The Passive Immunoprotective Activity Using Egg Yolk IgY Antibodies of Live or Inactivated Aeromonas veronii Against Major Pathogenic Bacteria (A. veronii and A. hydrophila) in Fish
Simple Summary
Abstract
1. Introduction
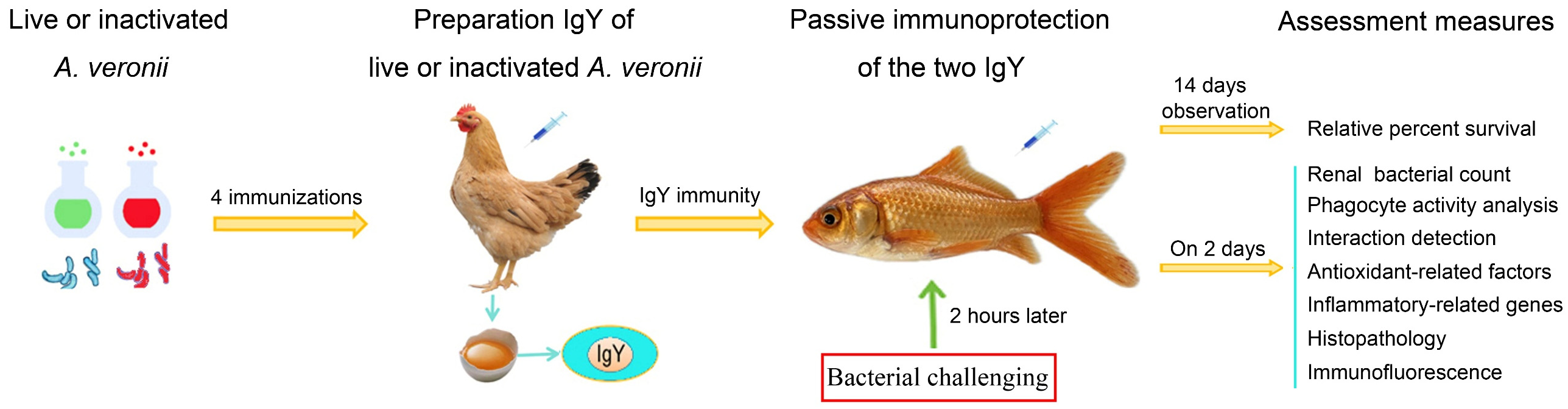
2. Materials and Methods
2.1. Bacterial Strains
2.2. Animals and Breeding
2.3. Preparation of IgY Antibodies
2.4. The Detection of In Vitro Interactions of IgY or C. auratus Serum with Pathogenic Bacteria
2.5. Median Lethal Dose (LD50) of A. veronii or A. hydrophila in C. auratus
2.6. Passive Protection and Passive Cross-Protection Rate of IgY Antibodies
2.7. Renal Bacterial Count
2.8. Analysis of the Phagocyte Phagocytic Activity
2.9. Analysis of Antioxidant Factors
2.10. mRNA Expression of Inflammatory Factors
2.11. Tissue Pathological Analysis
2.12. Renal Immunofluorescence Analysis
2.13. Statistical Analysis
3. Results
3.1. The Passive Protection and Passive Cross-Protection Rates of IgY Antibodies Against C. auratus
3.2. Determination of Bacterial Counts in the Kidney of C. auratus
3.3. Detection of Phagocytic Activity of Phagocyte from C. auratus
3.4. Detection of Antioxidant-Related Factors (SOD, CAT, and GSH-Px) in the Serum of C. auratus
3.5. Detection of the Expression of Inflammation-Related Genes in C. auratus
3.6. The Detection of In Vitro Interactions of IgY or C. auratus Serum with Pathogenic Bacteria
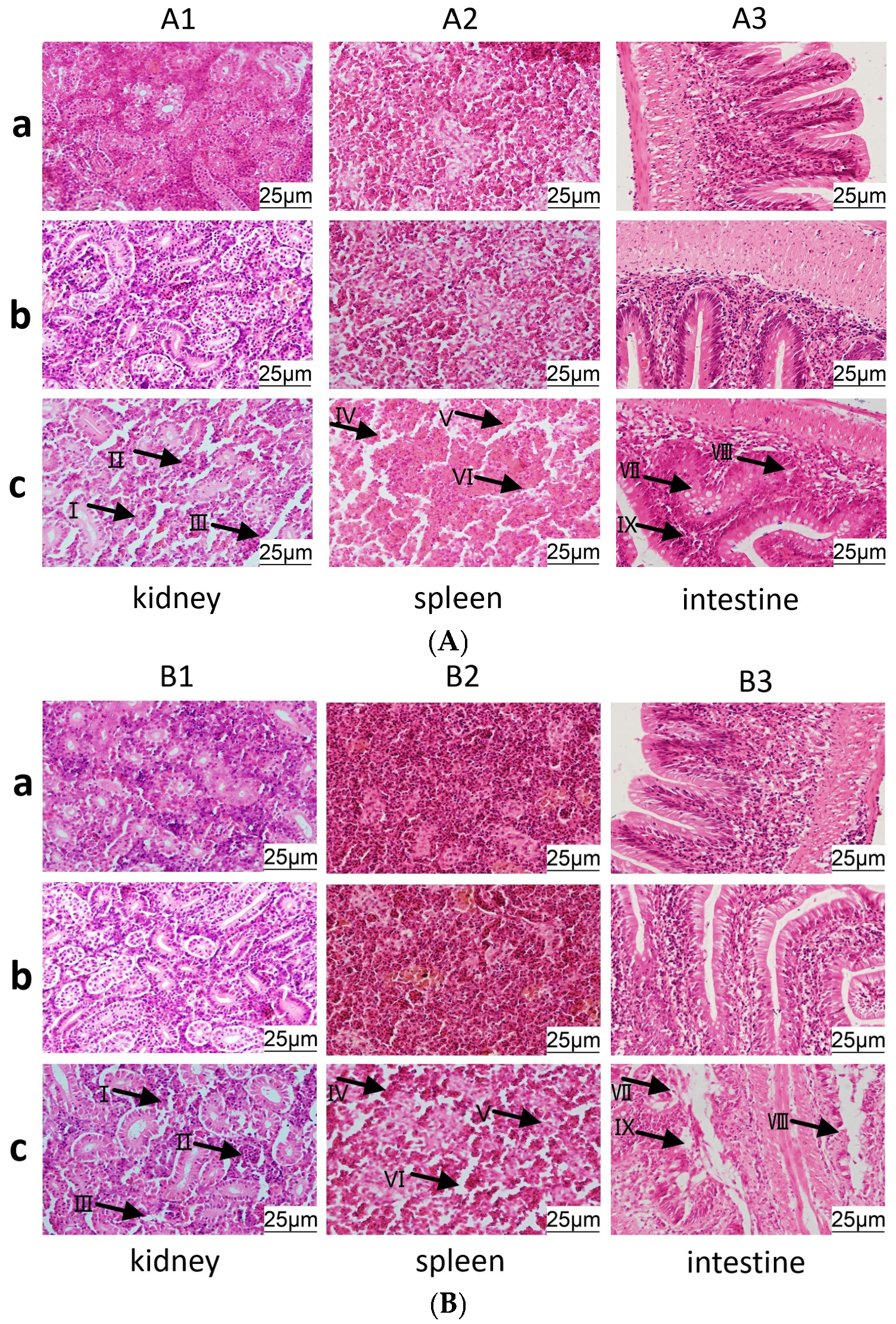
3.7. Histopathological Observation of C. auratus Tissue Morphology
3.8. Immunofluorescence Analysis on Kidney Tissues of C. auratus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.Á.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The Future of Food from the Sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef]
- Sasikumar, R.; Saranya, S.; Lourdu Lincy, L.; Thamanna, L.; Chellapandi, P. Genomic Insights into Fish Pathogenic Bacteria: A Systems Biology Perspective for Sustainable Aquaculture. Fish Shellfish Immunol. 2024, 154, 109978. [Google Scholar] [CrossRef]
- Du, H.F.; Zhang, Y.H.; Zhang, M.; Liu, Q.A.; Zhu, H.J.; Cao, F. Marine Fungal Metabolites as a Source of Drug Leads against Aquatic Pathogens. Appl. Microbiol. Biotechnol. 2022, 106, 3337–3350. [Google Scholar] [CrossRef]
- Liu, Y.M.; Li, X.T.; Zhang, C.Y.; Li, C.H.; Wang, H.Y.; Zhang, D.X.; Zhang, L.; Sun, W.-W.; Tao, L.T.; Shan, X.F. IgT-Mediated Mucosal Immunity and Microbiota Dynamics in Snakehead (Channa argus) Post Aeromonas veronii TH0426 and Aeromonas hydrophila TPS Infection: Implications for Aquaculture Disease Management. Int. Microbiol. 2024, 28, 777–793. [Google Scholar] [CrossRef]
- Du, Y.; Hu, X.; Miao, L.; Chen, J. Current Status and Development Prospects of Aquatic Vaccines. Front. Immunol. 2022, 13, 1040336. [Google Scholar] [CrossRef]
- Ma, R.; Yang, Y.; Cao, H.; Li, P. Editorial: Aquaculture Animal Diseases: Pathogens and Control. Front. Cell. Infect. Microbiol. 2023, 13, 1223046. [Google Scholar] [CrossRef]
- Cedeño-Muñoz, J.S.; Aransiola, S.A.; Reddy, K.V.; Ranjit, P.; Victor-Ekwebelem, M.O.; Oyedele, O.J.; Pérez-Almeida, I.B.; Maddela, N.R.; Rodríguez-Díaz, J.M. Antibiotic Resistant Bacteria and Antibiotic Resistance Genes as Contaminants of Emerging Concern: Occurrences, Impacts, Mitigations and Future Guidelines. Sci. Total Environ. 2024, 952, 175906. [Google Scholar] [CrossRef]
- Wu, D.; Bai, H.; He, L.Y.; He, L.X.; Gao, F.Z.; Liu, C.X.; Van den Brink, P.J.; Smidt, H.; Ying, G.G. From river to groundwater: Antibiotics Pollution, Resistance Prevalence, and Source Tracking. Environ. Int. 2025, 196, 109305. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Korma, S.A.; Salem, H.M.; Abd, E.T.A.; Alkafaas, S.S.; Elsalahaty, M.I.; Elkafas, S.S.; Mosa, W.F.A.; Ahmed, A.E.; et al. Garlic Bioactive Substances and Their Therapeutic Applications for Improving Human Health: A Comprehensive Review. Front. Immunol. 2024, 15, 1277074. [Google Scholar] [CrossRef]
- Qin, X.; Wu, Y.; Zhao, Y.; Qin, S.; Ji, Q.; Jia, J.; Huo, M.; Zhao, X.; Ma, Q.; Wang, X.; et al. Revealing Active Constituents within Traditional Chinese Medicine Used for Treating Bacterial Pneumonia, with Emphasis on the Mechanism of Baicalein against Multi-Drug Resistant Klebsiella Pneumoniae. J. Ethnopharmacol. 2024, 321, 117488. [Google Scholar] [CrossRef]
- He, P.; Cui, W.; Peng, L. Biocontrol Efficacy of Bacillus Velezensis HC-8 against Powdery Mildew of Honeysuckle Caused by Erysiphe lonicerae Var. Lonicerae. Biol. Control 2022, 166, 104834. [Google Scholar] [CrossRef]
- Kisa, O.; Oksuz, L.; Servi, H.; Aysal, A.I. Antibacterial Activity of Hypericum perforatum L. (St. John’s Wort) Extracts against Gram-Positive Bacteria and Characterisation of Its Secondary Metabolites. Nat. Prod. Res. 2025, 39, 1019–1026. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, Y.; Li, M.; Chen, Z.; Wu, Z.; Ji, W.; Wang, J.; Zhang, Y. Structural Elucidation and Immunomodulatory Activities in Vitro of Type I and II Arabinogalactans from Different Origins of Astragalus membranaceus. Carbohydr. Polym. 2024, 333, 121974. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Chen, Y.; Liu, Q.; Zhou, S.; Li, N.; Wu, Y.; Yuan, J. Houttuynia cordata Thunb. Alleviates Inflammatory Bowel Disease by Modulating Intestinal Microenvironment: A research review. Front. Immunol. 2023, 14, 1306375. [Google Scholar] [CrossRef]
- Kayser, V.; Ramzan, I. Vaccines and Vaccination: History and Emerging Issues. Hum. Vaccines Immunother. 2021, 17, 5255–5268. [Google Scholar] [CrossRef]
- Miryala, K.R.; Swain, B. Advances and Challenges in Aeromonas hydrophila Vaccine Development: Immunological Insights and Future Perspectives. Vaccines 2025, 13, 202. [Google Scholar] [CrossRef]
- Lee, W.; Syed Atif, A.; Tan, S.C.; Leow, C.H. Insights into the Chicken IgY with Emphasis on the Generation and Applications of Chicken Recombinant Monoclonal Antibodies. J. Immunol. Methods 2017, 447, 71–85. [Google Scholar] [CrossRef]
- Eriksson, M.; Nylén, S.; Grönvik, K.O. Passive Immunization of Mice with IgY Anti-H5N1 Protects against Experimental Influenza Virus Infection and Allows Development of Protective Immunity. Vaccine 2024, 42, 126133. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, H.; Chao, J.; Jian, S.; Wu, X.; Lu, J.; Wang, J.; Chen, C.; Liu, Y. Polyvalent Passive Vaccine Candidates from Egg Yolk Antibodies (IgY) of Important Outer Membrane Proteins (PF1380 and ExbB) of Pseudomonas fluorescens in Fish. Fish Shellfish Immunol. 2023, 143, 109211. [Google Scholar] [CrossRef]
- Mira, A.; Garro, C.J.; de Alba, P.; Monti, D.; Lang, M.C.; Vivas, A.; Medina, E.; Franco, J.C.; Gutierrez, Á.; Schnittger, L.; et al. P23-Specific IgY Significantly Reduces Diarrhea and Oocyst Shedding in Calves Experimentally Infected with Cryptosporidium parvum. Vaccines 2025, 13, 162. [Google Scholar] [CrossRef]
- Liang, Z.; Ning, Y.; Cao, J.; Liu, S.; Liang, X.; Peng, X.; Huang, Y.; Wei, J.; Xiao, S.; Qin, Q.; et al. The Protective Effect of Specific Yolk Antibody against Nervous Necrosis Virus Infection in Mandarin Fish (Siniperca chuatsi). Fish Shellfish Immunol. 2024, 155, 109996. [Google Scholar] [CrossRef] [PubMed]
- Cova, L. DNA-Designed Avian IgY Antibodies: Novel Tools for Research, Diagnostics and Therapy. J. Clin. Virol. 2005, 34, 70–74. [Google Scholar] [CrossRef]
- Yakhkeshi, S.; Wu, R.; Chelliappan, B.; Zhang, X. Trends in Industrialization and Commercialization of IgY Technology. Front. Immunol. 2022, 13, 991931. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, Y.; Liu, A.; Lei, S.; He, P. Preparation of Bispecific IgY-scFvs Inhibition Adherences of Enterotoxigenic Escherichia coli (K88 and F18) to Porcine IPEC-J2 Cell. Int. J. Mol. Sci. 2024, 25, 3638. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Duan, S.; Cui, P.; Chen, J.; Che, X.; Lu, J.; Wang, J.; Zhu, G.; Liu, Y.; Liu, X. Polyvalent Immunoprotection of Protein, DNA and IgY Antibody Vaccines of Vibrio fluvialis Outer Membrane Protein VF08100 in Fish. Fish Shellfish Immunol. 2025, 161, 110260. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, J.; Cui, P.; Che, X.; Wu, X.; Lu, J.; Zhu, G.; Liu, Y.; Liu, X. Evaluation of the Multivalent Immunoprotective Effects of Protein, DNA, and IgY Vaccines Against Vibrio fluvialis Outer Membrane Protein VF14355 in Carassius auratus. Int. J. Mol. Sci. 2025, 26, 3379. [Google Scholar] [CrossRef]
- Ren, H.; Yang, W.; Thirumalai, D.; Zhang, X.; Schade, R. A Comparative Evaluation of Six Principal IgY Antibody Extraction Methods. Altern. Lab. Anim. 2016, 44, 11–20. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Mousavi Gargari, S.L.; Talei, D. Anti-Flagellin IgY Antibodies Protect against Pseudomonas aeruginosa Infection in Both Acute Pneumonia and Burn Wound Murine Models in a Non-Type-Specific Mode. Mol. Immunol. 2021, 136, 118–127. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, H.; Cui, P.; Chen, J.; Chao, J.; Wu, X.; Lu, J.; Zhang, X.; Xu, G.; Liu, Y. Differential Polyvalent Passive Immune Protection of Egg Yolk Antibodies (IgY) against Live and Inactivated Vibrio fluvialis in Fish. Fish Shellfish Immunol. 2024, 151, 109751. [Google Scholar] [CrossRef]
- Rabb, H.; Mendiola, C.C.; Dietz, J.; Saba, S.R.; Issekutz, T.B.; Abanilla, F.; Bonventre, J.V.; Ramirez, G. Role of CD11a and CD11b in Ischemic Acute Renal Failure in Rats. Am. J. Physiol. 1994, 267, 1052–1058. [Google Scholar] [CrossRef]
- Deng, W.; Abliz, A.; Xu, S.; Sun, R.Z.; Guo, W.Y.; Shi, Q.; Yu, J.; Wang, W.X. Severity of Pancreatitis-associated Intestinal Mucosal Barrier Injury is Reduced Following Treatment with the NADPH Oxidase Inhibitor Apocynin. Mol. Med. Rep. 2016, 14, 3525–3534. [Google Scholar] [CrossRef]
- Eriksson, M.; Larsson, A. Avian Antibodies as Potential Therapeutic Tools. Antibodies 2025, 14, 18. [Google Scholar] [CrossRef]
- Calvert, R.A.; Nyamboya, R.A.; Beavil, A.J.; Sutton, B.J. The Evolution of Flexibility and Function in the Fc Domains of IgM, IgY, and IgE. Front. Immunol. 2024, 15, 1389494. [Google Scholar] [CrossRef]
- El-Kafrawy, S.A.; Abbas, A.T.; Oelkrug, C.; Tahoon, M.; Ezzat, S.; Zumla, A.; Azhar, E.I. IgY Antibodies: The Promising Potential to Overcome Antibiotic Resistance. Front. Immunol. 2023, 14, 1065353. [Google Scholar] [CrossRef]
- Zhang, L.; Hong, Y.; Sun, K.; Zhao, S.; Bai, Y.; Yang, S.; Tao, J.; Shi, F.; Zhan, F.; Lin, L.; et al. Passive Protection of Chicken Egg Yolk Immunoglobulin (IgY) against Streptococcus agalactiae Infection in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2024, 154, 109923. [Google Scholar] [CrossRef]
- Klobuch, S.; Seijkens, T.T.P.; Schumacher, T.N.; Haanen, J.B.A.G. Tumour-Infiltrating Lymphocyte Therapy for Patients with Advanced-Stage Melanoma. Nat. Rev. Clin. Oncol. 2024, 21, 173–184. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, T.; Su, L.; Wang, H.; Zhang, B.; Su, Y. Effects of srtA Variation on Phagocytosis Resistance and Immune Response of Streptococcus equi. Infect. Genet. Evol. 2021, 89, 104732. [Google Scholar] [CrossRef]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond Binding: Antibody Effector Functions in Infectious Diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef]
- van ’t Land, F.R.; Willemsen, M.; Bezemer, K.; van der Burg, S.H.; van den Bosch, T.P.P.; Doukas, M.; Fellah, A.; Kolijn, P.M.; Langerak, A.W.; Moskie, M.; et al. Dendritic Cell-Based Immunotherapy in Patients with Resected Pancreatic Cancer. J. Clin. Oncol. 2024, 42, 3083–3093. [Google Scholar] [CrossRef]
- Chen, D.; Yu, J.; Wang, J.; Li, Z.; Dong, C.; Liang, Y.; Huang, Y.; Chen, Y.; Xue, T.; Lin, C. IgY Antibodies as A Non-antibiotic Approach to Combat Vibrio vulnificus Infection and Gut Microbiota Dysbiosis in Zebrafish. Fish Shellfish Immunol. 2025, 166, 110637. [Google Scholar] [CrossRef]
- Rizkiantino, R.; Pasaribu, F.H.; Soejoedono, R.D.; Arnafia, W.; Reisinta, D.; Yadiansyah, R.I.; Halalludin, B.; Ardini, Y.; Khanaria, G.; Wibawan, I.W.T. Chicken Enterococcus faecalis-induced Immunoglobulin Y as A Prophylactic and Therapeutic Agent against streptococcosis in Red Tilapia (Oreochromis hybrid). Vet. World 2023, 16, 175–186. [Google Scholar] [CrossRef]
- Vuscan, P.; Kischkel, B.; Joosten, L.A.B.; Netea, M.G. Trained Immunity: General and Emerging Concepts. Immunol. Rev. 2024, 323, 164–185. [Google Scholar] [CrossRef]
- Fajrin, F.A.; Sulistyowaty, M.I.; Ghiffary, M.L.; Zuhra, S.A.; Panggalih, W.R.; Pratoko, D.K.; Christianty, F.M.; Matsunami, K.; Indrianingsih, A.W. Immunomodulatory Effect from Ethanol Extract and Ethyl Acetate Fraction of Curcuma heyneana Valeton and Zijp: Transient Receptor Vanilloid Protein Approach. Heliyon 2023, 9, e15582. [Google Scholar] [CrossRef] [PubMed]
- Bunnoy, A.; Thompson, K.D.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Pirarat, N.; Kitiyodom, S.; Thangsunan, P.; Sukkarun, P.; Prukbenjakul, P.; et al. Development of a Bivalent Mucoadhesive Nanovaccine to Prevent Francisellosis and Columnaris Diseases in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2023, 138, 108813. [Google Scholar] [CrossRef] [PubMed]
- Hayrapetyan, H.; Tran, T.; Tellez-Corrales, E.; Madiraju, C. Enzyme-Linked Immunosorbent Assay: Types and Applications. Methods Mol. Biol. 2023, 2612, 1–17. [Google Scholar]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, Oxidative Stress and Degenerative Diseases: Mechanisms, Complications and Emerging Therapeutic Strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, G.; Liu, P.; Hu, Y.; Chen, Y.; Fang, Y.; Sun, G.; Huang, H.; Wu, J. Hyaluronic Acid-Based Glucose-Responsive Antioxidant Hydrogel Platform for Enhanced Diabetic Wound Repair. Acta Biomater. 2022, 147, 147–157. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Amador-Martínez, I.; Medina-Campos, O.N.; Garcia-Garcia, M.; Bernabe-Yepes, B.; León-Contreras, J.C.; Hernández-Pando, R.; Aparicio-Trejo, O.E.; Sánchez-Lozada, L.G.; et al. Sulforaphane Protects from Kidney Damage during the Release of Unilateral Ureteral Obstruction (RUUO) by Activating Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2): Role of Antioxidant, Anti-Inflammatory, and Antiapoptotic Mechanisms. Free Radic. Biol. Med. 2024, 212, 49–64. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, Y.; Tao, J.; Yang, S.; Tu, C.; Liu, L.; Huang, X.; Li, L.; Qin, Z. Effects of Feeding Chicken Egg Yolk Antibodies on Intestinal Cell Apoptosis, Oxidative Stress and Microbial Flora of Tilapia (Oreochromis niloticus) Infected with Streptococcus agalactiae. Fish Shellfish Immunol. 2024, 150, 109596. [Google Scholar] [CrossRef]
- Chadda, K.R.; Puthucheary, Z. Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS): A Review of Definitions, Potential Therapies, and Research Priorities. Br. J. Anaesth. 2024, 132, 507–518. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Qiao, M.; Sun, X.; Li, G. Resveratrol Alleviates Inflammation and ER Stress Through SIRT1/NRF2 to Delay Ovarian Aging in a Short-Lived Fish. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 596–602. [Google Scholar] [CrossRef]
- Shi, X.; Xu, T.; Gao, M.; Bi, Y.; Wang, J.; Yin, Y.; Xu, S. Combined Exposure of Emamectin Benzoate and Microplastics Induces Tight Junction Disorder, Immune Disorder and Inflammation in Carp Midgut via Lysosome/ROS/Ferroptosis Pathway. Water Res. 2024, 257, 121660. [Google Scholar] [CrossRef]
- Al-Kharashi, L.A.; Alqarni, S.A.; Ahmad, S.F.; Al-Harbi, N.O.; Alsanea, S.; Ibrahim, K.E.; Algahtani, M.M.; Alhazzani, K.; Shazly, G.A.; Al-Harbi, M.M.; et al. BALB/c and C57BL/6 Mice Differ in Oxidant and Antioxidant Responses in Innate and Adaptive Immune Cells in an Asthma Model Induced by Cockroach Allergens. Int. Immunopharmacol. 2023, 124, 110892. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Asp. Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, R.; Han, L.; Kuerban, K.; Ye, L.; Pan, S.; Li, S.; Yuan, Y. Activation of Autophagy Reverses Gemcitabine-Induced Immune Inhibition of RAW264.7 Macrophages by Promoting TNF-alpha, IL-6 and MHC-II Expression. Immunol. Res. 2021, 69, 352–362. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Balasundaram, C.; Arockiaraj, J.; Jagruthi, C. Efficacy of Ulvan on Immune Response and Immuno-Antioxidant Gene Modulation in Labeo rohita against Columnaris Disease. Fish Shellfish Immunol. 2021, 117, 262–273. [Google Scholar] [CrossRef]
- Cui, A.; Huang, T.; Li, S.; Ma, A.; Pérez, J.L.; Sander, C.; Keskin, D.B.; Wu, C.J.; Fraenkel, E.; Hacohen, N. Dictionary of Immune Responses to Cytokines at Single-Cell Resolution. Nature 2024, 625, 377–384. [Google Scholar] [CrossRef]
- Malhotra, S.; Hayes, D.J.; Wozniak, D.J. Cystic Fibrosis and Pseudomonas aeruginosa: The Host-Microbe Interface. Clin. Microbiol. Rev. 2019, 32, e00138-18. [Google Scholar] [CrossRef]
- Neurath, M.F. Strategies for Targeting Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2024, 24, 559–576. [Google Scholar] [CrossRef]
- Abhiraman, G.C.; Householder, K.D.; Rodriguez, G.E.; Glassman, C.R.; Saxton, R.A.; Breuer, C.B.; Wilson, S.C.; Su, L.; Yen, M.; Hsu, C.; et al. Redirecting Immune Signaling with Cytokine Adaptors. Nat. Commun. 2025, 16, 2432. [Google Scholar] [CrossRef] [PubMed]
- Latonen, L.; Koivukoski, S.; Khan, U.; Ruusuvuori, P. Virtual Staining for Histology by Deep Learning. Trends Biotechnol. 2024, 42, 1177–1191. [Google Scholar] [CrossRef]
- Fu, Z.; Shen, X.; Deng, C.; Cao, H.; Jin, Y.; Zheng, Q.; Yang, Y.; Qian, B.; Yuan, C.; Wang, W.; et al. Prediction of the Pathological Subtypes by Intraoperative Frozen Section for Patients with cT1N0M0 Invasive Lung Adenocarcinoma (ECTOP-1015): A Prospective Multicenter Study. Int. J. Surg. 2024, 110, 5444–5451. [Google Scholar] [CrossRef]
- Qiao, N.; Swearingen, B.; Hedley-Whyte, E.T.; Tritos, N.A. The Utility of Intraoperative Cytological Smear and Frozen Section in the Surgical Management of Patients with Cushing’s Disease Due to Pituitary Microadenomas. Endocr. Pathol. 2019, 30, 180–188. [Google Scholar] [CrossRef]
- Getnet, M.A.; Mekonnen, M.Y.; Yimam, H.M.; Berihun, A.M.; Malede, B.A. Histopathology Based Study of Nile Tilapia Fish (Oreochromis niloticus) as a Biomarker for Water Pollution Evaluation in the Southern Gulf of Lake Tana, Ethiopia. BMC Vet. Res. 2024, 20, 409. [Google Scholar] [CrossRef] [PubMed]
- Harms, P.W.; Frankel, T.L.; Moutafi, M.; Rao, A.; Rimm, D.L.; Taube, J.M.; Thomas, D.; Chan, M.P.; Pantanowitz, L. Multiplex Immunohistochemistry and Immunofluorescence: A Practical Update for Pathologists. Mod. Pathol. 2023, 36, 100197. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Liu, T.; Li, Y.; Wang, Y.; Hu, W.; Zhu, Z.; Sun, Y. Identification of Fish Spermatogenic Cells through High-Throughput Immunofluorescence against Testis with an Antibody Set. Front. Endocrinol. 2023, 14, 1044318. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Deng, Y.; Feng, J.; Liu, J.; Yang, M.; Pu, Z.; Zhang, S.; Wu, Z.; Ji, N.; Park, D.M.; et al. NAD+ Metabolic Enzyme Inhibitor as Radiosensitizer for Malignant Meningioma and Its Modulation of P53 Expression. Mol. Cancer Ther. 2024, 23, 1586–1596. [Google Scholar] [CrossRef]
- Kumar, P.; Hassan, M.; Tacke, F.; Engelmann, C. Delineating the Heterogeneity of Senescence-induced-functional Alterations in Hepatocytes. Cell. Mol. Life Sci. 2024, 81, 200. [Google Scholar] [CrossRef]






| Bacteria | IgY Antibody | No. | Survival (no.) | Death (no.) | Cumulative Mortality Rate (%) | RPS (%) | RPS (p-Value) |
|---|---|---|---|---|---|---|---|
| A. veronii | NC | 15 | 0 | 15 | 100 | -- | -- |
| NE-C | 15 | 0 | 15 | 100 | 0 | -- | |
| Live bacteria | 15 | 9 | 6 | 40.00 | 60.00 ** | 0.0007 | |
| Inactivated bacteria | 15 | 8 | 7 | 46.67 | 53.33 ** | 0.0003 | |
| A. hydrophila | NC | 15 | 2 | 13 | 86.67 | -- | -- |
| NE-C | 15 | 1 | 14 | 93.33 | −7.68 | 0.5430 | |
| Live bacteria | 15 | 12 | 3 | 20.00 | 76.92 ** | 0.0002 | |
| Inactivated bacteria | 15 | 8 | 7 | 46.47 | 46.15 * | 0.018 |
| Bacteria | Group (IgY) | PP% | PI% | PP% (p-Value) | PI% (p-Value) |
|---|---|---|---|---|---|
| Challenged with A. veronii | Control | 31.53 ± 3.01 a | 70.27 ± 14.10 a | -- | -- |
| Live bacteria | 60.80 ± 2.03 c | 130.40 ± 13.21 b | 0.0005 | 0.0004 | |
| Inactivated bacteria | 50.70 ± 1.27 b | 142.65 ± 8.34 b | 0.0004 | 0.0003 | |
| Challenged with A. hydrophila | Control | 32.35 ± 1.36 a | 71.32 ± 7.36 a | -- | -- |
| Live bacteria | 58.55 ± 0.89 c | 130.30 ± 4.70 b | 0.0006 | 0.0005 | |
| Inactivated bacteria | 52.38 ± 3.00 b | 139.50 ± 20.40 b | 0.0003 | 0.0004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Cui, P.; Xiao, H.; Wu, X.; Lu, J.; Liu, Y.; Liu, X. The Passive Immunoprotective Activity Using Egg Yolk IgY Antibodies of Live or Inactivated Aeromonas veronii Against Major Pathogenic Bacteria (A. veronii and A. hydrophila) in Fish. Vet. Sci. 2025, 12, 831. https://doi.org/10.3390/vetsci12090831
Chen J, Cui P, Xiao H, Wu X, Lu J, Liu Y, Liu X. The Passive Immunoprotective Activity Using Egg Yolk IgY Antibodies of Live or Inactivated Aeromonas veronii Against Major Pathogenic Bacteria (A. veronii and A. hydrophila) in Fish. Veterinary Sciences. 2025; 12(9):831. https://doi.org/10.3390/vetsci12090831
Chicago/Turabian StyleChen, Jing, Pan Cui, Huihui Xiao, Xiaoqing Wu, Juan Lu, Yong Liu, and Xiang Liu. 2025. "The Passive Immunoprotective Activity Using Egg Yolk IgY Antibodies of Live or Inactivated Aeromonas veronii Against Major Pathogenic Bacteria (A. veronii and A. hydrophila) in Fish" Veterinary Sciences 12, no. 9: 831. https://doi.org/10.3390/vetsci12090831
APA StyleChen, J., Cui, P., Xiao, H., Wu, X., Lu, J., Liu, Y., & Liu, X. (2025). The Passive Immunoprotective Activity Using Egg Yolk IgY Antibodies of Live or Inactivated Aeromonas veronii Against Major Pathogenic Bacteria (A. veronii and A. hydrophila) in Fish. Veterinary Sciences, 12(9), 831. https://doi.org/10.3390/vetsci12090831




