Molecular Survey for Major Canine Enteric Viral Pathogens in Wild Carnivores, Northwestern Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Nucleic Acids Extraction and Molecular Investigations
2.3. SISPA, ONT Library Preparation and Sequencing
3. Results
3.1. Molecular Screening and Sanger Sequencing
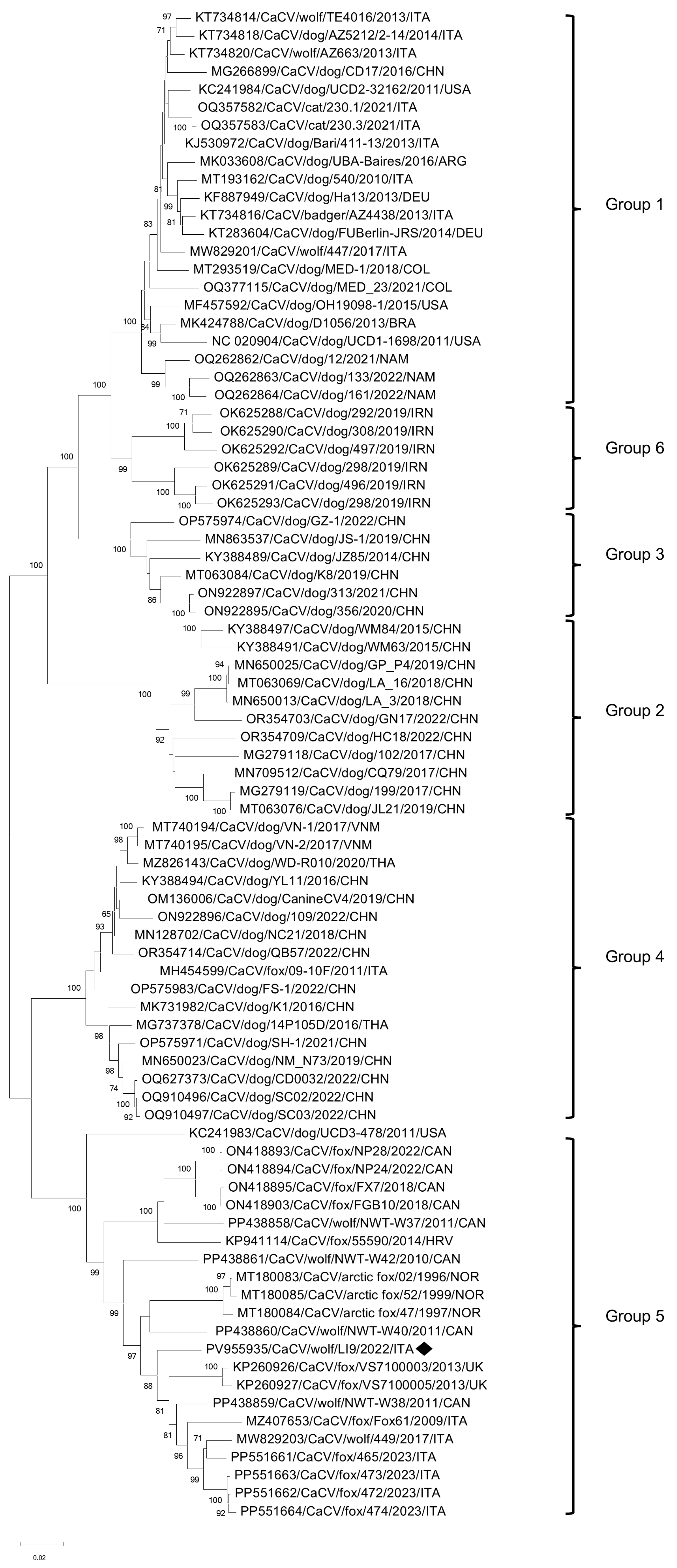
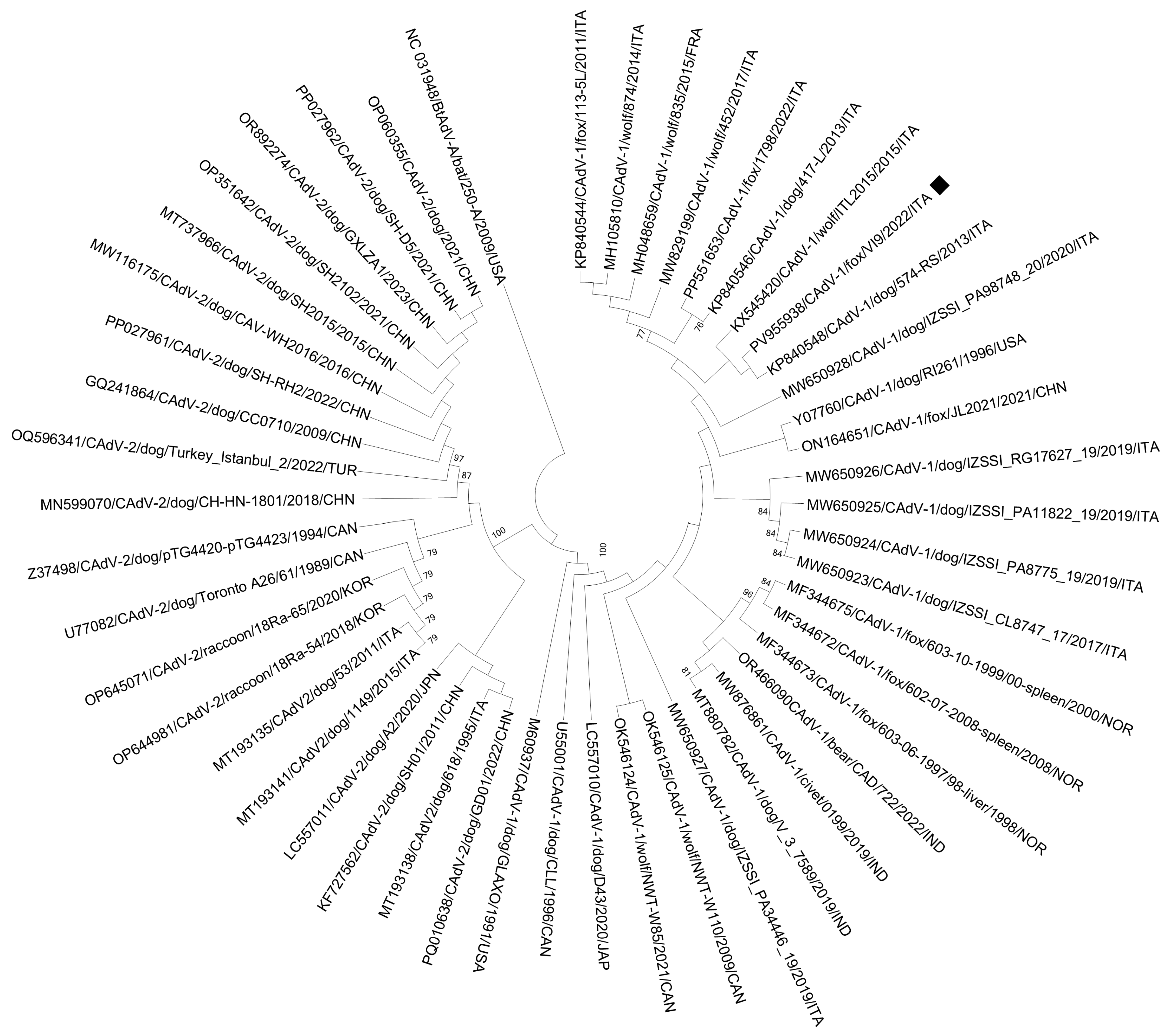
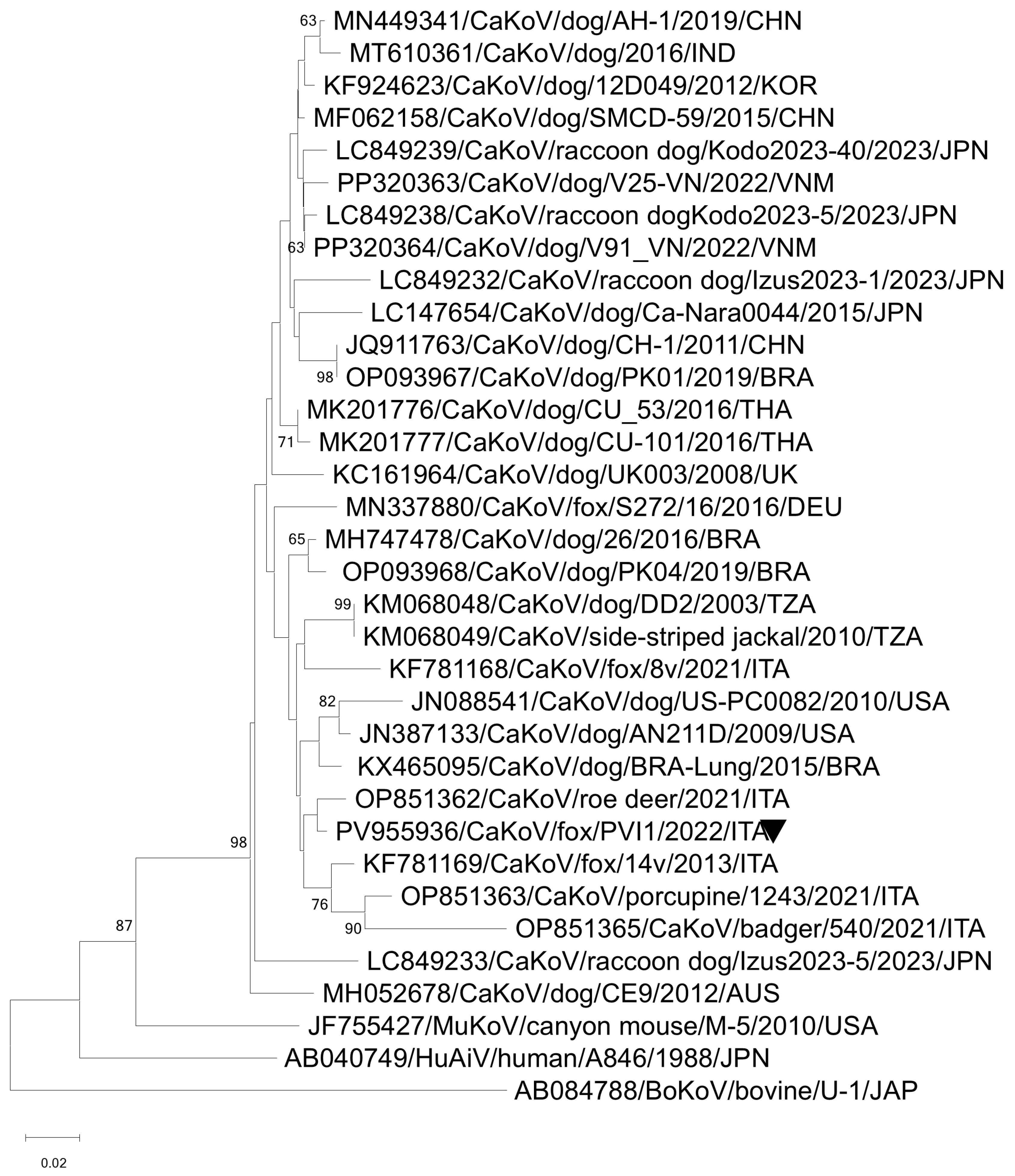
3.2. ONT Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roemer, G.W.; Gompper, M.E.; Van Valkenburgh, B. The Ecological Role of the Mammalian Mesocarnivore. BioScience 2009, 59, 165–173. [Google Scholar] [CrossRef]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P.; et al. Status and Ecological Effects of the World’s Largest Carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef] [PubMed]
- Henle, K.; Davies, K.F.; Kleyer, M.; Margules, C.; Settele, J. Predictors of Species Sensitivity to Fragmentation. Biodivers. Conserv. 2004, 13, 207–251. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife—Threats to Biodiversity and Human Health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Belsare, A.V.; Vanak, A.T.; Gompper, M.E. Epidemiology of Viral Pathogens of Free-Ranging Dogs and Indian Foxes in a Human-Dominated Landscape in Central India. Transbound. Emerg. Dis. 2014, 61, 78–86. [Google Scholar] [CrossRef]
- Murray, D.L.; Kapke, C.A.; Evermann, J.F.; Fuller, T.K. Infectious Disease and the Conservation of Free-Ranging Large Carnivores. Anim. Conserv. 1999, 2, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.B.; Jones, K.E.; Nunn, C.L.; Altizer, S. Infectious Diseases and Extinction Risk in Wild Mammals. Conserv. Biol. 2007, 21, 1269–1279. [Google Scholar] [CrossRef]
- Martins, N.B.; Neves de Almeida, J.C.; Gonçalves, M.S.S.; Gila, L.I.; Yogui, D.R.; Alves, M.H.; Desbiez, A.L.J.; Brandão, P.E.; da Hora, A.S. Occurrence of Typical Domestic Animal Viruses in Wild Carnivorans: An Emerging Threat to the Conservation of Endangered Species. Transbound. Emerg. Dis. 2024, 71, e3931047. [Google Scholar] [CrossRef]
- Pénzes, J.J.; Söderlund-Venermo, M.; Canut, M.; Eis-Hübinger, A.M.; Hughes, J.; Cotmore, S.F.; Harrach, B. Reorganizing the Family Parvoviridae: A Revised Taxonomy Independent of the Canonical Approach Based on Host Association. Arch. Virol. 2020, 165, 2133–2146. [Google Scholar] [CrossRef]
- Allison, A.B.; Harbison, C.E.; Pagan, I.; Stucker, K.M.; Kaelber, J.T.; Brown, J.D.; Ruder, M.G.; Keel, M.K.; Dubovi, E.J.; Holmes, E.C.; et al. Role of Multiple Hosts in the Cross-Species Transmission and Emergence of a Pandemic Parvovirus. J. Virol. 2012, 86, 865–872. [Google Scholar] [CrossRef]
- Canuti, M.; Fry, K.; Cluff, H.D.; Mira, F.; Fenton, H.; Lang, A.S. Co-Circulation of Five Species of Dog Parvoviruses and Canine Adenovirus Type 1 among Gray Wolves (Canis lupus) in Northern Canada. Transbound. Emerg. Dis. 2022, 69, e1407–e1433. [Google Scholar] [CrossRef]
- Kurucay, H.N.; Tamer, C.; Muftuoglu, B.; Elhag, A.E.; Gozel, S.; Cicek-Yildiz, Y.; Demirtas, S.; Ozan, E.; Albayrak, H.; Okur-Gumusova, S.; et al. First Isolation and Molecular Characterization of Canine Parvovirus Type 2b (CPV-2b) from Red Foxes (Vulpes vulpes) in Turkey. Virol. J. 2023, 20, 27. [Google Scholar] [CrossRef]
- Calatayud, O.; Esperón, F.; Velarde, R.; Oleaga, Á.; Llaneza, L.; Ribas, A.; Negre, N.; de la Torre, A.; Rodríguez, A.; Millán, J. Genetic Characterization of Carnivore Parvoviruses in Spanish Wildlife Reveals Domestic Dog and Cat-Related Sequences. Transbound. Emerg. Dis. 2020, 67, 626–634. [Google Scholar] [CrossRef]
- Ndiana, L.A.; Lanave, G.; Desario, C.; Berjaoui, S.; Alfano, F.; Puglia, I.; Fusco, G.; Colaianni, M.L.; Vincifori, G.; Camarda, A.; et al. Circulation of Diverse Protoparvoviruses in Wild Carnivores, Italy. Transbound. Emerg. Dis. 2021, 68, 2489–2502. [Google Scholar] [CrossRef] [PubMed]
- Diakoudi, G.; Lanave, G.; Berjaoui, S.; Desario, C.; Di Teodoro, G.; Vasinioti, V.I.; Pellegrini, F.; Defourny, S.V.P.; Salucci, S.; Cocco, A.; et al. Feline Panleukopenia Virus in a Marsican Brown Bear and Crested Porcupine, Italy, 2022-2023. Emerg. Infect. Dis. 2024, 30, 2655–2659. [Google Scholar] [CrossRef]
- Battilani, M.; Scagliarini, A.; Tisato, E.; Turilli, C.; Jacoboni, I.; Casadio, R.; Prosperi, S. Analysis of Canine Parvovirus Sequences from Wolves and Dogs Isolated in Italy. J. Gen. Virol. 2001, 82, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Balboni, A.; Urbani, L.; Delogu, M.; Musto, C.; Fontana, M.C.; Merialdi, G.; Lucifora, G.; Terrusi, A.; Dondi, F.; Battilani, M. Integrated Use of Molecular Techniques to Detect and Genetically Characterise DNA Viruses in Italian Wolves (Canis lupus italicus). Animals 2021, 11, 2198. [Google Scholar] [CrossRef]
- Duarte, M.D.; Henriques, A.M.; Barros, S.C.; Fagulha, T.; Mendonça, P.; Carvalho, P.; Monteiro, M.; Fevereiro, M.; Basto, M.P.; Rosalino, L.M.; et al. Snapshot of Viral Infections in Wild Carnivores Reveals Ubiquity of Parvovirus and Susceptibility of Egyptian Mongoose to Feline Panleukopenia Virus. PLoS ONE 2013, 8, e59399. [Google Scholar] [CrossRef]
- Barlow, A.M.; Schock, A.; Bradshaw, J.; Mullineaux, E.; Dastjerdi, A.; Everest, D.J.; McGowan, S.; Steinbach, F.; Cowen, S. Parvovirus enteritis in Eurasian badgers (Meles meles). Vet. Rec. 2012, 170, 416. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, M.; Santoro, M.; Clausi, M.T.; Cozzolino, L.; Decaro, N.; Colaianni, M.L.; Fusco, G. Molecular Detection and Characterization of Carnivore Parvoviruses in Free-Ranging Eurasian Otters (Lutra lutra) in Southern Italy. Transbound. Emerg. Dis. 2019, 66, 1864–1872. [Google Scholar] [CrossRef]
- Magliocca, M.; Taddei, R.; Urbani, L.; Bertasio, C.; Facile, V.; Gallina, L.; Sampieri, M.; Rugna, G.; Rubini, S.; Maioli, G.; et al. Molecular Detection of Viral and Bacterial Pathogens in Red Foxes (Vulpes vulpes) from Italy. Animals 2024, 14, 1969. [Google Scholar] [CrossRef]
- Leopardi, S.; Milani, A.; Cocchi, M.; Bregoli, M.; Schivo, A.; Leardini, S.; Festa, F.; Pastori, A.; De Zan, G.; Gobbo, F.; et al. Carnivore Protoparvovirus 1 (CPV-2 and FPV) Circulating in Wild Carnivores and in Puppies Illegally Imported into North-Eastern Italy. Viruses 2022, 14, 2612. [Google Scholar] [CrossRef] [PubMed]
- Wasieri, J.; Schmiedeknecht, G.; Förster, C.; König, M.; Reinacher, M. Parvovirus Infection in a Eurasian Lynx (Lynx lynx) and in a European Wildcat (Felis silvestris silvestris). J. Comp. Pathol. 2009, 140, 203–207. [Google Scholar] [CrossRef]
- Campoy, A.; Gomez-Lucia, E.; Garcia, T.; Crespo, E.; Olmeda, S.; Valcarcel, F.; Fandiño, S.; Domenech, A. First Description of a Carnivore Protoparvovirus Associated with a Clinical Case in the Iberian Lynx (Lynx pardinus). Animals 2025, 15, 1026. [Google Scholar] [CrossRef]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef]
- Decaro, N.; Martella, V.; Buonavoglia, C. Canine Adenoviruses and Herpesvirus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Green, R.G.; Ziegler, N.R.; Breen, B.B. Epizootic Fox Encephalitis. I. General Description. Am. J. Epidemiol. 1930, 12, 109–129. [Google Scholar] [CrossRef]
- Junge, R.E.; Bauman, K.; King, M.; Gompper, M.E. A Serologic Assessment of Exposure to Viral Pathogens and Leptospira in an Urban Raccoon (Procyon lotor) Population Inhabiting a Large Zoological Park. J. Zoo Wildl. Med. 2007, 38, 18–26. [Google Scholar] [CrossRef]
- Balboni, A.; Verin, R.; Morandi, F.; Poli, A.; Prosperi, S.; Battilani, M. Molecular Epidemiology of Canine Adenovirus Type 1 and Type 2 in Free-Ranging Red Foxes (Vulpes vulpes) in Italy. Vet. Microbiol. 2013, 162, 551–557. [Google Scholar] [CrossRef]
- Pizzurro, F.; Marcacci, M.; Zaccaria, G.; Orsini, M.; Cito, F.; Rosamilia, A.; Di Renzo, L.; Malatesta, D.; Di Sabatino, D.; Lorusso, A. Genome Sequence of Canine Adenovirus Type 1 Isolated from a Wolf (Canis lupus) in Southern Italy. Genome Announc. 2017, 5, e00225-17. [Google Scholar] [CrossRef]
- Dowgier, G.; Lahoreau, J.; Lanave, G.; Losurdo, M.; Varello, K.; Lucente, M.S.; Ventriglia, G.; Bozzetta, E.; Martella, V.; Buonavoglia, C.; et al. Sequential Circulation of Canine Adenoviruses 1 and 2 in Captive Wild Carnivores, France. Vet. Microbiol. 2018, 221, 67–73. [Google Scholar] [CrossRef]
- García Marín, J.F.; Royo, L.J.; Oleaga, A.; Gayo, E.; Alarcia, O.; Pinto, D.; Martínez, I.Z.; González, P.; Balsera, R.; Marcos, J.L.; et al. Canine adenovirus type 1 (CAdV-1) in free-ranging European brown bear (Ursus arctos arctos): A threat for Cantabrian population? Transbound. Emerg. Dis. 2018, 65, 2049–2056. [Google Scholar] [CrossRef]
- Ndiana, L.A.; Lanave, G.; Vasinioti, V.; Desario, C.; Martino, C.; Colaianni, M.L.; Pellegrini, F.; Camarda, A.; Berjaoui, S.; Sgroi, G.; et al. Detection and Genetic Characterization of Canine Adenoviruses, Circoviruses, and Novel Cycloviruses from Wild Carnivores in Italy. Front. Vet. Sci. 2022, 9, 851987. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, A.; Balseiro, A.; Espí, A.; Royo, L.J. Wolf (Canis lupus) as Canine Adenovirus Type 1 (CAdV-1) Sentinel for the Endangered Cantabrian Brown Bear (Ursus arctos arctos). Transbound. Emerg. Dis. 2022, 69, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, C.E.; Smoglica, C.; Paoletti, B.; Angelucci, S.; Innocenti, M.; Antonucci, A.; Di Domenico, G.; Marsilio, F. Detection of Selected Pathogens in Apennine Wolf (Canis lupus italicus) by a Non-Invasive GPS-Based Telemetry Sampling of Two Packs from Majella National Park, Italy. Eur. J. Wildl. Res. 2019, 65, 84. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.Y.; Kim, Y.S.; Na, E.J.; Park, J.S.; Oem, J.K. Genetic Characteristics of Canine Adenovirus Type 2 Detected in Wild Raccoon Dogs (Nyctereutes procyonoides) in Korea (2017–2020). Vet. Sci. 2022, 9, 591. [Google Scholar] [CrossRef]
- Buonavoglia, C.; Martella, V.; Pratelli, A.; Tempesta, M.; Cavalli, A.; Buonavoglia, D.; Bozzo, G.; Elia, G.; Decaro, N.; Carmichael, L.E. Evidence for Evolution of Canine Parvovirus Type 2 in Italy. J. Gen. Virol. 2001, 82, 3021–3025. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.L.; Huang, G.; Qiu, W.; Zhong, Z.H.; Xia, X.Z.; Yin, Z. Detection and Differentiation of CAV-1 and CAV-2 by Polymerase Chain Reaction. Vet. Res. Commun. 2001, 25, 77–84. [Google Scholar] [CrossRef]
- Reyes, G.R.; Kim, J.P. Sequence-Independent Single-Primer Amplification (SISPA) of Complex DNA Populations. Mol. Cell Probes 1991, 5, 473–481. [Google Scholar] [CrossRef]
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a Human Parvovirus by Molecular Screening of Respiratory Tract Samples. Proc. Natl. Acad. Sci. USA 2005, 102, 12891–12896. [Google Scholar] [CrossRef]
- Djikeng, A.; Halpin, R.; Kuzmickas, R.; Depasse, J.; Feldblyum, J.; Sengamalay, N.; Afonso, C.; Zhang, X.; Anderson, N.G.; Ghedin, E.; et al. Viral Genome Sequencing by Random Priming Methods. BMC Genom. 2008, 9, 5. [Google Scholar] [CrossRef]
- Vilsker, M.; Moosa, Y.; Nooij, S.; Fonseca, V.; Ghysens, Y.; Dumon, K.; Pauwels, R.; Alcantara, L.C.; Vanden Eynden, E.; Vandamme, A.-M.; et al. Genome Detective: An Automated System for Virus Identification from High-Throughput Sequencing Data. Bioinformatics 2019, 35, 871–873. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Canuti, M.; Britton, A.P.; Graham, S.M.; Lang, A.S. Epidemiology and Molecular Characterization of Protoparvoviruses Infecting Wild Raccoons (Procyon lotor) in British Columbia, Canada. Virus Res. 2017, 242, 85–89. [Google Scholar] [CrossRef]
- Canuti, M.; McDonald, E.; Graham, S.M.; Rodrigues, B.; Bouchard, É.; Neville, R.; Pitcher, M.; Whitney, H.G.; Marshall, H.D.; Lang, A.S. Multi-Host Dispersal of Known and Novel Carnivore Amdoparvoviruses. Virus Evol. 2020, 6, veaa072. [Google Scholar] [CrossRef]
- Mira, F.; Canuti, M.; Purpari, G.; Cannella, V.; Di Bella, S.; Occhiogrosso, L.; Schirò, G.; Chiaramonte, G.; Barreca, S.; Pisano, P.; et al. Molecular Characterization and Evolutionary Analyses of Carnivore Protoparvovirus1 NS1 Gene. Viruses 2019, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Carrino, M.; Tassoni, L.; Campalto, M.; Cavicchio, L.; Mion, M.; Corrò, M.; Natale, A.; Beato, M.S. Molecular Investigation of Recent Canine Parvovirus-2 (CPV-2) in Italy Revealed Distinct Clustering. Viruses 2022, 14, 917. [Google Scholar] [CrossRef] [PubMed]
- Demeter, Z.; Palade, E.A.; Soós, T.; Farsang, A.; Jakab, C.; Rusvai, M. Misleading Results of the MboII-Based Identification of Type 2a Canine Parvovirus Strains from Hungary Reacting as Type 2c Strains. Virus Genes 2010, 41, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ohneiser, S.A.; Hills, S.F.; Cave, N.J.; Passmore, D.; Dunowska, M. Canine Parvoviruses in New Zealand Form a Monophyletic Group Distinct from the Viruses Circulating in Other Parts of the World. Vet. Microbiol. 2015, 178, 190–200. [Google Scholar] [CrossRef]
- Zhao, M.; Yue, C.; Yang, Z.; Li, Y.; Zhang, D.; Zhang, J.; Yang, S.; Shen, Q.; Su, X.; Qi, D.; et al. Viral Metagenomics Unveiled Extensive Communication of Viruses within Giant Pandas and Their Associated Organisms in the Same Ecosystem. Sci. Total Environ. 2022, 820, 153317. [Google Scholar] [CrossRef]
- Mira, F.; Purpari, G.; Lorusso, E.; Di Bella, S.; Gucciardi, F.; Desario, C.; Macaluso, G.; Decaro, N.; Guercio, A. Introduction of Asian Canine Parvovirus in Europe through Dog Importation. Transbound. Emerg. Dis. 2018, 65, 16–21. [Google Scholar] [CrossRef]
- Alam, S.; Chowdhury, Q.M.M.K.; Roy, S.; Chowdhury, M.S.R.; Hasan, M.; Mamun, M.A.; Uddin, M.B.; Hossain, M.M.; Rahman, M.M.; Rahman, M.M. Molecular Detection and Phylogenetic Analysis of Canine Parvovirus (CPV) in Diarrhoeic Pet Dogs in Bangladesh. Vet. Anim. Sci. 2021, 14, 100224. [Google Scholar] [CrossRef]
- Zhuang, Q.Y.; Qiu, Y.; Pan, Z.H.; Wang, S.C.; Wang, B.; Wu, W.K.; Yu, J.M.; Yi, Y.; Sun, F.L.; Wang, K.C. Genome Sequence Characterization of Canine Parvoviruses Prevalent in the Sichuan Province of China. Transbound. Emerg. Dis. 2019, 66, 897–907. [Google Scholar] [CrossRef]
- Wardhani, S.W.; Wongsakul, B.; Kasantikul, T.; Piewbang, C.; Techangamsuwan, S. Molecular and Pathological Investigations of Selected Viral Neuropathogens in Rabies-Negative Brains of Cats and Dogs Revealed Neurotropism of Carnivore Protoparvovirus1. Front. Vet. Sci. 2021, 8, 710701. [Google Scholar] [CrossRef]
- Kapiya, J.; Nalubamba, K.S.; Kaimoyo, E.; Changula, K.; Chidumayo, N.; Saasa, N.; Simuunza, M.C.; Takada, A.; Mweene, A.S.; Chitanga, S.; et al. First Genetic Detection and Characterization of Canine Parvovirus from Diarrheic Dogs in Zambia. Arch. Virol. 2019, 164, 303–307. [Google Scholar] [CrossRef]
- Yang, Y.; Geng, Y.; Peng, Q.; Li, Y.; Guo, H.; Deng, H.; Hou, R.; Lai, W.; Zhang, D.; Liu, S. Identification of a Feline Panleukopenia Virus from Captive Giant Pandas (Ailuropoda melanoleuca) and Its Phylogenetic Analysis. Transbound. Emerg. Dis. 2023, 70, e1486–e1491. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Liu, H.; Du, J.; Zhou, F.; Dong, Y.; He, X.; Li, Y.; Wang, C. Recombinant Feline Parvovirus Infection of Immunized Tigers in Central China. Emerg. Microbes Infect. 2017, 6, e42. [Google Scholar] [CrossRef]
- Walker, D.; Fee, S.A.; Hartley, G.; Learmount, J.; O’Hagan, M.J.; Meredith, A.L.; Bronsvoort, B.M.D.C.; Porphyre, T.; Sharp, C.P.; Philbey, A.W. Serological and Molecular Epidemiology of Canine Adenovirus Type 1 in Red Foxes (Vulpes vulpes) in the United Kingdom. Sci. Rep. 2016, 6, 36051. [Google Scholar] [CrossRef]
- Pacini, M.I.; Mazzei, M.; Sgorbini, M.; D’Alfonso, R.; Papini, R.A. A One-Year Retrospective Analysis of Viral and Parasitological Agents in Wildlife Animals Admitted to a First Aid Hospital. Animals 2023, 13, 931. [Google Scholar] [CrossRef]
- Wong, M.; Woolford, L.; Hasan, N.H.; Hemmatzadeh, F. A Novel Recombinant Canine Adenovirus Type 1 Detected from Acute Lethal Cases of Infectious Canine Hepatitis. Viral Immunol. 2017, 30, 258–263. [Google Scholar] [CrossRef]
- Mira, F.; Puleio, R.; Schirò, G.; Condorelli, L.; Di Bella, S.; Chiaramonte, G.; Purpari, G.; Cannella, V.; Balboni, A.; Randazzo, V.; et al. Study on the Canine Adenovirus Type 1 (CAdV-1) Infection in Domestic Dogs in Southern Italy. Pathogens 2022, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M.; Delwart, E.; Rosario, K.; Segalés, J.; Varsani, A.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Circoviridae. J. Gen. Virol. 2017, 98, 1997–1998. [Google Scholar] [CrossRef]
- Kapoor, A.; Dubovi, E.J.; Henriquez-Rivera, J.A.; Lipkin, W.I. Complete Genome Sequence of the First Canine Circovirus. J. Virol. 2012, 86, 7018. [Google Scholar] [CrossRef]
- Li, L.; McGraw, S.; Zhu, K.; Leutenegger, C.M.; Marks, S.L.; Kubiski, S.; Gaffney, P.; Dela Cruz, F.N., Jr.; Wang, C.; Delwart, E.; et al. Circovirus in Tissues of Dogs with Vasculitis and Hemorrhage. Emerg. Infect. Dis. 2013, 19, 534–541. [Google Scholar] [CrossRef]
- Niu, L.; Wang, Z.; Zhao, L.; Wang, Y.; Cui, X.; Shi, Y.; Chen, H.; Ge, J. Detection and Molecular Characterization of Canine Circovirus Circulating in Northeastern China during 2014-2016. Arch. Virol. 2020, 165, 137–143. [Google Scholar] [CrossRef]
- Beikpour, F.; Ndiana, L.A.; Sazmand, A.; Capozza, P.; Nemati, F.; Pellegrini, F.; Zafari, S.; Zolhavarieh, S.M.; Cardone, R.; Faraji, R.; et al. Detection and Genomic Characterization of Canine Circovirus in Iran. Animals 2022, 12, 507. [Google Scholar] [CrossRef]
- Balboni, A.; Dondi, F.; Agnoli, C.; Verin, R.; Gruarin, M.; Morini, M.; Battilani, M. Novel Sequence Variants of Viral Hexon and Fibre Genes in Two Dogs with Canine Adenovirus Type 1-Associated Disease. Vet. J. 2017, 223, 73–75. [Google Scholar] [CrossRef]
- Steinel, A.; Parrish, C.R.; Bloom, M.E.; Truyen, U. Parvovirus Infections in Wild Carnivores. J. Wildl. Dis. 2001, 37, 594–607. [Google Scholar] [CrossRef]
- Allison, A.B.; Kohler, D.J.; Fox, K.A.; Brown, J.D.; Gerhold, R.W.; Dubovi, E.J.; Parrish, C.R.; Holmes, E.C. Frequent Cross-Species Transmission of Parvoviruses among Diverse Carnivore Hosts. J. Virol. 2013, 87, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Truyen, U. Emergence and Recent Evolution of Canine Parvovirus. Vet. Microbiol. 1999, 69, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Santos, N.; Parrish, C.R.; Thompson, G. Genetic Characterization of Canine Parvovirus in Sympatric Free-Ranging Wild Carnivores in Portugal. J. Wildl. Dis. 2017, 53, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zeng, W.; Zhang, X.; Li, S. The Genetic Evolution of Canine Parvovirus—A New Perspective. PLoS ONE 2017, 12, e0175035. [Google Scholar] [CrossRef]
- Martella, V.; Decaro, N.; Elia, G.; Buonavoglia, C. Surveillance Activity for Canine Parvovirus in Italy. J. Vet. Med. B 2005, 52, 312–315. [Google Scholar] [CrossRef]
- Jager, M.C.; Tomlinson, J.E.; Lopez-Astacio, R.A.; Parrish, C.R.; Van de Walle, G.R. Small but Mighty: Old and New Parvoviruses of Veterinary Significance. Virol. J. 2021, 18, 210. [Google Scholar] [CrossRef]
- Ogbu, K.I.; Chukwudi, I.C.; Mira, F.; Eze, U.U.; Di Bella, S.; Olaolu, O.S.; Tion, M.T.; Purpari, G.; Cannella, V.; Nwosuh, I.C.; et al. Current Status and Risk Factors of Canine Parvovirus Type 2 in North Central Nigeria. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101578. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C. Canine Parvovirus: A Review of Epidemiological and Diagnostic Aspects, with Emphasis on Type 2c. Vet. Microbiol. 2012, 155, 1–12. [Google Scholar] [CrossRef]
- Li, Z.; Cai, J.; Feng, C.; Wang, Y.; Fang, S.; Xue, X. Two Novel Sites Determine Genetic Relationships Between CPV-2 and FPV: An Epidemiological Survey of Canine and Feline Parvoviruses in Changchun, China (2020). Front. Vet. Sci. 2024, 11, 1444984. [Google Scholar] [CrossRef]
- Callaway, H.M.; Welsch, K.; Weichert, W.; Allison, A.B.; Hafenstein, S.L.; Huang, K.; Iketani, S.; Parrish, C.R. Complex and Dynamic Interactions between Parvovirus Capsids, Transferrin Receptors, and Antibodies Control Cell Infection and Host Range. J. Virol. 2018, 92, e00460-18. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yoon, S.-W.; Jang, J.H.; Jeong, D.G.; Lee, B.J.; Kim, H.K. Genetic Characterization of Feline Parvovirus Isolate Fe-P2 in Korean Cat and Serological Evidence on Its Infection in Wild Leopard Cat and Asian Badger. Front. Vet. Sci. 2021, 8, 650866. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Hartmann, K.; Leutenegger, C.M.; Proksch, A.L.; Mueller, R.S.; Unterer, S. Role of canine circovirus in dogs with acute haemorrhagic diarrhoea. Vet. Rec. 2017, 180, 542. [Google Scholar] [CrossRef] [PubMed]
- Dowgier, G.; Lorusso, E.; Decaro, N.; Desario, C.; Mari, V.; Lucente, M.S.; Lanave, G.; Buonavoglia, C.; Elia, G. A molecular survey for selected viral enteropathogens revealed a limited role of Canine circovirus in the development of canine acute gastroenteritis. Vet. Microbiol. 2017, 204, 54–58. [Google Scholar] [CrossRef]
- Urbani, L.; Tryland, M.; Ehrich, D.; Fuglei, E.; Battilani, M.; Balboni, A. Ancient Origin and Genetic Segregation of Canine Circovirus Infecting Arctic Foxes (Vulpes lagopus) in Svalbard and Red Foxes (Vulpes vulpes) in Northern Norway. Transbound. Emerg. Dis. 2021, 68, 1283–1293. [Google Scholar] [CrossRef]
- Bexton, S.; Wiersma, L.C.; Getu, S.; van Run, P.R.; Verjans, G.M.; Schipper, D.; Schapendonk, C.M.; Bodewes, R.; Oldroyd, L.; Haagmans, B.L.; et al. Detection of Circovirus in Foxes with Meningoencephalitis, United Kingdom, 2009-2013. Emerg. Infect Dis. 2015, 21, 1205–1208. [Google Scholar] [CrossRef]
- De Arcangeli, S.; Balboni, A.; Kaehler, E.; Urbani, L.; Verin, R.; Battilani, M. Genomic Characterization of Canine Circovirus Detected in Red Foxes (Vulpes vulpes) from Italy using a New Real-Time PCR Assay. J. Wildl. Dis. 2020, 56, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Canuti, M.; King, A.V.L.; Franzo, G.; Cluff, H.D.; Larsen, L.E.; Fenton, H.; Dufour, S.C.; Lang, A.S. Diverse Fox Circovirus (Circovirus canine) Variants Circulate at High Prevalence in Grey Wolves (Canis lupus) from the Northwest Territories, Canada. Peer Community J. 2024, 4, e73. [Google Scholar] [CrossRef]
- Zaccaria, G.; Malatesta, D.; Scipioni, G.; Di Felice, E.; Campolo, M.; Casaccia, C.; Savini, G.; Di Sabatino, D.; Lorusso, A. Circovirus in Domestic and Wild Carnivores: An important Opportunistic Agent? Virology 2016, 490, 69–74. [Google Scholar] [CrossRef]
- Thaiwong, T.; Wise, A.G.; Maes, R.K.; Mullaney, T.; Kiupel, M. Canine Circovirus 1 (CaCV-1) and Canine Parvovirus 2 (CPV-2): Recurrent Dual Infections in a Papillon Breeding Colony. Vet. Pathol. 2016, 53, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Menandro, M.L.; Tucciarone, C.M.; Barbierato, G.; Crovato, L.; Mondin, A.; Libanora, M.; Obber, F.; Orusa, R.; Robetto, S.; et al. Canine Circovirus in Foxes from Northern Italy: Where Did It All Begin? Pathogens 2021, 10, 1002. [Google Scholar] [CrossRef]
- de Villiers, L.; Molini, U.; Coetzee, L.M.; Visser, L.; Spangenberg, J.; de Villiers, M.; Berjaoui, S.; Khaiseb, S.; Lorusso, A.; Franzo, G. Molecular Epidemiology of Canine Circovirus in Domestic Dogs and Wildlife in Namibia, Africa. Infect. Genet. Evol. 2023, 112, 105458. [Google Scholar] [CrossRef]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Kapoor, A.; Simmonds, P.; Dubovi, E.J.; Qaisar, N.; Henriquez, J.A.; Medina, J.; Shields, S.; Lipkin, W.I. Characterization of a Canine Homolog of Human Aichivirus. J. Virol. 2011, 85, 11520–11525. [Google Scholar] [CrossRef]
- Li, L.; Pesavento, P.A.; Shan, T.; Leutenegger, C.M.; Wang, C.; Delwart, E. Viruses in Diarrhoeic Dogs Include Novel Kobuviruses and Sapoviruses. J. Gen. Virol. 2011, 92, 2534–2541. [Google Scholar] [CrossRef]
- Carmona-Vicente, N.; Buesa, J.; Brown, P.A.; Merga, J.Y.; Darby, A.C.; Stavisky, J.; Sadler, L.; Gaskell, R.M.; Dawson, S.; Radford, A.D. Phylogeny and Prevalence of Kobuviruses in Dogs and Cats in the UK. Vet. Microbiol. 2013, 164, 246–252. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Felice, E.; Ceci, C.; Di Profio, F.; Marsilio, F. Canine Kobuviruses in Diarrhoeic Dogs in Italy. Vet. Microbiol. 2013, 166, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Oem, J.K.; Choi, J.W.; Lee, M.H.; Lee, K.K.; Choi, K.S. Canine Kobuvirus Infections in Korean Dogs. Arch. Virol. 2014, 159, 2751–2755. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, F.M.; Ribeiro, J.; Alfieri, A.F.; Alfieri, A.A. Detection of Canine Kobuvirus RNA in Diarrheic Fecal Samples of Dogs with Parvoviruses. Braz. J. Microbiol. 2019, 50, 871–874. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Li, Y.; Wang, X.; Yang, K.; Zhang, D.; Zhao, L.; Bai, C.; Jiang, S.; Li, Y. Identification and Full-Genome Sequencing of Canine Kobuvirus in Canine Fecal Samples Collected from Anhui Province, Eastern China. Arch. Virol. 2020, 165, 2495–2501. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, B.; Di Profio, F.; Melegari, I.; Robetto, S.; Di Felice, E.; Orusa, R.; Marsilio, F. Molecular Evidence of Kobuviruses in Free-Ranging Red Foxes (Vulpes vulpes). Arch. Virol. 2014, 159, 1803–1806. [Google Scholar] [CrossRef]
- Kaiser, F.K.; van Dyck, L.; Jo, W.K.; Schreiner, T.; Pfankuche, V.M.; Wohlsein, P.; Baumann, I.; Peters, M.; Baumgärtner, W.; Osterhaus, A.D.M.E.; et al. Detection of Systemic Canine Kobuvirus Infection in Peripheral Tissues and the Central Nervous System of a Fox Infected with Canine Distemper Virus. Microorganisms 2021, 9, 2521. [Google Scholar] [CrossRef]
- Melegari, I.; Sarchese, V.; Di Profio, F.; Robetto, S.; Carella, E.; Bermudez Sanchez, S.; Orusa, R.; Martella, V.; Marsilio, F.; Di Martino, B. First Molecular Identification of Kobuviruses in Wolves (Canis lupus) in Italy. Arch. Virol. 2018, 163, 509–513. [Google Scholar] [CrossRef]
- Olarte-Castillo, X.A.; Heeger, F.; Mazzoni, C.J.; Greenwood, A.D.; Fyumagwa, R.; Moehlman, P.D.; Hofer, H.; East, M.L. Molecular Characterization of Canine Kobuvirus in Wild Carnivores and the Domestic Dog in Africa. Virology 2015, 477, 89–97. [Google Scholar] [CrossRef] [PubMed]
- He, W.T.; Hou, X.; Zhao, J.; Sun, J.; He, H.; Si, W.; Wang, J.; Jiang, Z.; Yan, Z.; Xing, G.; et al. Virome Characterization of Game Animals in China Reveals a Spectrum of Emerging Pathogens. Cell 2022, 185, 1117–1129.e8. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, T.; Kasantikul, T.; Piewbang, C.; Techangamsuwan, S. Evolutionary Dynamics of Canine Kobuvirus in Vietnam and Thailand Reveal the Evidence of Viral Ability to Evade Host Immunity. Sci. Rep. 2024, 14, 12037. [Google Scholar] [CrossRef] [PubMed]
- de Deus, D.R.; Siqueira, J.A.M.; Maués, M.A.C.; de Fátima Mesquita de Figueiredo, M.J.; Júnior, E.C.S.; da Silva Bandeira, R.; da Costa Pinheiro, K.; Teixeira, D.M.; da Silva, L.D.; de Fátima Dos Santos Guerra, S.; et al. Analysis of Viral Diversity in Dogs With Acute Gastroenteritis From Brazilian Amazon. Infect. Genet. Evol. 2024, 123, 105637. [Google Scholar] [CrossRef]
- Decaro, N.; Buonavoglia, C.; Barrs, V.R. Canine Parvovirus Vaccination and Immunisation Failures: Are We Far from Disease Eradication? Vet. Microbiol. 2020, 247, 108760. [Google Scholar] [CrossRef]
- Costanzi, L.; Brambilla, A.; Di Blasio, A.; Dondo, A.; Goria, M.; Masoero, L.; Gennero, M.S.; Bassano, B. Beware of dogs! Domestic animals as a threat for wildlife conservation in Alpine protected areas. Eur. J. Wildl. Res. 2021, 67, 70. [Google Scholar] [CrossRef]
- Verin, R.; Forzan, M.; Schulze, C.; Rocchigiani, G.; Balboni, A.; Poli, A.; Mazzei, M. Multicentric Molecular and Pathologic Study On Canine Adenovirus Type 1 in Red Foxes (Vulpes vulpes) in Three European Countries. J. Wildl. Dis. 2019, 55, 935–939. [Google Scholar] [CrossRef]

| Animal Species Tested | No. of Animals | Virus % (Positive/Total) | Total % (Positive/Total) | |
|---|---|---|---|---|
| FPV/CPV-2 | CAdV-1/CAdV-2 | |||
| Wolf (Canis lupus) | 47 | 6.4% (3/47) | 0% (0/47) | 6.4% (3/47) |
| Fox (Vulpes vulpes) | 55 | 0% (0/55) | 1.8% (1/55) | 1.8% (1/55) |
| Stone marten (Martes foina) | 15 | 0% (0/15) | 0% (0/15) | 0.0% (0/15) |
| Eurasian badger (Meles meles) | 23 | 4.3% (1/23) | 0% (0/23) | 4.3% (1/23) |
| Total % (Positive/Total) | 140 | 2.9% (4/140) | 0.7% (1/140) | 3.6% (5/140) |
| Strain ID | Virus Species | Virus Name | Reads Assembled | Total Reads | No. of Contigs | Coverage Length (nt) | Reference Sequence |
|---|---|---|---|---|---|---|---|
| CPV-2/Wolf/LI6/2021/ITA | Protoparvovirus carnivoran1 | CPV-2a | 131,239 | 188,291 | 1 | 4492 | NC001539 |
| CPV-2/Wolf/LI8/2021/ITA | CPV-2a | 489,633 | 886,740 | 1 | 4492 | NC001539 | |
| CPV-2/Wolf/LI9/2022/ITA | CPV-2c | 831,287 | 1,191,652 | 1 | 4492 | NC001539 | |
| FPV/Badger/TI6/2022/ITA | FPV | 344,382 | 399,962 | 1 | 4591 | NC001539 | |
| CaCV/Wolf/LI9/2022/ITA | Circovirus canine | CaCV | 588 | 1,191,652 | 1 | 2063 | NC020904 |
| CaKoV/Fox/PVI1/2021/ITA | Kobuvirus aichi | CaKoV | 2630 | 92,178 | 3 | 4759 | NC001918 |
| CAdV-1/Fox/VI9/2021/ITA | Mastadenovirus canidae | CAdV-1 | 7356 | 57,335 | 8 | 17,320 | NC001734 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarchese, V.; Di Profio, F.; Robetto, S.; Orusa, R.; Vuillermoz, B.; Pellegrini, F.; Marsilio, F.; Martella, V.; Di Martino, B. Molecular Survey for Major Canine Enteric Viral Pathogens in Wild Carnivores, Northwestern Italy. Vet. Sci. 2025, 12, 814. https://doi.org/10.3390/vetsci12090814
Sarchese V, Di Profio F, Robetto S, Orusa R, Vuillermoz B, Pellegrini F, Marsilio F, Martella V, Di Martino B. Molecular Survey for Major Canine Enteric Viral Pathogens in Wild Carnivores, Northwestern Italy. Veterinary Sciences. 2025; 12(9):814. https://doi.org/10.3390/vetsci12090814
Chicago/Turabian StyleSarchese, Vittorio, Federica Di Profio, Serena Robetto, Riccardo Orusa, Beatrice Vuillermoz, Francesco Pellegrini, Fulvio Marsilio, Vito Martella, and Barbara Di Martino. 2025. "Molecular Survey for Major Canine Enteric Viral Pathogens in Wild Carnivores, Northwestern Italy" Veterinary Sciences 12, no. 9: 814. https://doi.org/10.3390/vetsci12090814
APA StyleSarchese, V., Di Profio, F., Robetto, S., Orusa, R., Vuillermoz, B., Pellegrini, F., Marsilio, F., Martella, V., & Di Martino, B. (2025). Molecular Survey for Major Canine Enteric Viral Pathogens in Wild Carnivores, Northwestern Italy. Veterinary Sciences, 12(9), 814. https://doi.org/10.3390/vetsci12090814







