emerg. Infect. Dis. 2011, 17, 1143–1144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neveau, M.N.; Zeller, M.A.; Kaplan, B.S.; Souza, C.K.; Gauger, P.C.; Vincent, A.L.; Anderson, T.K. Genetic and antigenic characterization of an expanding H3 influenza a virus clade in U.S. swine visualized by Nextstrain. MSphere 2022, 7, e00994. [Google Scholar] [CrossRef]
- Van Reeth, K.; Nicoll, A. A human case of swine influenza virus infection in Europe--implications for human health and research. Eurosurveillance 2009, 14, 19124. [Google Scholar] [CrossRef] [PubMed]
- Gregory, V.; Bennett, M.; Thomas, Y.; Kaiser, L.; Wunderli, W.; Matter, H.; Hay, A.; Lin, Y.P. Human infection by a Swine Influenza A (H1N1) virus in Switzerland. Arch. Virol. 2003, 148, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Adiego Sancho, B.; Omenaca Teres, M.; Martinez Cuenca, S.; Rodrigo Val, P.; Sanchez Villanueva, P.; Casas, I.; Pozo, F.; Perez Brena, P. Human case of swine influenza A (H1N1), Aragon, Spain, November 2008. Eurosurveillance 2009, 14, 19120. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Groth, M.; Krumbholz, A.; Lange, J.; Philipps, A.; Dürrwald, R. Cocirculation of Swine H1N1 Influenza A Virus Lineages in Germany. Viruses 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heider, A.; Wedde, M.; Weinheimer, V.; Döllinger, S.; Monazahian, M.; Dürrwald, R.; Wolff, T.; Schweiger, B. Characteristics of two zoonotic swine influenza A(H1N1) viruses isolated in Germany from diseased patients. Int. J. Med. Microbiol. 2024, 314, 151609. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S. Human Swine Influenza A (h1N1) Infection in the United States. Surveillance and Epidemiology Branch. Centre for Health Protection. Hong Kong Department of Health. 2009. Available online: www.dh.gov.hk/chs/useful/useful_ld/files/ltod20090424.pdf (accessed on 30 July 2025).
- Komadina, N.; Roque, V.; Thawatsupha, P.; Rimando-Magalong, J.; Waicharoen, S.; Bomasang, E.; Sawanpanyalert, P.; Rivera, M.; Iannello, P.; Hurt, A.C.; et al. Genetic analysis of two Influenza A (H1) Swine viruses isolated from humans in Thailand and the Philippines. Virus Genes 2007, 35, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Ikranbegijn, R.; Kuznetsova, T.V.; Grudinin, M.P.; Kiselev, O.I.; Glebova, T.I.; Bajmakhanova, B.B.; Shamenova, M.G.; Pisareva, M.M.; Komissarov, A.B.; Elpaeva, E.A.; et al. Molecular-genetic peculiarities of the pandemic virus H1N1v features which circulated on the territory of Kazakhstan (2009–2010). Vestnik NSU 2012, 10, 80–86. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Swine origin influenza A (H3N2) virus infection in two children--Indiana and Pennsylvania, July-August 2011. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1213–1215. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines-five states, 2011. MMWR Morb. Mortal. Wkly. Rep. 2012, 60, 1741–1744. [Google Scholar] [PubMed]
- Szablewski, C.M.; McBride, D.S.; Trock, S.C.; Habing, G.G.; Hoet, A.E.; Nelson, S.W.; Nolting, J.M.; Bowman, A.S. Evolution of influenza A viruses in exhibition swine and transmission to humans, 2013-2015. Zoonoses Public Health 2024, 71, 281–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Centers for Disease Control and Prevention (CDC). Case count: Detected U.S. Human Infections with H3N2v by State Since August 2011. Atlanta: CDC. 2013. Available online: https://archive.cdc.gov/www_cdc_gov/flu/swineflu/variant/h3n2v-situation.htm (accessed on 3 July 2025).
- Epperson, S.; Jhung, M.; Richards, S.; Quinlisk, P.; Ball, L.; Moll, M.; Boulton, R.; Haddy, L.; Biggerstaff, M.; Brammer, L.; et al. Human infections with influenza A(H3N2) variant virus in the United States, 2011–2012. Clin. Infect. Dis. 2013, 57 (Suppl. S1), 4–11. [Google Scholar] [CrossRef] [PubMed]
- Jhung, M.; Epperson, S.; Biggerstaff, M.; Allen, D.; Balish, A.; Barnes, N.; Beaudoin, A.; Berman, L.; Bidol, S.; Blanton, L.; et al. Outbreak of variant influenza A(H3N2) virus in the United States. Clin. Infect. Dis. 2013, 57, 1703–1712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schicker, R.S.; Rossow, J.; Eckel, S.; Fisher, N.; Bidol, S.; Tatham, L.; Matthews-Greer, J.; Sohner, K.; Bowman, A.S.; Avrill, J.; et al. Outbreak of Influenza A(H3N2) Variant Virus Infections Among Persons Attending Agricultural Fairs Housing Infected Swine-Michigan and Ohio, July-August 2016. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.S.; Walia, R.R.; Nolting, J.M.; Vincent, A.L.; Killian, M.L.; Zentkovich, M.M.; Lorbach, J.N.; Lauterbach, S.E.; Anderson, T.K.; Davis, C.T.; et al. Influenza A(H3N2) Virus in Swine at Agricultural Fairs and Transmission to Humans, Michigan and Ohio, USA, 2016. Emerg. Infect. Dis. 2017, 23, 1551–1555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parys, A.; Vandoorn, E.; King, J.; Graaf, A.; Pohlmann, A.; Beer, M.; Harder, T.; Van Reeth, K. Human Infection with Eurasian Avian-Like Swine Influenza A(H1N1) Virus, the Netherlands, September 2019. Emerg. Infect. Dis. 2021, 27, 939–943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, W.; Zhang, H.; Xiang, X.; Zhong, L.; Yang, L.; Guo, J.; Xie, Y.; Li, F.; Deng, Z.; Feng, H.; et al. Reassortant Eurasian Avian-Like Influenza A(H1N1) Virus from a Severely Ill Child, Hunan Province, China, 2015. Emerg. Infect. Dis. 2016, 22, 1930–1936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, J.; Yi, L.; Jing, Y.; Tan, H.; Mai, W.; Song, Y.; Zou, L.; Liang, L.; Xiao, H.; Kang, M.; et al. A human infection with a novel reassortant H3N2 swine virus in China. J. Infect. 2019, 79, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Liu, C.; Cheng, Y.; Kong, M.; Yang, L.; Zhuang, Z.; Liu, J.; Zou, M.; Dong, X.; et al. Human infection with a novel reassortant Eurasian-avian lineage swine H1N1 virus in northern China. Emerg. Microbes Infect. 2019, 8, 1535–1545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glebova, T.; Klivleyeva, N.; Baimukhametova, A.; Lukmanova, G.; Saktaganov, N.; Ongarbayeva, N.; Baimakhanova, B.; Kassymova, G.; Sagatova, M.; Rachimbayeva, A.; et al. Acute Respiratory and Influenza Viruses Circulating in Kazakhstan During 2018–2024. Pathogens 2025, 14, 493. [Google Scholar] [CrossRef]
- Kydyrmanov, A.; Karamendin, K.; Klivleyeva, N.; Sabyrzhan, T.; Suleimen, B.; Ismailov, E.; Glebova, T. Genome Sequence of Influenza B Epidemic Strain B/Almaty/8/2018. Microbiol. Resour. Announc. 2022, 11, e0054422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, Y.M.; Iannello, P.; Smith, I.; Watson, J.; Barr, I.G.; Daniels, P.; Komadina, N.; Harrower, B.; Wong, F.Y. Transmission of influenza A(H1N1) 2009 pandemic viruses in Australian swine. Influenza Other Respir. Viruses 2012, 6, e42–e47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, D.W.; Barr, I.G.; Loh, R.; Levy, A.; Tempone, S.; O’Dea, M.; Watson, J.; Wong, F.Y.K.; Effler, P.V. Respiratory Illness in a Piggery Associated with the First Identified Outbreak of Swine Influenza in Australia: Assessing the Risk to Human Health and Zoonotic Potential. Trop. Med. Infect. Dis. 2019, 4, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, Y.M.; Wong, F.Y.K.; Spirason, N.; Kaye, M.; Beazley, R.; Grau, M.L.L.; Shan, S.; Stevens, V.; Subbarao, K.; Sullivan, S.; et al. Locally Acquired Human Infection with Swine-Origin Influenza A(H3N2) Variant Virus, Australia, 2018. Emerg. Infect. Dis. 2020, 26, 143–147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Influenza at the Human-Animal Interface: Summary and Assessment, 22 January to 12 February 2019. 2019. Available online: https://cdn.who.int/media/docs/default-source/influenza/human-animal-interface-risk-assessments/influenza_summary_ira_ha_interface_12_02_2019.pdf?sfvrsn=5f7dd172_12.pdf (accessed on 2 April 2019).
- Anjorin, A.A.; Sausy, A.; Muller, C.P.; Hübschen, J.M.; Omilabu, S.A.; Snoeck, C.J. Human Seasonal Influenza Viruses in Swine Workers in Lagos, Nigeria: Consequences for Animal and Public Health. Viruses 2023, 15, 1219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osoro, E.M.; Lidechi, S.; Nyaundi, J.; Marwanga, D.; Mwatondo, A.; Muturi, M.; Ng’ang’a, Z.; Njenga, K. Detection of pandemic influenza A/H1N1/pdm09 virus among pigs but not in humans in slaughterhouses in Kenya, 2013-2014. BMC Res. Notes. 2019, 12, 628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osoro, E.M.; Lidechi, S.; Marwanga, D.; Nyaundi, J.; Mwatondo, A.; Muturi, M.; Ng’aNg’a, Z.; Njenga, K. Seroprevalence of influenza A virus in pigs and low risk of acute respiratory illness among pig workers in Kenya. Environ. Health Prev. Med. 2019, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Resende, P.C.; Junqueira, D.M.; Tochetto, C.; Ogrzewalska, M.; Motta, F.C.; Lopes, J.; Appolinario, L.; Macedo, L.; Caetano, B.; Matos, A.; et al. Zoonotic transmission of novel Influenza A variant viruses detected in Brazil during 2020 to 2023. Nat. Commun. 2024, 15, 10748. [Google Scholar] [CrossRef]
- Dürrwald, R.; Wedde, M.; Biere, B.; Oh, D.Y.; Heßler-Klee, M.; Geidel, C.; Volmer, R.; Hauri, A.M.; Gerst, K.; Thürmer, A.; et al. Zoonotic infection with swine A/H1avN1 influenza virus in a child, Germany, June 2020. Eurosurveillance 2020, 25, 2001638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, H.; Xiao, Y.; Liu, J.; Wang, D.; Li, F.; Wang, C.; Li, C.; Zhu, J.; Song, J.; Sun, H.; et al. Prevalent Eurasian avian-like H1N1 swine influenza virus with 2009 pandemic viral genes facilitating human infection. Proc. Natl. Acad. Sci. USA 2020, 117, 17204–17210, Erratum in: Proc. Natl. Acad. Sci. USA 2020, 117, 23194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersen, K.M.; Vestergaard, L.S.; Nissen, J.N.; George, S.J.; Ryt-Hansen, P.; Hjulsager, C.K.; Krog, J.S.; Skov, M.N.; Alexandersen, S.; Larsen, L.E.; et al. Severe Human Case of Zoonotic Infection with Swine-Origin Influenza A Virus, Denmark, 2021. Emerg. Infect. Dis. 2022, 28, 2561–2564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, J.R.; Kuo, C.Y.; Yu, I.L.; Kung, F.Y.; Wu, F.T.; Lin, J.S.; Liu, M.T. Human infection with a reassortant swine-origin influenza A(H1N2)v virus in Taiwan, 2021. Virol. J. 2022, 19, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Garten, R.J.; Davis, C.T.; Russell, C.A.; Shu, B.; Lindstrom, S.; Balish, A.; Sessions, W.M.; Xu, X.; Skepner, E.; Deyde, V. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009, 325, 197–201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mena, I.; Nelson, M.I.; Quezada-Monroy, F.; Dutta, J.; Cortes-Fernandez, R.; Lara-Puente, J.H.; Castro-Peralta, F.; Cunha, L.F.; Trovao, N.S.; Lozano-Dubernard, B.; et al. Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. eLife 2016, 5, e16777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Domínguez-Cherit, G.; Lapinsky, S.E.; Macias, A.E.; Pinto, R.; Espinosa-Perez, L.; de la Torre, A.; Poblano-Morales, M.; Baltazar-Torres, J.A.; Bautista, E.; Martinez, A.; et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009, 302, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Strategic Action Plan for Pandemic Influenza 2006-2007//WHO/CDC/EPR/GIP/2006. Available online: https://iris.who.int/bitstream/handle/10665/70038/WHO_CDS_EPR_GIP_2006.2a_eng.pdf (accessed on 2 April 2025).
- Nelson, M.I.; Wentworth, D.E.; Culhane, M.R.; Vincent, A.L.; Viboud, C.; LaPointe, M.P.; Lin, X.; Holmes, E.C.; Detmer, S.E. Introductions and evolution of human-origin seasonal influenza A viruses in multinational swine populations. J. Virol. 2014, 88, 10110–10119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, W.; Belisle, S.E.; Mosier, D.; Li, X.; Stigger-Rosser, E.; Liu, Q.; Qiao, C.; Elder, J.; Webby, R.; Katze, M.G.; et al. 2009 pandemic H1N1 influenza virus causes disease and upregulation of genes related to inflammatory and immune responses, cell death, and lipid metabolism in pigs. J. Virol. 2011, 85, 11626–11637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, M.I.; Vincent, A.L. Reverse zoonosis of influenza to swine: New perspectives on the human-animal interface. Trends Microbiol. 2015, 23, 142–153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markin, A.; Ciacci Zanella, G.; Arendsee, Z.W.; Zhang, J.; Krueger, K.M.; Gauger, P.C.; Vincent Baker, A.L.; Anderson, T.K. Reverse-zoonoses of 2009 H1N1 pandemic influenza A viruses and evolution in United States swine results in viruses with zoonotic potential. PLoS Pathog. 2023, 19, e1011476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Global status report on noncommunicable diseases 2010. Available online: https://iris.who.int/bitstream/handle/10665/44579/9789240686458_eng.pdf?sequence=1 (accessed on 2 April 2025).
- Kuroda, M.; Usui, T.; Shibata, C.; Nishigaki, H.; Yamaguchi, T. Possible bidirectional human-swine and subsequent human-human transmission of influenza virus A(H1N1)/2009 in Japan. Zoonoses Public Health 2022, 69, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Jallow, M.M.; Barry, M.A.; Fall, A.; Ndiaye, N.K.; Kiori, D.; Sy, S.; Goudiaby, D.; Niang, M.N.; Fall, G.; Fall, M.; et al. Influenza A Virus in Pigs in Senegal and Risk Assessment of Avian Influenza Virus (AIV) Emergence and Transmission to Human. Microorganisms 2023, 11, 1961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karpova, L.S.; Marinich, I.G.; Popovtseva, N.M.; Stolyarova, T.P. Epidemiology of influenza A/California/07/09 (H1N1) in population of 49 cities in Russia in 2009-2010. J. Microbiol. Epidemiol. Immunobiol. 2011, 88, 14–20. [Google Scholar]
- Nelson, M.I.; Stucker, K.M.; Schobel, S.A.; Trovão, N.S.; Das, S.R.; Dugan, V.G.; Nelson, S.W.; Sreevatsan, S.; Killian, M.L.; Nolting, J.M.; et al. Introduction, Evolution, and Dissemination of Influenza A Viruses in Exhibition Swine in the United States during 2009 to 2013. J. Virol. 2016, 90, 10963–10971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorbach, J.N.; Fitzgerald, T.; Nolan, C.; Nolting, J.M.; Treanor, J.J.; Topham, D.J.; Bowman, A.S. Gaps in serologic immunity against contemporary swine-origin influenza A viruses among healthy individuals in the United States. Viruses 2021, 13, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saenz, R.A.; Hethcote, H.W.; Gray, G.C. Confined animal feeding operations as amplifiers of influenza. Vector Borne Zoonotic Dis. 2006, 6, 338–346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gambhir, M.; Swerdlow, D.L.; Finelli, L.; Van Kerkhove, M.D.; Biggerstaff, M.; Cauchemez, S.; Ferguson, N.M. Multiple contributory factors to the age distribution of disease cases: A modeling study in the context of influenza A(H3N2v). Clin. Infect. Dis. 2013, 57 (Suppl. S1), 23–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rambo-Martin, B.L.; Keller, M.W.; Wilson, M.M.; Nolting, J.M.; Anderson, T.K.; Vincent, A.L.; Bagal, U.R.; Jang, Y.; Neuhaus, E.B.; Davis, C.T.; et al. Influenza A Virus Field Surveillance at a Swine-Human Interface. mSphere 2020, 5, e00822-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. One Health. Available online: https://www.who.int/features/qa/one-health/en/ (accessed on 2 April 2025).
- One Health Initiative. Available online: http://www.onehealthinitiative.com (accessed on 3 September 2018).
- Gray, G.C.; Baker, W.S. The importance of including swine and poultry workers in influenza vaccination programs. Clin. Pharmacol. Ther. 2007, 82, 638–641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gray, G.C.; Kayali, G. Facing pandemic influenza threats: The importance of including poultry and swine workers in preparedness plans. Poult. Sci. 2009, 88, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.C.; Trampel, D.W.; Roth, J.A. Pandemic influenza planning: Shouldn’t swine and poultry workers be included? Vaccine 2007, 25, 4376–4381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Webster, R.G. The importance of animal influenza for human disease. Vaccine 2002, 20 (Suppl. S2), 16–20. [Google Scholar] [CrossRef] [PubMed]
- Lorbach, J.N.; Nelson, S.W.; Lauterbach, S.E.; Nolting, J.M.; Kenah, E.; McBride, D.S.; Culhane, M.R.; Goodell, C.; Bowman, A.S. Influenza Vaccination of Swine Reduces Public Health Risk at the Swine-Human Interface. mSphere 2021, 6, e0117020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Year | Continents and Countries | Influenza A Virus Transmitted from Swine to Humans | References |
|---|---|---|---|
| 1958 | Europe: Czechoslovakia | H1N1 | [78,79,80] |
| 1970–1980 | America: Tennessee, Wisconsin, Virginia, New Jersey, Missouri, Minnesota, Texas | H1N1 | [78,81,82,83,84,85,86,87,88,89,90,91,92] |
| 1981–1990 | America: Wisconsin, Nevada Europe: Bulgaria, Switzerland, Netherlands Asia: Kazakhstan | H1N1, cH1N1 | [78,86,93,94,95,96,97,98,99,100] |
| 1991–2000 | America: Maryland, Wisconsin, Minnesota Europe: Netherlands Asia: Hong Kong | H1N1, H3N2 | [101,102,103,104,105,106,107] |
| 2001–2010 | America: Canada, Kansas Europe: Switzerland, Spain, Germany Asia: Hong Kong, Philippine, Thailand, Kazakhstan | H1N1, (H1N1)v, (H1N1)pdm09, H1N2, H3N2, (H3N2)v | [108,109,110,111,112,113,114,115,116,117,118,119] |
| 2011–2020 | America: Indiana, Pennsylvania, Atlanta, Hawaii, Illinois, Maryland, Michigan, Minnesota, Ohio, West Virginia, Wisconsin, New Jersey and Iowa Europe: Germany, Netherlands Asia: China, Kazakhstan Australia: Queensland, Melbourne, Victoria, Western Australia, Perth, South Australia Africa: Kenya, Nigeria | H1N1, (H1N1)pdm09 (H3N2)v, H1N2, H3N2, (H3N2)v | [25,26,27,116,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140] |
| 2020–2024 | America: Brazil, Paraná Europe: Germany, Denmark Asia: Taiwan | H1N1, (H1N1)v, (H1N1)pdm09, A/sw/H1avN1 1C.2.2, (H1N2)v (H3N2)v | [25,26,27,132,133,141,142,143,144,145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klivleyeva, N.; Glebova, T.; Saktaganov, N.; Webby, R. Cases of Interspecies Transmission of Influenza A Virus from Swine to Humans. Vet. Sci. 2025, 12, 873. https://doi.org/10.3390/vetsci12090873
Klivleyeva N, Glebova T, Saktaganov N, Webby R. Cases of Interspecies Transmission of Influenza A Virus from Swine to Humans. Veterinary Sciences. 2025; 12(9):873. https://doi.org/10.3390/vetsci12090873
Chicago/Turabian StyleKlivleyeva, Nailya, Tatyana Glebova, Nurbol Saktaganov, and Richard Webby. 2025. "Cases of Interspecies Transmission of Influenza A Virus from Swine to Humans" Veterinary Sciences 12, no. 9: 873. https://doi.org/10.3390/vetsci12090873
APA StyleKlivleyeva, N., Glebova, T., Saktaganov, N., & Webby, R. (2025). Cases of Interspecies Transmission of Influenza A Virus from Swine to Humans. Veterinary Sciences, 12(9), 873. https://doi.org/10.3390/vetsci12090873