Flavonoids of S. suberectus Dunn Regulate Cyclophosphamide-Induced Immunosuppression Through NF-κB Pathways
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. S. suberectus Dunn Sample Pretreatment
2.3. UPLC Conditions
2.4. ESI-QTRAP-MS/MS
2.5. Acquisition of Target Data
2.6. Network Construction
2.7. Molecular Docking Studies
2.8. Animals and Treatments
2.9. Measurement of Body Weights and Immune Organs Indexes
2.10. Histopathological Observation
2.11. Hematology Analysis
2.12. ELISA Assay
2.13. RT-qPCR Assay
2.14. Statistical Analysis
3. Results
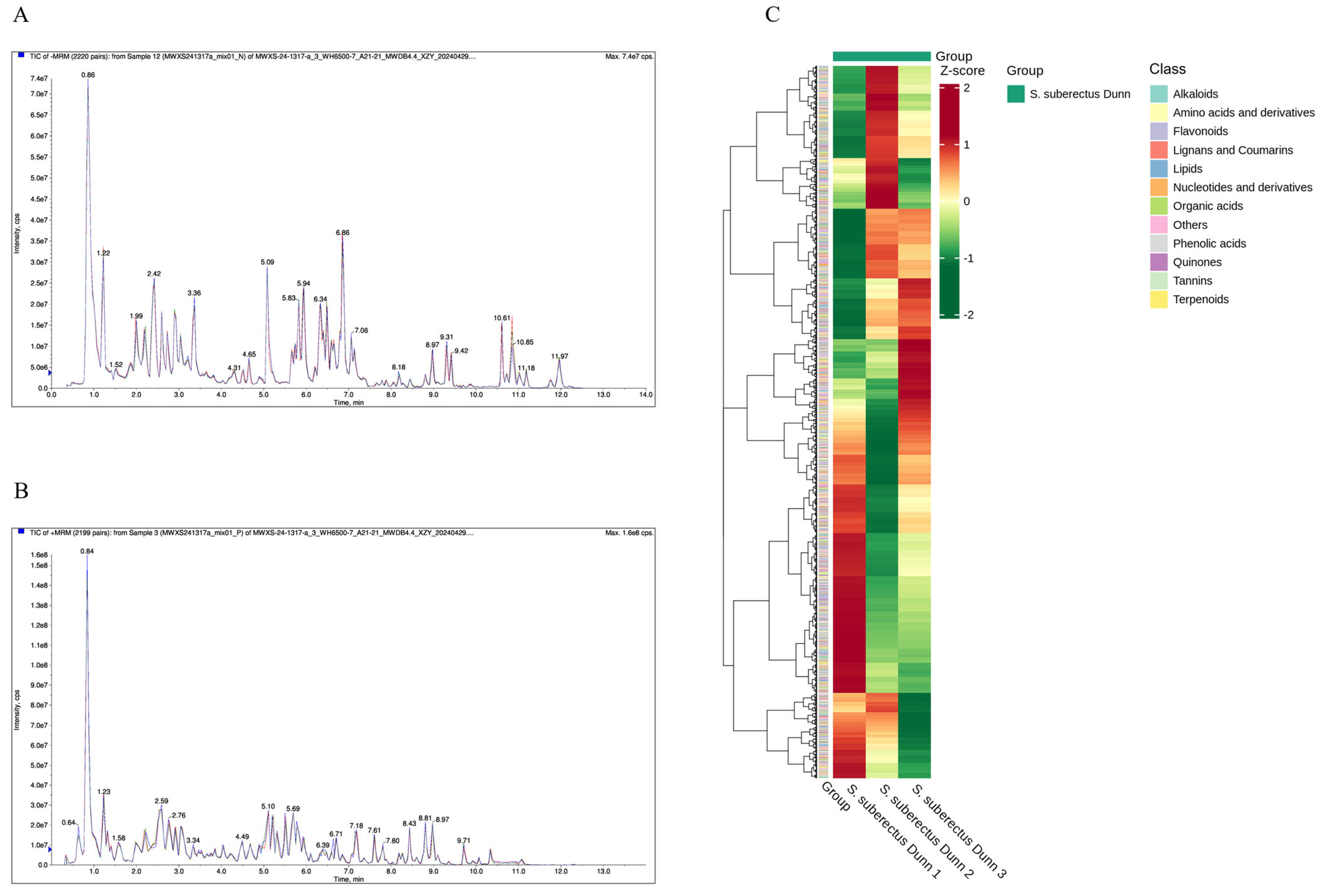
3.1. Metabolomics Results of S. suberectus Dun
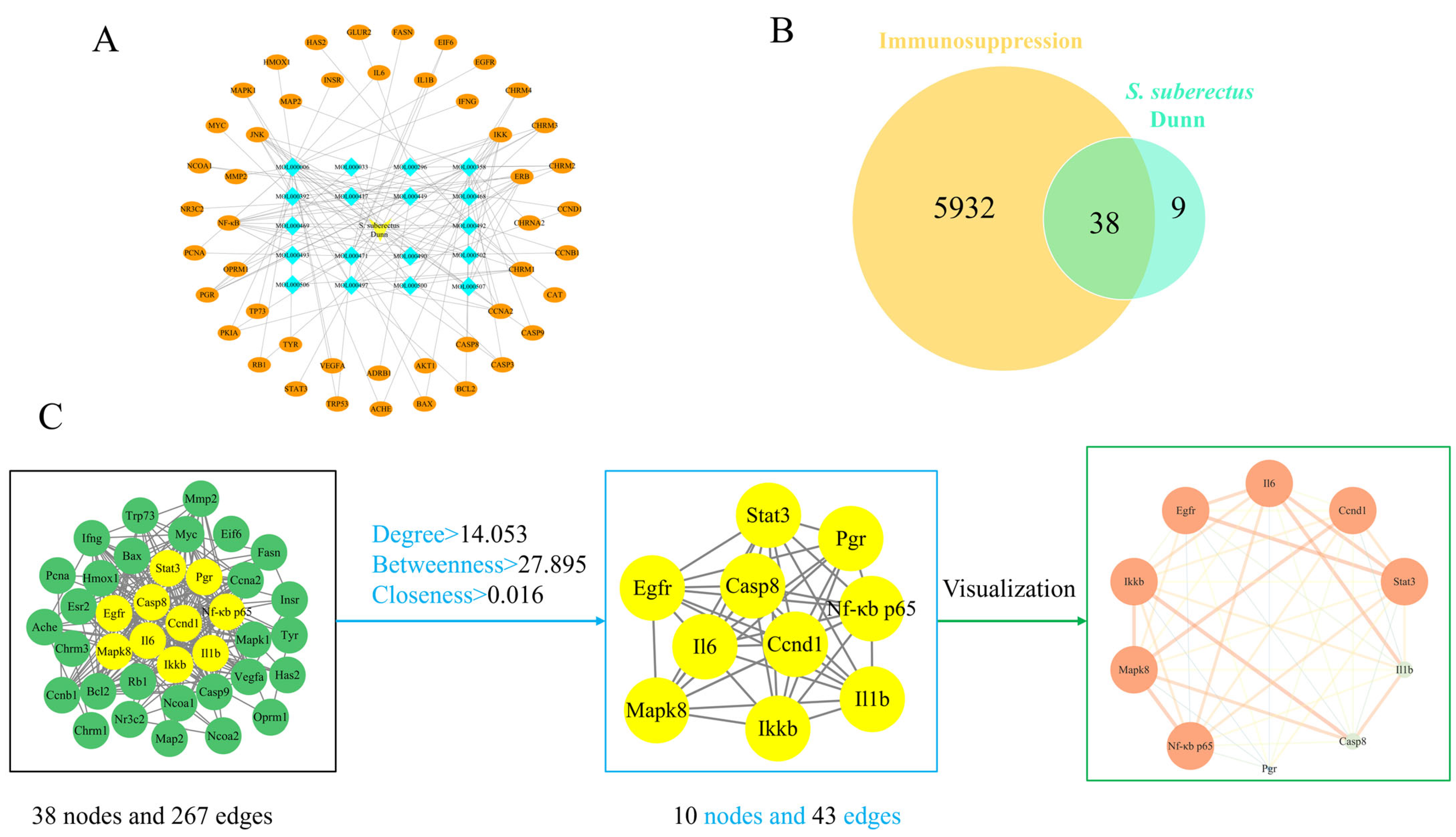
3.2. Compound-Target Network and PPI Network Construction
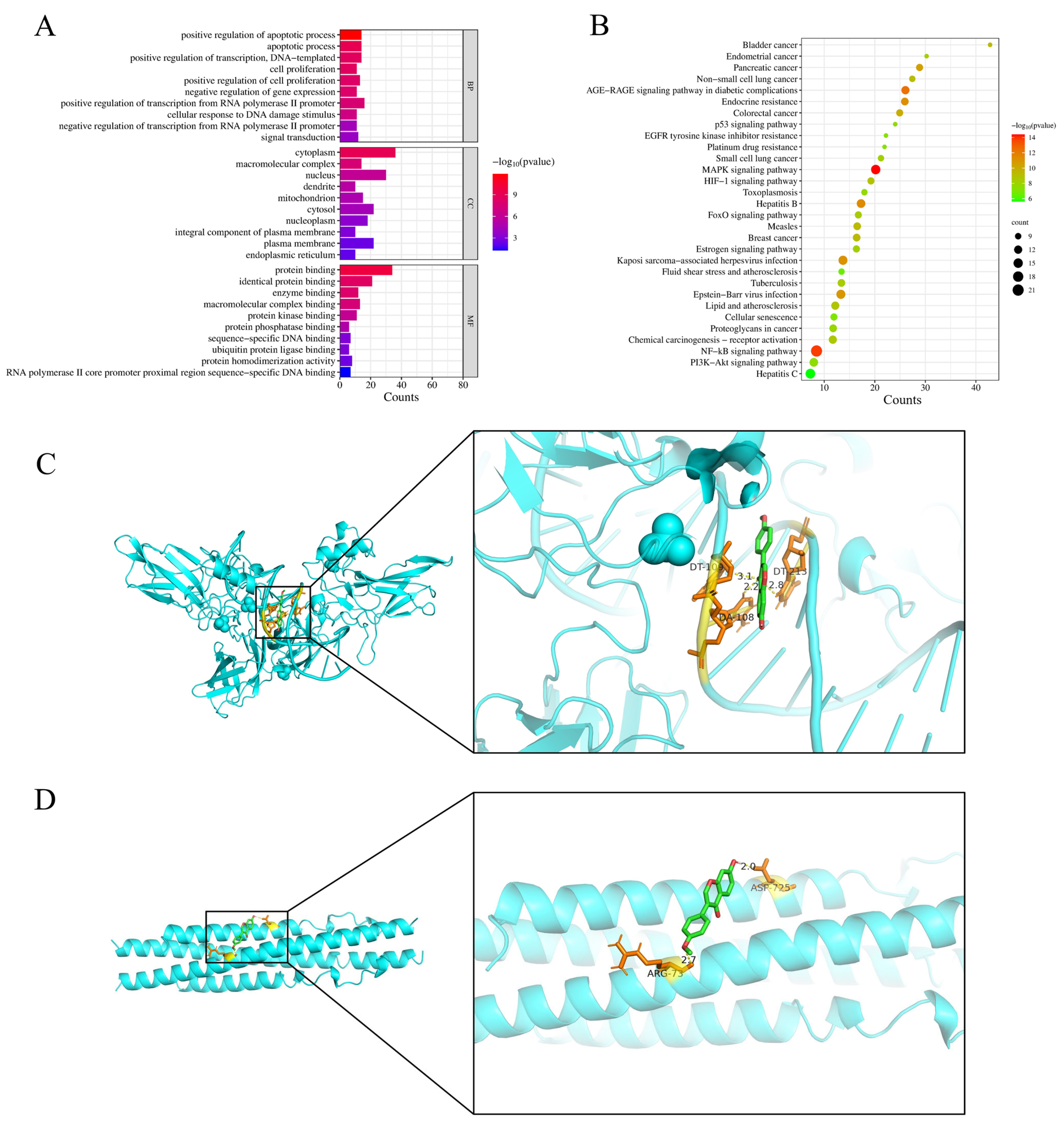
3.3. GO Enrichment, KEGG Pathway Analysis, and Molecular Docking Results
3.4. TFSD and FMN Ameliorated the Clinical Performance of CTX-Administered Mice
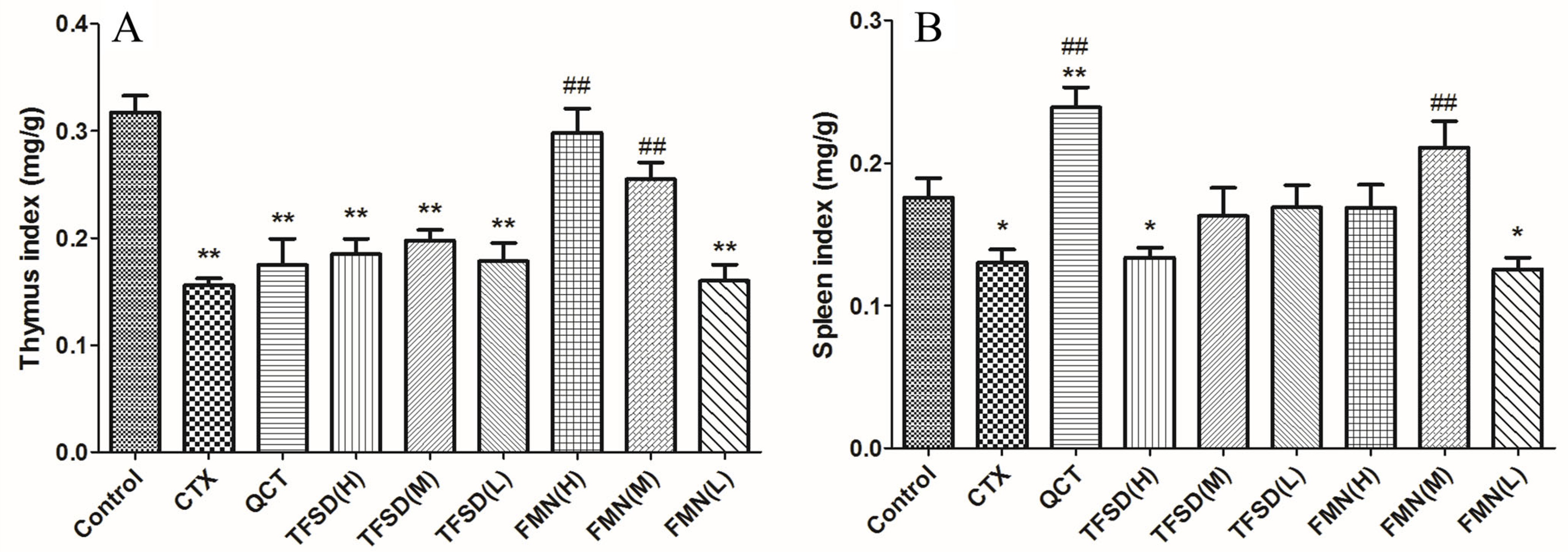
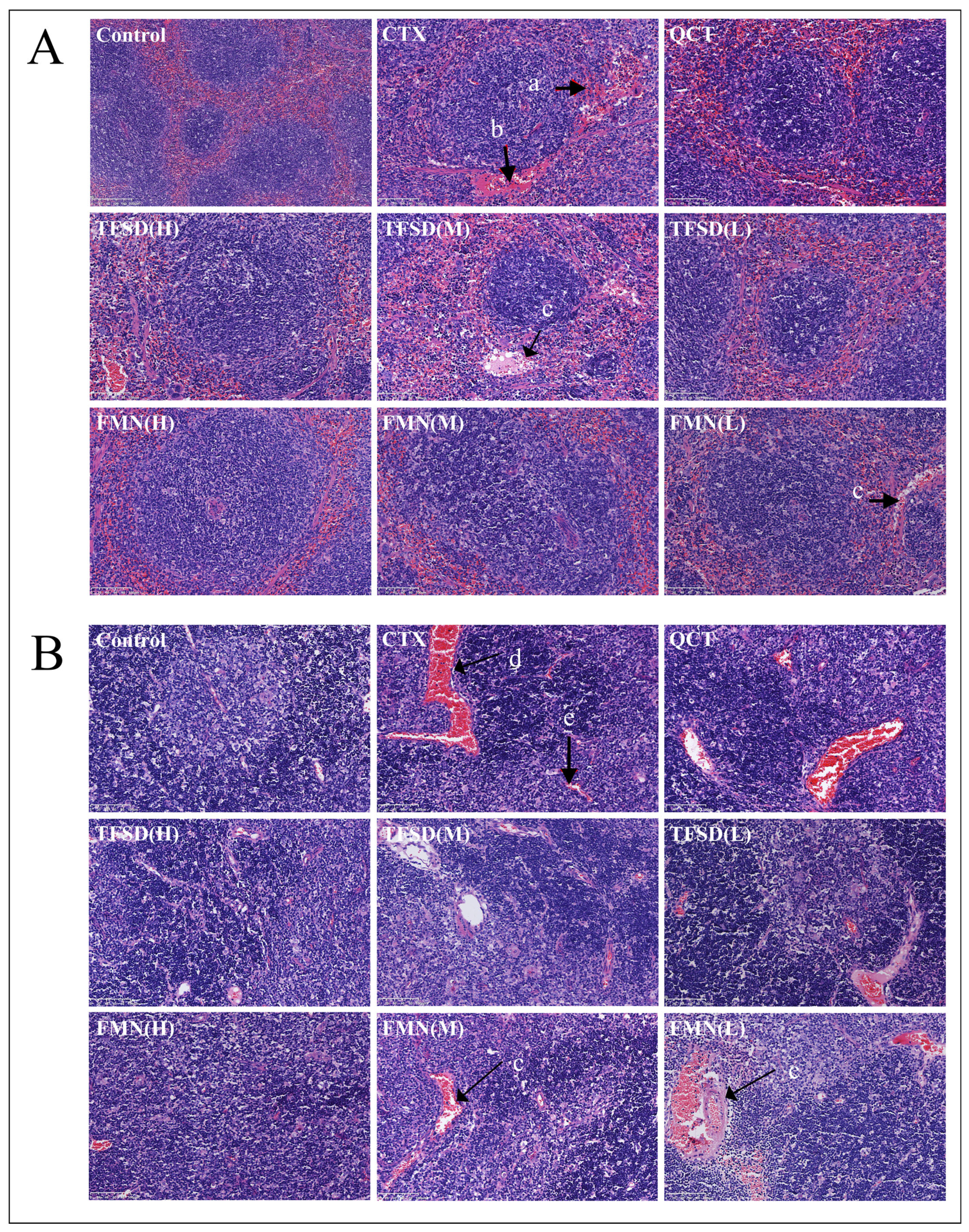
3.5. TFSD and FMN Alleviated CTX-Induced Injury in Immune Organs
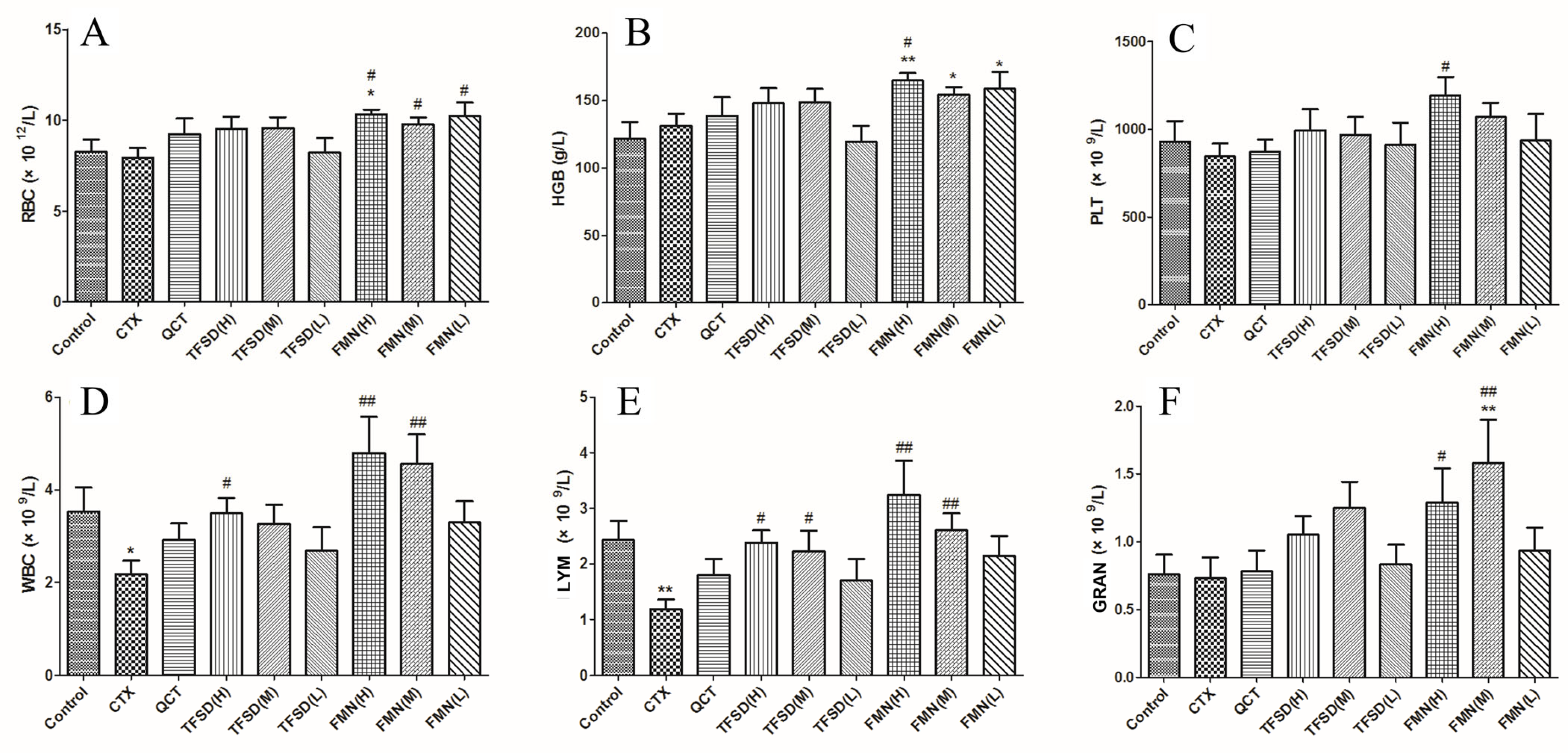
3.6. TFSD and FMN Modulated Biomarker Levels in the Blood Routine
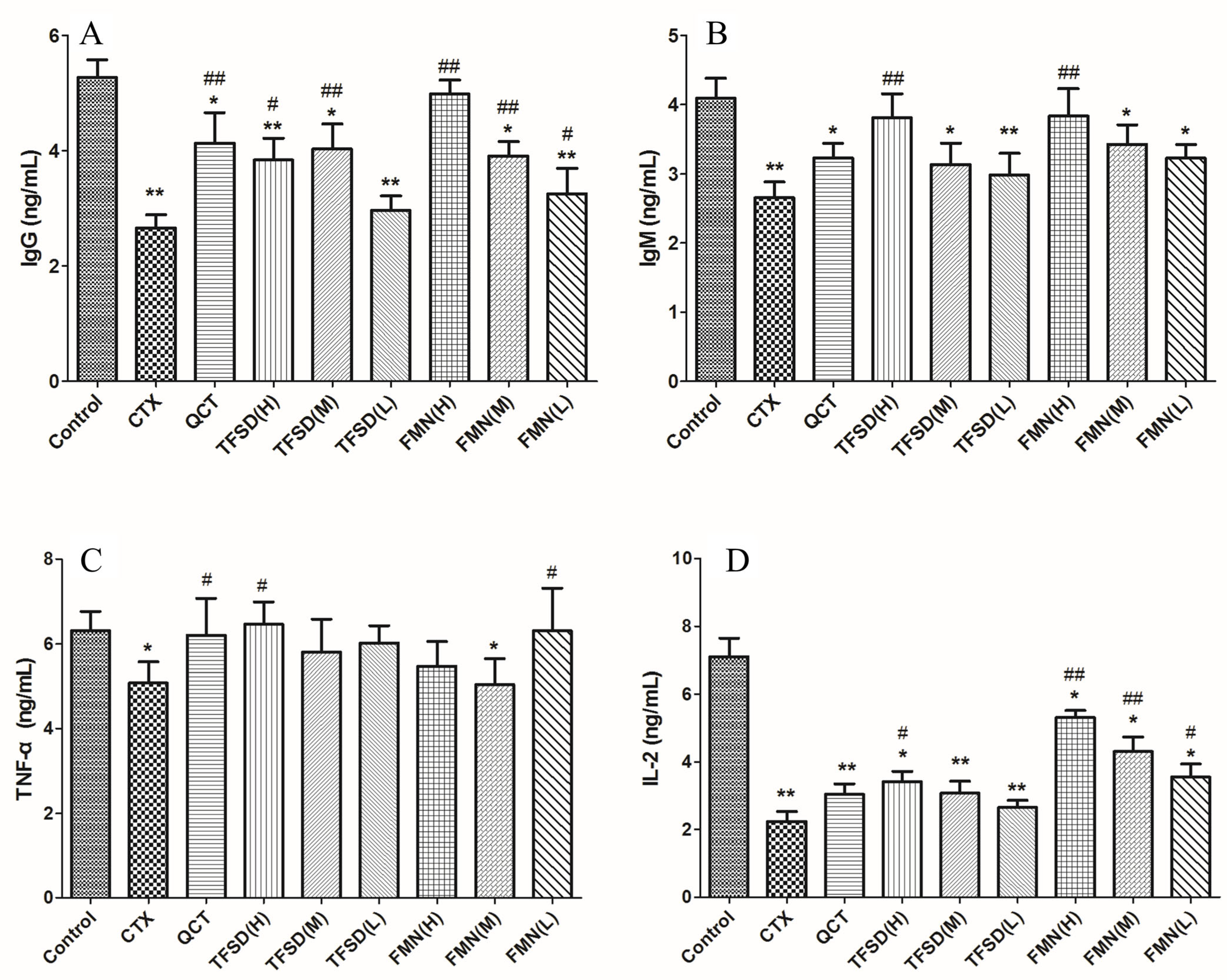
3.7. TFSD and FMN Promote Secretion of Immunoglobulins and Key Cytokines
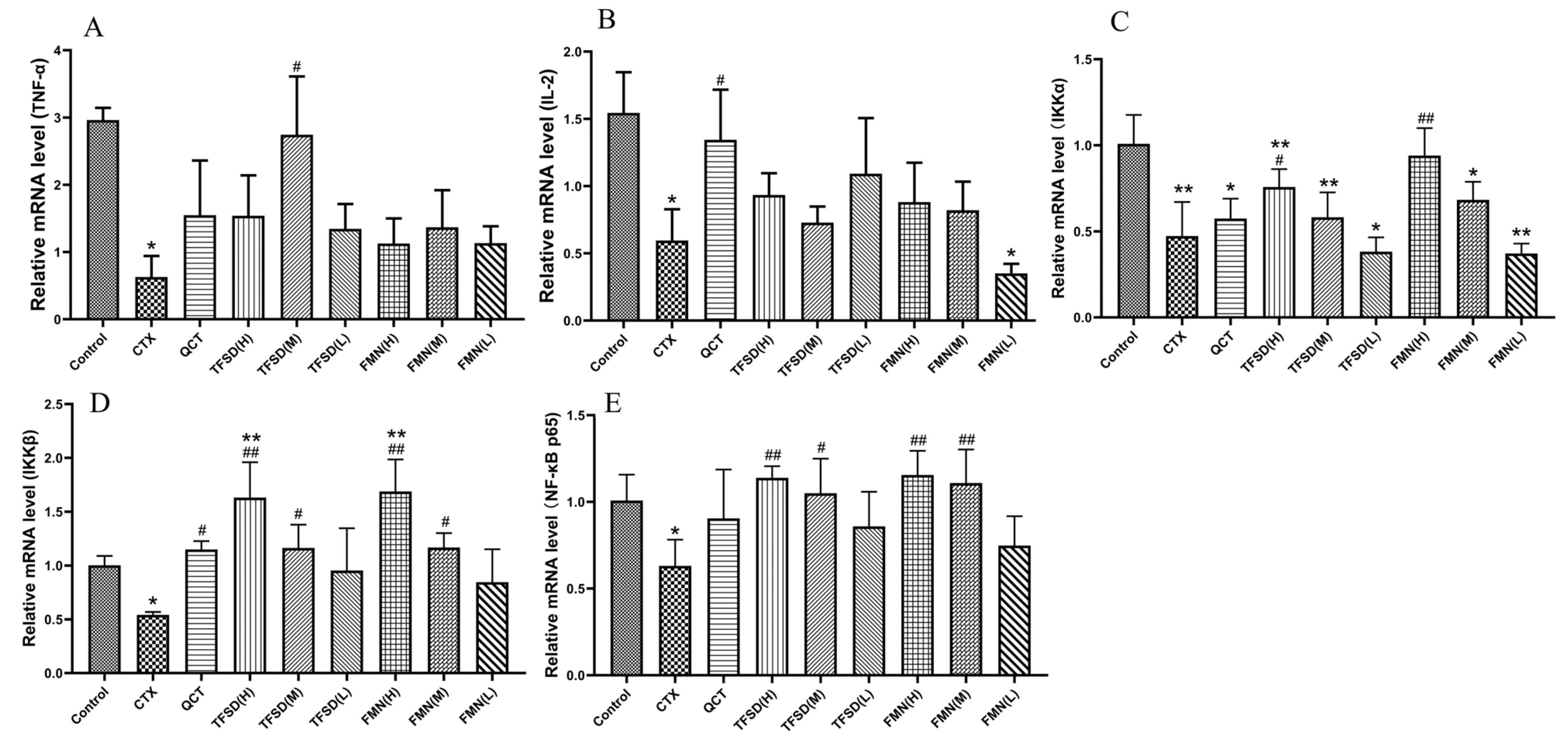
3.8. TFSD and FMN Regulated the mRNA Expression of Key Cytokines in the Spleen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TFSD | Total flavonoid of Spatholobus suberctus Dunn |
| CTX | Cyclophosphamide |
| NF-κB | Nuclear factor kappa-B |
| IKK | I Kappa B Kinase |
| QCT | Quercetin |
| TNF-α | Tumor necrosis factor-ɑ |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| IL-2 | Interleukin-2 |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| BP | Biological processes |
| CC | Cellular components |
| MF | Molecular functions |
| TIC | Total ion current |
| QC | Quality control |
| OB | Oral bioavailability |
| DL | Drug-likeness |
| PPI | Protein–protein interaction |
References
- Meng, F.; Xu, P.; Wang, X.; Huang, Y.; Wu, L.; Chen, Y.; Teng, L.; Wang, D. Investigation on the immunomodulatory activities of Sarcodon imbricatus extracts in a cyclophosphamide (CTX)-induced immunosuppressanted mouse model. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2017, 25, 460–463. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Zaccone, G.; Hussein, M.T. Morphological and ultrastructural insights into the goldfish (Carassius auratus) spleen: Immune organization and cellular composition. Vet. Sci. 2025, 12, 517. [Google Scholar] [CrossRef]
- Lussi, C.; Schweizer, M. What can pestiviral endonucleases teach us about innate immunotolerance? Cytokine Growth Factor Rev. 2016, 29, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Lu, L.M.; Wu, X.C.; Yang, T. Etiology and control measures for major immunosuppressive diseases in chickens. Anim. Ind. Environ. 2025, 53, 34–36. [Google Scholar]
- Luo, Y.; Li, S.; Sun, Y.; Qiu, H.J. Classical swine fever in China: A minireview. Vet. Microbiol. 2014, 172, 1–6. [Google Scholar] [CrossRef]
- Taira, O.; Kato, A.; Tsutsumi, N.; Sugiura, K. Epidemiological review of porcine reproductive and respiratory syndrome virus (PRRSV) in Japan: From discovery and spread to economic losses and future prospects. Vet. Sci. 2025, 12, 554. [Google Scholar] [CrossRef]
- Li, Z.L. The impact of common immunosuppressive diseases on pig health and prevention and control strategies. Beifang Muye 2025, 10, 34. [Google Scholar]
- Nazki, S.; Reddy, V.; Kamble, N.; Sadeyen, J.R.; Iqbal, M.; Behboudi, S.; Shelton, H.; Broadbent, A.J. CD4(+)TGFβ(+) cells infiltrated the bursa of Fabricius following IBDV infection, and correlated with a delayed viral clearance, but did not correlate with disease severity, or immunosuppression. Front. Immunol. 2023, 14, 1197746. [Google Scholar] [CrossRef]
- Miller, M.M.; Schat, K.A. Chicken infectious anemia virus: An example of the ultimate host-parasite relationship. Avian Dis. 2004, 48, 734–745. [Google Scholar] [CrossRef]
- Gimeno, I.M.; Schat, K.A. Virus-induced immunosuppression in chickens. Avian Dis. 2018, 62, 272–285. [Google Scholar] [CrossRef]
- Bakinowska, E.; Kiełbowski, K.; Pawlik, A. The role of extracellular vesicles in the pathogenesis and treatment of rheumatoid arthritis and osteoarthritis. Cells 2023, 12, 2716. [Google Scholar] [CrossRef]
- Kim, W.; Chu, T.H.; Nienhüser, H.; Jiang, Z.; Del Portillo, A.; Remotti, H.E.; White, R.A.; Hayakawa, Y.; Tomita, H.; Fox, J.G.; et al. PD-1 signaling promotes tumor-infiltrating myeloid-derived suppressor cells and gastric tumorigenesis in mice. Gastroenterology 2021, 160, 781–796. [Google Scholar] [CrossRef]
- Salazar Sandoval, S.; Díaz-Saldívar, P.; Araya, I.; Celis, F.; Cortés-Arriagada, D.; Riveros, A.; Rojas-Romo, C.; Jullian, C.; Silva, N.; Yutronic, N.; et al. Controlled release of the anticancer drug cyclophosphamide from a superparamagnetic β-Cyclodextrin nanosponge by local hyperthermia generated by an alternating magnetic field. ACS Appl. Mater. Interfaces 2025, 17, 13001–13017. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- He, Y.N.; Amer, H.M.; Xu, Z.H.; Liu, L.; Wu, S.J.; He, B.H.; Liu, J.Q.; Kai, G.Y. Exploration of the underlying mechanism of Astragaloside III in attenuating immunosuppression via network pharmacology and vitro/vivo pharmacological validation. J. Ethnopharmacol. 2024, 330, 1182–1196. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, M.; Zhang, K.; Niu, H.; Li, H.; Wu, W. Ginsenosides modulate immunity via TLR4/MyD88/NF-κB pathway and gut microbiota. Phytomedicine Int. J. Phytother. Phytopharm. 2025, 142, 156763. [Google Scholar] [CrossRef]
- Li, Y.; Lei, Z.; Guo, Y.; Liu, Y.; Guo, X.; Wang, X.; Che, J.; Yuan, J.; Wang, C.; Li, M. Fermentation of Ganoderma lucidum and Raphani Semen with a probiotic mixture attenuates cyclophosphamide-induced immunosuppression through microbiota-dependent or -independent regulation of intestinal mucosal barrier and immune responses. Phytomed. Int. J. Phytother. Phytopharm. 2023, 121, 1582–1601. [Google Scholar] [CrossRef]
- Cao, B.; Wei, G.L. Progress on the pharmacological effects of flavonoids in Spatholobus suberectus Dunn. Intern. Med. 2017, 12, 341–343. [Google Scholar]
- Fu, Y.F.; Jiang, L.H.; Zhao, W.D.; Xi-Nan, M.; Huang, S.Q.; Yang, J.; Hu, T.J.; Chen, H.L. Immunomodulatory and antioxidant effects of total flavonoids of Spatholobus suberectus Dunn on PCV2 infected mice. Sci. Rep. 2017, 7, 867–876. [Google Scholar] [CrossRef]
- Huang, S.Q. Regulatory Effect of Flavonoids Form Spatholobus suberectus Dunn on Escherichia coli Sepsis. Master’s Thesis, Guangxi University, Nanning, China, 2019. [Google Scholar]
- Chen, Q.P.; Xie, Y.N.; Xu, S.X.; Hu, X.Y.; Qing, Z.X.; Yu, D.L.; Xiao, P.; Xi, Y.L. Effect of adding Spatholobus suberectus extract in diet on the growth performance and immune function of Lingshan ma chicks. Mod. J. Anim. Husb. Vet. Med. 2025, 6, 34–38. [Google Scholar]
- Yang, N.N.; Zhang, Y.; Du, X.K.; Liu, N.; Zhang, J.; Yang, Q.; Sun, L.D.; Zhu, X.X.; Li, Q. Effect of Spatholobi Caulis extract from ethyl acetate on immune killing function of NK cells. China J. Chin. Mater. Medica 2024, 49, 1335–1342. [Google Scholar]
- Hu, T.J.; He, C.M.; Zhong, J. Preparation and anti-bacterial activities of extracts from Millettia reticulate. Prog. Vet. Med. 2010, 31, 33–36. [Google Scholar]
- Li, J.-X.; Li, R.Z.; Sun, A.; Zhou, H.; Neher, E.; Yang, J.S.; Huang, J.M.; Zhang, Y.Z.; Jiang, Z.B.; Liang, T.L.; et al. Metabolomics and integrated network pharmacology analysis reveal tricin as the active anti-cancer component of Weijing decoction by suppression of PRKCA and sphingolipid signaling. Pharmacol. Res. 2021, 171, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.D.; Meng, X.N.; Fu, Y.F.; Hu, T.J.; Huang, S.Q.; Chen, H.L. Effect of total flavonoids of Spatholobus suberectus Dunn on LPS-induced oxidative stress in RAW264.7 cells. Anim. Husb. Vet. 2017, 49, 47–51. [Google Scholar]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13–16. [Google Scholar] [CrossRef]
- Pichler, K.; Warner, K.; Magrane, M. SPIN: Submitting sequences determined at protein level to UniProt. Curr. Protoc. Bioinform. 2018, 62, e52–e60. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Gan, X.X.; Zhong, L.K.; Shen, F.; Feng, J.H.; Li, Y.Y.; Li, S.J.; Cai, W.S.; Xu, B. Network pharmacology to explore the molecular mechanisms of prunella vulgaris for treating hashimoto’s thyroiditis. Front. Pharmacol. 2021, 12, 700896. [Google Scholar] [CrossRef]
- Huang, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Res. 2017, 45, D331–D338. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Shi, X.R.; Chen, Y.K.; Chen, Z.T.; Zhao, W.D.; Jiang, M.S.; Chen, H.L. Protective Effects of total flavonoids from Spatholobus suberectus Dunn on liver injury induced by cyclophosphamide in mice. Anim. Husb. Feed. Sci. 2022, 43, 8–13. [Google Scholar]
- Li, M.; Jiang, C.; Chen, J.; Wang, J. Formononetin enhances the antitumor effect of H22 hepatoma transplanted mice. Chin. J. Cell. Mol. Immunol. 2023, 39, 1063–1068. [Google Scholar]
- Hsin, K.Y.; Ghosh, S.; Kitano, H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS ONE 2013, 8, e83922. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Pan, C.L.; Yao, J.X.; Huang, X.Y.; Yu, L.Y. Research progress on chemical constituents and pharmacological action of Spatholobus suberectus Dunn. Res. Pract. Chin. Med. 2021, 35, 96–102. [Google Scholar]
- Gao, H.; Kang, N.; Hu, C.; Zhang, Z.; Xu, Q.; Liu, Y.; Yang, S. Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine Int. J. Phytother. Phytopharm. 2020, 69, 153197. [Google Scholar] [CrossRef]
- Jia, D.; Lu, W.; Wang, C.; Sun, S.; Cai, G.; Li, Y.; Wang, G.; Liu, Y.; Zhang, M.; Wang, D. Investigation on immunomodulatory activity of calf spleen extractive injection in cyclophosphamide-induced immunosuppressed mice and underlying mechanisms. Scand. J. Immunol. 2016, 84, 20–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Tang, A.; Zeng, Z.; Zheng, W.; Luo, Y.; Huang, Y.; Dai, X.; Lu, W.; Fan, L.; et al. Cow placenta extract ameliorates cyclophosphamide-induced intestinal damage by enhancing the intestinal barrier, improving immune function, and restoring intestinal microbiota. Vet. Sci. 2024, 11, 505. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, L.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Immunomodulatory activity of carboxymethyl pachymaran on immunosuppressed mice induced by cyclophosphamide. Molecules 2021, 26, 5733. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Li, G.; Xue, S.; Zhang, G.; Dang, Y.; Wang, H. Modulating the gut microbiota is involved in the effect of low-molecular-weight Glycyrrhiza polysaccharide on immune function. Gut Microbes 2023, 15, 2276–2281. [Google Scholar] [CrossRef]
- Wang, J.; Tong, X.; Li, P.; Cao, H.; Su, W. Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 2012, 139, 788–795. [Google Scholar] [CrossRef]
- Xu, X.; Shao, T.; Meng, Y.; Liu, C.; Zhang, P.; Chen, K. Immunomodulatory mechanisms of an acidic polysaccharide from the fermented burdock residue by Rhizopus nigricans in RAW264.7 cells and cyclophosphamide-induced immunosuppressive mice. Int. J. Biol. Macromol. 2023, 252, 1264–1272. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Wang, Y.; Zhang, C.; Xu, S.; Luo, M.; Yang, S. Caragana sinica (Buc’hoz) Rehd. (jin ji er) polysaccharide regulates the immune function and intestinal microbiota of cyclophosphamide (CTX) induced immunosuppressed mice. J. Ethnopharmacol. 2023, 26, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Joss, J.; Mohammad, M.G. Functional analyses of lymphocytes and granulocytes isolated from the thymus, spiral valve intestine, spleen, and kidney of juvenile Australian lungfish, Neoceratodus forsteri. Fish Shellfish. Immunol. 2013, 35, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, J.; Huang, L. A study on the antitumour effect of total flavonoids from Pteris multifida Poir in H22 tumour-bearing mice. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, Y.; Zhao, S.; Zhang, M.; Yan, X.; Gao, X.; Li, J.; Gao, Y.; Zhang, A.; Gao, Y. Antitumor and immunomodulatory activities of total flavonoids extract from persimmon leaves in H (22) liver tumor-bearing mice. Sci. Rep. 2018, 8, 105–113. [Google Scholar] [CrossRef]
- Vidarsson, G.; Sigurdardottir, S.T.; Gudnason, T.; Kjartansson, S.; Kristinsson, K.G.; Ingolfsdottir, G.; Jonsson, S.; Valdimarsson, H.; Schiffman, G.; Schneerson, R.; et al. Isotypes and opsonophagocytosis of pneumococcus type 6B antibodies elicited in infants and adults by an experimental pneumococcus type 6B-tetanus toxoid vaccine. Infect. Immun. 1998, 66, 2866–2870. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Pan, L.; Guo, N.; Li, B.; Yao, J.; Wang, M.; Qi, C.; Zhang, G.; Liu, Z. Improvement of icaritin on hematopoietic function in cyclophosphamide-induced myelosuppression mice. Immunopharmacol. Immunotoxicol. 2018, 40, 25–34. [Google Scholar] [CrossRef]
- Starr, R.; Willson, T.A.; Viney, E.M.; Murray, L.J.; Rayner, J.R.; Jenkins, B.J.; Gonda, T.J.; Alexander, W.S.; Metcalf, D.; Nicola, N.A.; et al. A family of cytokine-inducible inhibitors of signalling. Nature 1997, 387, 917–921. [Google Scholar] [CrossRef]
- Kato, K.; Fukunaga, K.; Kamikozuru, K.; Kashiwamura, S.; Hida, N.; Ohda, Y.; Takeda, N.; Yoshida, K.; Iimuro, M.; Yokoyama, Y.; et al. Infliximab therapy impacts the peripheral immune system of immunomodulator and corticosteroid naïve patients with Crohn’s disease. Gut Liver 2011, 5, 37–45. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Pillars article: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 2011, 186, 3808–3821. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.R.; Galli, S.J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature 1990, 346, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Elkhalifa, A.; Weiner, H. Cyclophosphamide Treatment of MS: Current Therapeutic Approaches and Treatment Regimens. Int. MS J. 2010, 17, 12–18. [Google Scholar] [PubMed]
- Logani, M.K.; Alekseev, S.; Bhopale, M.K.; Slovinsky, W.S.; Ziskin, M.C. Effect of millimeter waves and cyclophosphamide on cytokine regulation. Immunopharmacol. Immunotoxicol. 2012, 34, 107–112. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Shen, C.Y.; Yang, L.; Jiang, J.G.; Zheng, C.Y.; Zhu, W. Immune enhancement effects and extraction optimization of polysaccharides from Citrus aurantium L. var. amara. Engl. Food Funct. 2017, 8, 796–807. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, I.C.; Ko, J.W.; Moon, C.; Kim, S.H.; Shin, I.S.; Seo, Y.W.; Kim, H.C.; Kim, J.C. Diallyl disulfide prevents cyclophosphamide-induced hemorrhagic cystitis in rats through the inhibition of oxidative damage, mapks, and nf-κb pathways. Biomol. Ther. 2015, 23, 180–188. [Google Scholar] [CrossRef]
- Taguchi, L.; Pinheiro, N.M.; Olivo, C.R.; Choqueta-Toledo, A.; Grecco, S.S.; Lopes, F.D.; Caperuto, L.C.; Martins, M.A.; Tiberio, I.F.; Câmara, N.O.; et al. A flavanone from Baccharis retusa (Asteraceae) prevents elastase-induced emphysema in mice by regulating NF-κB, oxidative stress and metalloproteinases. Respir. Res. 2015, 16, 79–84. [Google Scholar] [CrossRef]
- Sha, J.Y.; Chen, K.C.; Liu, Z.B.; Li, W.; Lu, Y.S.; Liu, S.; Ma, J.K.; Qu, D.; Sun, Y.S. Ginseng-DF ameliorates intestinal mucosal barrier injury and enhances immunity in immunosuppressed mice by regulating MAPK/NF-κB signaling pathways. Eur. J. Nutr. 2024, 63, 1487–1500. [Google Scholar] [CrossRef]
- Mou, M.S. Effects of Scutellaria baicalensis and Lactic Acid Bacteria on Growth Performance and Microflorain Ileum and Caecum of Broilers. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2009. [Google Scholar]
- Li, T.; Zou, Q.P.; Mao, Z.W.; Wu, S.L.; He, H.P.; Li, Y.P. Therapeutic effect of flavonoids from Desmodium triflorum on an ulcerative colitis mouse model and the influence on intestinal flora. Chin. J. Comp. Med. 2022, 32, 29–38. [Google Scholar]
| Group | D1 to D7 | D7 |
|---|---|---|
| Control | PBS 0.2 mL/mouse | PBS 0.2 mL/mouse |
| CTX | PBS 0.2 mL/mouse | CTX 200 mg/kg.bw |
| QCT | QCT 100 mg/kg.bw | CTX 200 mg/kg.bw |
| TFSD(H) | TFSD 100 mg/kg.bw | CTX 200 mg/kg.bw |
| TFSD(M) | TFSD 50 mg/kg.bw | CTX 200 mg/kg.bw |
| TFSD(L) | TFSD 25 mg/kg.bw | CTX 200 mg/kg.bw |
| FMN(H) | FMN 50 mg/kg.bw | CTX 200 mg/kg.bw |
| FMN(M) | FMN 25 mg/kg.bw | CTX 200 mg/kg.bw |
| FMN(L) | FMN 12.5 mg/kg.bw | CTX 200 mg/kg.bw |
| Gene | Primers Sequences (5′-3′) | Products Size (bp) | Accession Number |
|---|---|---|---|
| β-actin | F: 5′-ATCACTATTGGCAACGAGCG-3′ | 191 | NM_007393.5 |
| R: 5′-TCAGCAATGCCTGGGTACAT-3′ | |||
| TNF-α | F: 5′-AGCACAGAAAGCATGATCCG-3′ | 212 | NM_013693.3 |
| R: 5′-CTGATGAGAGGGAGGCCATT-3′ | |||
| IL-2 | F: 5′-GATGGATAGCCTTCTGTC-3′ | 82 | NM_008366.3 |
| R: 5′-GAGAGCCTTATGTGTTGT-3′ | |||
| IKKα | F: 5′-AGTTCTGCCCGCTCTCTTGTAG-3′ | 100 | XM_030250732.2 |
| R: 5′-GAGGATGTTCACGGTCTGCTAATG-3′ | |||
| IKKβ | F: 5′-GCAGAAGAGCGAAGTGGACATC-3′ | 112 | NM_001424831.1 |
| R: 5′-CAGCCGTTCAGCCAAGACAC-3′ | |||
| NF-κB p65 | F: 5′-GACCTGGAGCAAGCCATTAG-3′ | 125 | NM_001402548.1 |
| R: 5′-CGCACTGTCACCTGGAAGC-3′ |
| Sample | Class I | S. suberectus Dunn 1 | S. suberectus Dunn 2 | S. suberectus Dunn 3 |
|---|---|---|---|---|
| Formononetin | Flavonoids | 5.32 × 107 | 6.16 × 107 | 5.80 × 107 |
| Isoliquiritigenin | Flavonoids | 4.65 × 107 | 4.79 × 107 | 4.64 × 107 |
| Quercetin | Flavonoids | 3.60 × 107 | 3.16 × 107 | 3.49 × 107 |
| Medicagol | Flavonoids | 3.45 × 107 | 3.46 × 107 | 3.42 × 107 |
| Peganone I | Quinones | 3.18 × 107 | 2.19 × 107 | 2.68 × 107 |
| Stearic Acid | Lipids | 3.14 × 107 | 5.27 × 107 | 4.54 × 107 |
| 3′,7-dihydroxy-4′-methoxyflavone | Flavonoids | 2.85 × 107 | 2.53 × 107 | 2.57 × 107 |
| L-Pipecolic Acid | Alkaloids | 2.77 × 107 | 3.01 × 107 | 1.93 × 107 |
| Protogenkwanone | Flavonoids | 2.73 × 107 | 2.66 × 107 | 2.85 × 107 |
| 5,7,2′-Trihydroxy-8-methoxyflavone | Flavonoids | 2.68 × 107 | 2.62 × 107 | 2.78 × 107 |
| Mol ID | Component | OB (%) | DL |
|---|---|---|---|
| MOL000483 | (Z)-3-(4-hydroxy-3-methoxy-phenyl)-N-[2-(4-hydroxyphenyl)ethyl]acrylamide | 118.35 | 0.26 |
| MOL000471 | aloe-emodin | 83.38 | 0.24 |
| MOL000500 | Vestitol | 74.66 | 0.21 |
| MOL000468 | 8-o-Methylreyusi | 70.32 | 0.27 |
| MOL000507 | Psi-Baptigenin | 70.12 | 0.31 |
| MOL000392 | Formononetin | 69.67 | 0.21 |
| MOL000502 | Cajinin | 68.8 | 0.27 |
| MOL000501 | Consume close grain | 68.12 | 0.27 |
| MOL000506 | Lupinidine | 61.89 | 0.21 |
| MOL000503 | Medicagol | 57.49 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, B.; Huang, S.; Deng, J.; Liang, Y.; He, J.; Hu, T.; Xie, L.; Chen, H.; Yu, M. Flavonoids of S. suberectus Dunn Regulate Cyclophosphamide-Induced Immunosuppression Through NF-κB Pathways. Vet. Sci. 2025, 12, 762. https://doi.org/10.3390/vetsci12080762
Zhang J, Zhang B, Huang S, Deng J, Liang Y, He J, Hu T, Xie L, Chen H, Yu M. Flavonoids of S. suberectus Dunn Regulate Cyclophosphamide-Induced Immunosuppression Through NF-κB Pathways. Veterinary Sciences. 2025; 12(8):762. https://doi.org/10.3390/vetsci12080762
Chicago/Turabian StyleZhang, Jinwu, Bo Zhang, Shiqi Huang, Jianhao Deng, Yiying Liang, Jiakang He, Tingjun Hu, Liji Xie, Hailan Chen, and Meiling Yu. 2025. "Flavonoids of S. suberectus Dunn Regulate Cyclophosphamide-Induced Immunosuppression Through NF-κB Pathways" Veterinary Sciences 12, no. 8: 762. https://doi.org/10.3390/vetsci12080762
APA StyleZhang, J., Zhang, B., Huang, S., Deng, J., Liang, Y., He, J., Hu, T., Xie, L., Chen, H., & Yu, M. (2025). Flavonoids of S. suberectus Dunn Regulate Cyclophosphamide-Induced Immunosuppression Through NF-κB Pathways. Veterinary Sciences, 12(8), 762. https://doi.org/10.3390/vetsci12080762







