Traditional Transportation Methods and Their Influence on Local Chicken Welfare, Behavior, and Blood Profiles: A Policy Considerations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaire for Chickens’ Farmers
2.2. Chickens
2.3. Tonic Immobility Test and Observation of Pecking Behavior
2.4. Blood Parameters Measurements
2.5. Pro-Inflammatory Cytokines Measurement
2.6. Plasma Corticosterone, Total Cholesterol, and Blood Glucose Measurements
2.7. Oxidative and Antioxidant Indexes
2.8. Data Analysis
3. Results
3.1. Chicken Owner Response to Questionnaire
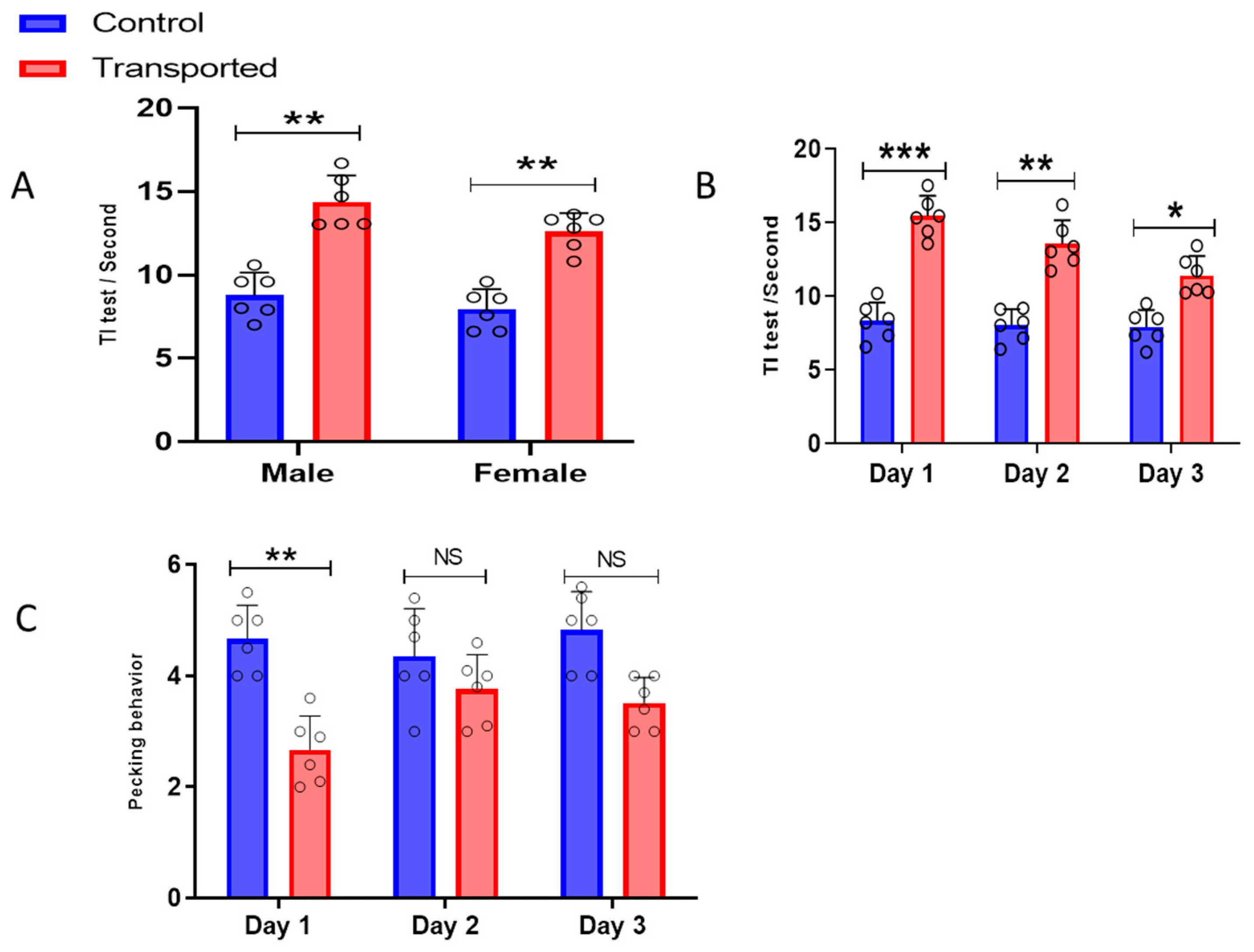
3.2. Tonic Immobility Test and Pecking
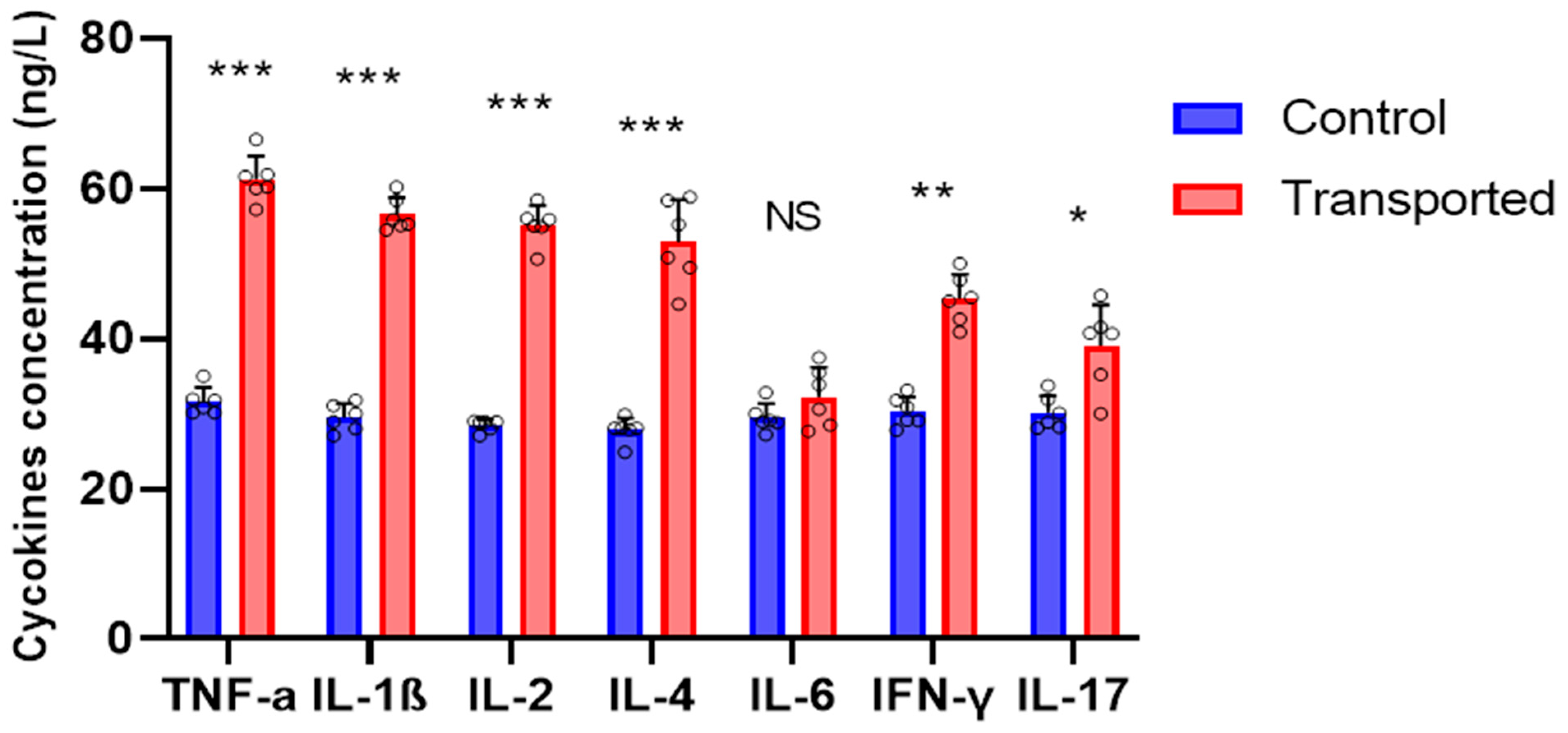
3.3. Blood Parameter and Pro-Inflammatory Cytokines Measurements
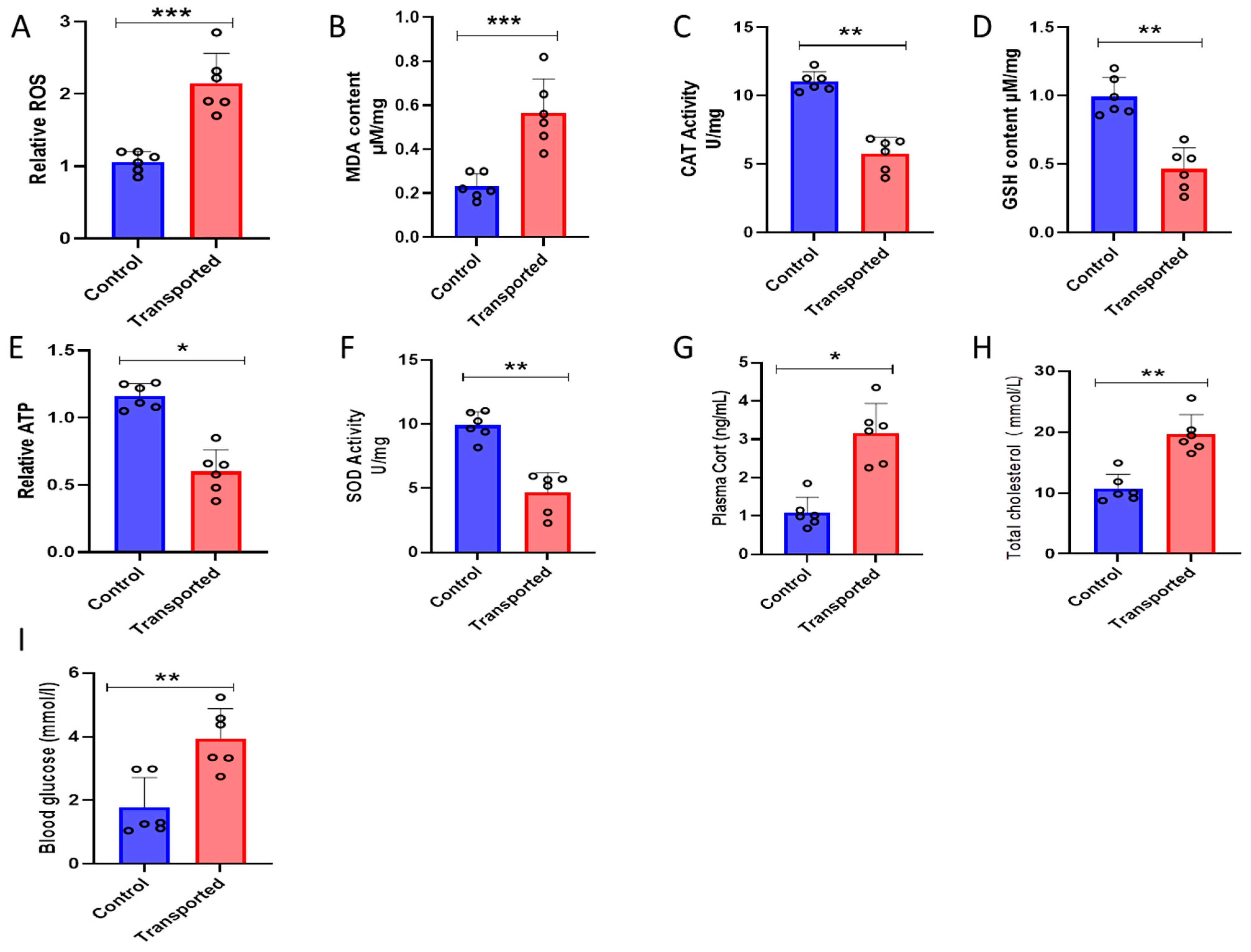
3.4. Oxidative Stress, Corticosterone, Total Cholesterol, and Blood Glucose in the Plasma of the Chickens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, M.; Kettlewell, P. Welfare of poultry during transport—A review. In Proceedings of the 8th European Symposium on Poultry Welfare, Cervia, Italy, 18–22 May 2009. [Google Scholar]
- Yousif, I.; Berima, M.; Ishag, I. Evaluation of Sudanese Native Chicken Production System and Major Constraints. J. Vet. Med. Anim. Prod. 2015, 6, 127–135. [Google Scholar]
- Afolabi, K.D. Local or Indigenous Chicken Production: A Key to Food Security, Poverty Alleviation, Disease Mitigation and Socio-Cultural Fulfilment in Africa. In Sustainable Food Security in the Era of Local and Global Environmental Change; Behnassi, M., Pollmann, O., Kissinger, G., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 217–229. [Google Scholar] [CrossRef]
- Leta, S.; Bekana, E. Survey on Village Based Chicken Production and Utilization System in Mid Rift Valley of Oromia, Ethiopia. Glob. Vet. 2010, 5, 198–203. [Google Scholar]
- Frimpong, S.; Gebresenbet, G.; Bobobee, E.; Aklaku, E.D.; Hamdu, I. Effect of Transportation and Pre-Slaughter Handling on Welfare and Meat Quality of Cattle: Case Study of Kumasi Abattoir, Ghana. Vet. Sci. 2014, 1, 174–191. [Google Scholar] [CrossRef]
- Al-Aqil, A.; Zulkifli, I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult. Sci. 2009, 88, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, Y.; Zhou, Z.; Habiba, U. Parameters of Physiological Responses and Meat Quality in Poultry Subjected to Transport Stress. Biol. Syst. Open Access 2017, 6, 175. [Google Scholar] [CrossRef]
- Zheng, A.; Lin, S.; Pirzado, S.A.; Chen, Z.; Chang, W.; Cai, H.; Liu, G. Stress Associated with Simulated Transport, Changes Serum Biochemistry, Postmortem Muscle Metabolism, and Meat Quality of Broilers. Animals 2020, 10, 1442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yue, H.Y.; Wu, S.G.; Xu, L.; Zhang, H.J.; Yan, H.J.; Cao, Y.L.; Gong, Y.S.; Qi, G.H. Transport stress in broilers. II. Superoxide production, adenosine phosphate concentrations, and mRNA levels of avian uncoupling protein, avian adenine nucleotide translocator, and avian peroxisome proliferator-activated receptor-gamma coactivator-1alpha in skeletal muscles. Poult. Sci. 2010, 89, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.Y.; Zhang, L.; Wu, S.G.; Xu, L.; Zhang, H.J.; Qi, G.H. Effects of transport stress on blood metabolism, glycolytic potential, and meat quality in meat-type yellow-feathered chickens. Poult. Sci. 2010, 89, 413–419. [Google Scholar] [CrossRef]
- Simon, E.J.; Dickey, J.L.; Reece, J.B. Campbell Essential Biology, 5th ed.; Benjamin Cummings Publishing Company Inc.: Redword City, UK, 2006. [Google Scholar]
- Guyton, C.A. Buku Ajar Fisiologi Kedokteran. In Terjemahan Dari: Textbook of Medical Physiology, 12th ed.; Irawati, S.A.T., Ken, S., Alex, P., Irawati, S., Eds.; Penerbit Buku Kedokteran EGC.: Jakarta, Indonesia, 2012. [Google Scholar]
- Ulupi, N.; Aryani, S.S.; Evni, F.T.; Nugraha, R. Effects of Transportation Duration on Broiler Chicken Physiology and Performance Factors. Int. J. Poult. Sci. 2018, 17, 197–204. [Google Scholar] [CrossRef]
- Botía, M.; Escribano, D.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Tecles, F.; López-Arjona, M.; Cerón, J.J. Different Types of Glucocorticoids to Evaluate Stress and Welfare in Animals and Humans: General Concepts and Examples of Combined Use. Metabolites 2023, 13, 106. [Google Scholar] [CrossRef]
- Lin, H.Y.; Song, G.; Lei, F.; Li, D.; Qu, Y. Avian corticosteroid-binding globulin: Biological function and regulatory mechanisms in physiological stress responses. Front. Zool. 2021, 18, 22. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Degroote, J.; Majdeddin, M.; Golian, A.; De Smet, S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Rimoldi, S.; Lasagna, E.; Sarti, F.M.; Marelli, S.P.; Cozzi, M.C.; Bernardini, G.; Terova, G. Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions. Meta Gene 2015, 6, 17–25. [Google Scholar] [CrossRef]
- Borges, S.A.; Fischer da Silva, A.V.; Majorka, A.; Hooge, D.M.; Cummings, K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram). Poult. Sci. 2004, 83, 1551–1558. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Zhou, Y.; Feng, J.; Zhang, M. Effects of Heat Stress on Gut-Microbial Metabolites, Gastrointestinal Peptides, Glycolipid Metabolism, and Performance of Broilers. Animals 2021, 11, 1286. [Google Scholar] [CrossRef]
- Garriga, C.; Hunter, R.R.; Amat, C.; Planas, J.M.; Mitchell, M.A.; Moretó, M. Heat stress increases apical glucose transport in the chicken jejunum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R195–R201. [Google Scholar] [CrossRef]
- Soleimani, A.F.; Zulkifli, I.; Omar, A.R.; Raha, A.R. Neonatal feed restriction modulates circulating levels of corticosterone and expression of glucocorticoid receptor and heat shock protein 70 in aged Japanese quail exposed to acute heat stress. Poult. Sci. 2011, 90, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, A.; Ashwell, C.M.; Persia, M.E.; Rothschild, M.F.; Schmidt, C.J.; Lamont, S.J. Quantitative trait loci identified for blood chemistry components of an advanced intercross line of chickens under heat stress. BMC Genom. 2016, 17, 287. [Google Scholar] [CrossRef] [PubMed]
- Wigham, E.; Grist, A.; Mullan, S.; Wotton, S.; Butterworth, A. The Influence of Welfare Training on Bird Welfare and Carcass Quality in Two Commercial Poultry Primary Processing Plants. Animals 2019, 9, 584. [Google Scholar] [CrossRef]
- Song, J.; Lei, X.; Luo, J.; Everaert, N.; Zhao, G.; Wen, J.; Yang, Y. The effect of Epigallocatechin-3-gallate on small intestinal morphology, antioxidant capacity and anti-inflammatory effect in heat-stressed broilers. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhang, M.; Huang, X.; Zheng, X.; Song, Z.; Zhang, L.; Wang, T. An evaluation of natural and synthetic vitamin E supplementation on growth performance and antioxidant capacity of broilers in early age. Can. J. Anim. Sci. 2017, 98, 187–193. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Oladokun, S.; Adewole, D.I. Biomarkers of heat stress and mechanism of heat stress response in Avian species: Current insights and future perspectives from poultry science. J. Therm. Biol. 2022, 110, 103332. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, H.Y.; Zhang, H.J.; Xu, L.; Wu, S.G.; Yan, H.J.; Gong, Y.S.; Qi, G.H. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult. Sci. 2009, 88, 2033–2041. [Google Scholar] [CrossRef]
- Campbell, D.L.M.; Dickson, E.J.; Lee, C. Application of open field, tonic immobility, and attention bias tests to hens with different ranging patterns. PeerJ 2019, 7, e8122. [Google Scholar] [CrossRef]
- Robertson, G.W.; Maxwell, M.H. Modified staining techniques for avian blood cells. Br. Poult. Sci. 1990, 31, 881–886. [Google Scholar] [CrossRef]
- Cotter, P. Stress Assessment by the Hemogram Method—Circulating Cells Complicating Reliance on Heterophil/Lymphocyte (H/L) Ratio. J. Vet. Med. Res. 2022, 9, 1–7. [Google Scholar] [CrossRef]
- Dandekar, S.P.; Rane, S.A. Practical and Viva in Medical Biochemistry; Elsevier/Reed Elsevier PVT Ltd.: New Delhi, India, 2004. [Google Scholar]
- Li, Z.Y.; Lin, J.; Sun, F.; Li, H.; Xia, J.; Li, X.N.; Ge, J.; Zhang, C.; Li, J.L. Transport stress induces weight loss and heart injury in chicks: Disruption of ionic homeostasis via modulating ion transporting ATPases. Oncotarget 2017, 8, 24142–24153. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, J.; Zhao, N.; Xue, G.; Zhang, R.; Li, J.; Bao, J. Effects of transport time and feeding type on weight loss, meat quality and behavior of broilers. Anim. Biosci. 2022, 35, 1039–1047. [Google Scholar] [CrossRef]
- Shevchuk, M.; Stoyanovskyy, V.; Kolomiiets, I. Technological stress in poultry. Sci. Messenger LNU Vet. Med. Biotechnol. 2018, 20, 63–68. [Google Scholar] [CrossRef]
- Fernandes, E.; Raymundo, A.; Martins, L.L.; Lordelo, M.; de Almeida, A.M. The Naked Neck Gene in the Domestic Chicken: A Genetic Strategy to Mitigate the Impact of Heat Stress in Poultry Production—A Review. Animals 2023, 13, 1007. [Google Scholar] [CrossRef]
- Sato, T.; Tezuka, K.; Shibuya, H.; Watanabe, T.; Kamata, H.; Shirai, W. Cold-induced ascites in broiler chickens and its improvement by temperature-controlled rearing. Avian Dis. 2002, 46, 989–996. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Kageyama, K.; Iwasaki, Y.; Daimon, M. Hypothalamic Regulation of Corticotropin-Releasing Factor under Stress and Stress Resilience. Int. J. Mol. Sci. 2021, 22, 12242. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Sharma, S. Physiology, Adrenocorticotropic Hormone (ACTH). In StatPearls; StatPearls: Treasure Island, FL, USA, 2018. [Google Scholar]
- Abo-Al-Ela, H.G.; El-Kassas, S.; El-Naggar, K.; Abdo, S.E.; Jahejo, A.R.; Al Wakeel, R.A. Stress and immunity in poultry: Light management and nanotechnology as effective immune enhancers to fight stress. Cell Stress Chaperones 2021, 26, 457–472. [Google Scholar] [CrossRef]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and Macrophages as Viral Targets and Reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Eubank, T.D.; Doseff, A.I. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2010, 2, 204–215. [Google Scholar] [CrossRef]
- Hoffband, A.V.; Moss, P.A.H. Essential Haemotology, 6th ed.; EGC Medical Publisher: London, UK, 2011. [Google Scholar]
- Herrera-Heredia, S.A.; Hsu, H.P.; Kao, C.Y.; Tsai, Y.H.; Yamaguchi, Y.; Roers, A.; Hsu, C.L.; Dzhagalov, I.L. Heparin is required for the formation of granules in connective tissue mast cells. Front. Immunol. 2022, 13, 1000405. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Scanes, C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016, 95, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Bigazzi, A.; Ausri, F.; Peddie, L.; Fitch, D.; Puterman, E. Physiological markers of traffic-related stress during active travel. Transp. Res. Part F Traffic Psychol. Behav. 2022, 84, 223–238. [Google Scholar] [CrossRef]
- Lentfer, T.L.; Pendl, H.; Gebhardt-Henrich, S.G.; Fröhlich, E.K.; Von Borell, E. H/L ratio as a measurement of stress in laying hens—Methodology and reliability. Br. Poult. Sci. 2015, 56, 157–163. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Q.; Liu, R.; Zheng, M.; Wen, J.; Zhao, G. Genome-Wide Association Study of H/L Traits in Chicken. Animals 2019, 9, 260. [Google Scholar] [CrossRef] [PubMed]
- Iyasere, O.S.; Ajadi, T.A.; Sobayo, R.A.; Logunleko, M.O.; Adebayo, A.O.; Durosaro, S.O.; Egbeyale, L.T.; Famosaya, O.O.; Ajiboye, O.O.; Akinbode, S.O.; et al. Influence of exogenous corticosterone on testicular function and mating behavior of Nigerian indigenous cocks. Anim. Reprod. 2022, 19, e20210026. [Google Scholar] [CrossRef]
- Khondowe, P.; Mutayoba, B.; Muhairwa, A.; Phiri, E. Effects of heat stress and a low energy diet on blood parameters and liver hsp70 and iNOS gene expressions in local chickens. Vet. Anim. Sci. 2021, 14, 100221. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.X.; Sun, N.; Ning, G.B.; Zhang, D.J.; Feng, J.; Lv, T.; Wang, Y.; Wang, H.; Wang, X.; Li, F. Effects of gallid herpesvirus 2 marek’s disease challenge virus and attenuated vaccine virus CVI988/rispens on immune adhesion of erythrocytes of chickens. Int. J. Poult. Sci. 2013, 12, 217–223. [Google Scholar] [CrossRef]
- Cunningham, J.G. Textbook of Veterinary Physiology, 3rd ed.; WB Saunders Company: Philadelphia, PA, USA, 2002. [Google Scholar]
- Aengwanich, W. Effects of High Environmental Temperature on Blood Indices of Thai Indigenous Chickens, Thai Indigenous Chickens Crossbred and Broilers. Int. J. Poult. Sci. 2007, 6, 427–430. [Google Scholar] [CrossRef][Green Version]
- Jian, C.; Wang-Ye, X.; Yuan, G.; Yi-Xi, T.; Xiang-Wen, X.; Xue-Nan, L.; Jin-Long, L. Inhibition of mtDNA-PRRs pathway-mediated sterile inflammation by astragalus polysaccharide protects against transport stress-induced cardiac injury in chicks. Poult. Sci. 2024, 103, 103638. [Google Scholar] [CrossRef]
- Jessica, L.S.; Lauren, T.W.; Pier, L.S.; Charles, R.L.; George, A.P.; Ron, D.R.; Ryon, W.; Thomas, H.W.; Sarah, H.W.-S. 30 Prenatally Stressed Brahman Bull Calves had Increased Pro-Inflammatory Cytokines and Transportation-Induced Mitochondrial Impairments. J. Anim. Sci. 2023, 101, 19–20. [Google Scholar] [CrossRef]
- Jatuporn, R.; Chananphat, T.; Anyarat, T.; Warangkana, K.; Ittidet, W.; Autchara, K.; Chaiwat, B. Pro-inflammatory cytokine release from chicken peripheral blood mononuclear cells stimulated with lipopolysaccharide. Vet. World 2022, 15, 885–889. [Google Scholar] [CrossRef]
- Qiongxia, L.; Shuxia, Z.; Ruqian, Z. Transportation stress alters the expression of immunoregulatory cytokines in the porcine thymus. Vet. J. 2011, 187, 229–233. [Google Scholar] [CrossRef]
- Ning, L.; Jingqing, C.; Yu, H.; Hai, J.; Da, J.; Shuai, L.; Ying, Y.; Zhaolai, D.; Zhenlong, W.; Guoyao, W. Effects of maternal l-proline supplementation on inflammatory cytokines at the placenta and fetus interface of mice. Amino Acids 2020, 52, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.Y.; Muniyappan, M.; Huang, H.; Ennab, W.; Liu, H.Y.; Ahmed, A.A.; Sun, M.A.; Dessie, T.; Kim, I.H.; Hu, Y.; et al. Dietary Organic Zinc Supplementation Modifies the Oxidative Genes via RORγ and Epigenetic Regulations in the Ileum of Broiler Chickens Exposed to High-Temperature Stress. Antioxidants 2024, 13, 1079. [Google Scholar] [CrossRef]
- Sevcikova, M.; Modra, H.; Slaninova, A.; Svobodova, Z. Metals as a cause of oxidative stress in fish: A review. Vet. Med. 2011, 56, 537–546. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, J.C. Effects of sub-chronic exposure to lead (Pb) and ascorbic acid in juvenile rockfish: Antioxidant responses, MT gene expression, and neurotransmitters. Chemosphere 2017, 171, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.S.; Zhao, W.; Spitz, D.R.; Robbins, M.E. Inhibiting catalase activity sensitizes 36B10 rat glioma cells to oxidative stress. Free Radic. Biol. Med. 2007, 42, 787–797. [Google Scholar] [CrossRef]
- Carlos, E.S.; Ricardo, B.; Carlos, C.C. Spontaneous recovery, time course, and circadian influence on habituation of the cardiovascular responses to repeated restraint stress in rats. Pflügers Arch. Eur. J. Physiol. 2020, 472, 1495–1506. [Google Scholar] [CrossRef]
- Ahmed, G.; Samar, T.; Khalid, M.; Shatha, G.F.; Asmaa, F.K.; Norhan, E.K.; Mariusz, J.; Mahmoud, M.; Mohammed, O.A.; Uthman, A.; et al. Heat shock proteins as a key defense mechanism in poultry production under heat stress conditions. Poult. Sci. 2024, 103, 103537. [Google Scholar] [CrossRef]
- Lei, W.; Xinyi, L.; Aijia, Z.; Huimin, C.; Ruqian, Z.; Yimin, J. Chronic corticosterone exposure disrupts hepatic and intestinal bile acid metabolism in chicken. Front. Vet. Sci. 2023, 10, 1147024. [Google Scholar] [CrossRef]
- Yujing, D.; Wenyan, F.; Song, W.; Yingdong, N.; Ruqian, Z. Cholesterol deregulation induced by chronic corticosterone (CORT) stress in pectoralis major of broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2014, 176, 59–64. [Google Scholar] [CrossRef]

| Variable | Level | Number | Percentage (%) |
|---|---|---|---|
| Sex | Male | 32 | 76.2 |
| Female | 10 | 23.8 | |
| Age | 18–30 years | 10 | 23.8 |
| 31–40 years | 16 | 38.1 | |
| >40 years | 16 | 38.1 | |
| Highest educational level achieved | Uneducated | 12 | 28.6 |
| Primary school | 24 | 57.1 | |
| Secondary school | 6 | 14.3 | |
| Job | Farmer | 32 | 76.2 |
| Teacher | 4 | 9.5 | |
| Other | 6 | 14.3 | |
| Did you hear about animal welfare? | Yes | 0 | 0.0 |
| No | 42 | 100.0 | |
| Did you hear about animal rights? | Yes | 13 | 31.0 |
| No | 29 | 69.0 | |
| Do you know animal protection law? | Know | 6 | 14.3 |
| Do not know | 36 | 85.7 | |
| Do you think that transporting animals requires a law to protect them? | Yes | 30 | 71.4 |
| To some extent | 12 | 28.6 | |
| Have you implemented any law to protect chickens during transportation? | Yes | 8 | 19.0 |
| To some extent | 14 | 33.3 | |
| No | 20 | 47.6 | |
| Do you think that chickens feel pain during transportation? | Yes | 30 | 71.4 |
| To some extent | 12 | 28.6 | |
| How do you feel when chickens are in pain? | Feel bad | 28 | 66.7 |
| Do not care | 14 | 33.3 | |
| What is the distance between your area and Nyala city? | 51–60 km | 14 | 33.3 |
| >60 km | 28 | 66.7 | |
| How long does it take between your area and Nyala city? | 2 h | 32 | 76.2 |
| 3 h | 10 | 23.8 | |
| Was the road paved? | Yes | 22 | 52.4 |
| No | 20 | 47.6 |
| Variable | Groups | |
|---|---|---|
| Control (n = 6) | Transported (n = 6) | |
| Mean TWBCs (range) | 1716.7 a (1200–2600) | 34,333.3 b (20,000–40,000) |
| Mean differential counts (range) | ||
| Heterophils | 18.7 a (10–25) | 83.7 b (70–90) |
| Lymphocytes | 70.0 a (60–75) | 28.2 b (18–40) |
| Monocytes | 4.8 a (1–10) | 8.8 b (5–15) |
| Basophils | 0 a | 1.5 a (0–4) |
| Eosinophils | 0.3 a (0–2) | 1.0 a (1–5) |
| Mean H/L (range) | 0.3 a (0.1–0.4) | 3.2 b (2.2–4.2) |
| Blood Parameters | Control | Transported | p-Value |
|---|---|---|---|
| RBC (×106) | 2.32 ± 0.07 a | 4.70 ± 0.05 b | 0.002 |
| Hb (g/dL) | 13.56 ± 0.70 a | 21.23 ± 0.42 b | 0.003 |
| HCT (%) | 36.35 ± 1.08 a | 39.51 ± 0.75 a | 0.076 |
| MCV (fl) | 112.86 ± 8.38 a | 130.46 ± 5.44 a | 0.082 |
| MCHC (%) | 39.15 ± 1.73 a | 54.67 ± 1.89 b | 0.001 |
| MCH (pg) | 50.30 ± 2.30 a | 59.34 ± 3.30 a | 0.061 |
| PLT (×108/L) | 0.26 ± 0.02 a | 0.554 ± 0.04 b | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, S.Y.; Ahmed, A.A.; Jammaa, M.H.; AL Makhmari, M.R.; Husien, H.M.; Essa, M.O.A.; Elwan, H.; Shehab-El-Deen, M.; Elnesr, S.S.; Saleh, A.A.; et al. Traditional Transportation Methods and Their Influence on Local Chicken Welfare, Behavior, and Blood Profiles: A Policy Considerations. Vet. Sci. 2025, 12, 798. https://doi.org/10.3390/vetsci12090798
Adam SY, Ahmed AA, Jammaa MH, AL Makhmari MR, Husien HM, Essa MOA, Elwan H, Shehab-El-Deen M, Elnesr SS, Saleh AA, et al. Traditional Transportation Methods and Their Influence on Local Chicken Welfare, Behavior, and Blood Profiles: A Policy Considerations. Veterinary Sciences. 2025; 12(9):798. https://doi.org/10.3390/vetsci12090798
Chicago/Turabian StyleAdam, Saber Y., Abdelkareem A. Ahmed, Mohammed H. Jammaa, Mohammed Rashid AL Makhmari, Hosameldeen Mohamed Husien, Mohamed Osman Abdalrahem Essa, Hamada Elwan, Mohamed Shehab-El-Deen, Shaaban S. Elnesr, Ahmed A. Saleh, and et al. 2025. "Traditional Transportation Methods and Their Influence on Local Chicken Welfare, Behavior, and Blood Profiles: A Policy Considerations" Veterinary Sciences 12, no. 9: 798. https://doi.org/10.3390/vetsci12090798
APA StyleAdam, S. Y., Ahmed, A. A., Jammaa, M. H., AL Makhmari, M. R., Husien, H. M., Essa, M. O. A., Elwan, H., Shehab-El-Deen, M., Elnesr, S. S., Saleh, A. A., & Cai, D. (2025). Traditional Transportation Methods and Their Influence on Local Chicken Welfare, Behavior, and Blood Profiles: A Policy Considerations. Veterinary Sciences, 12(9), 798. https://doi.org/10.3390/vetsci12090798









