Comparative Analysis of Chemotherapy Resistance Mechanisms in Humans and Companion Animals
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
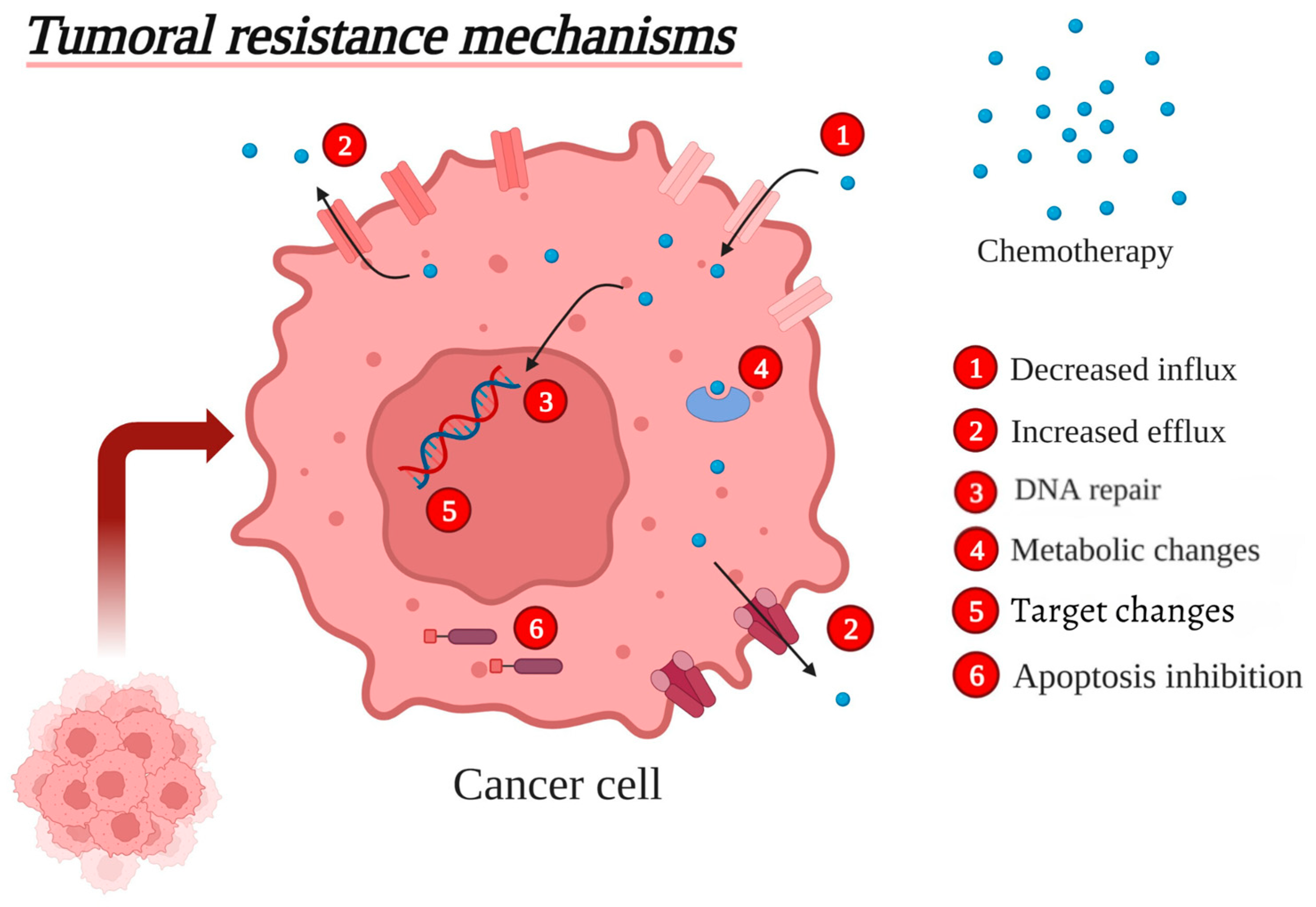
3. Major Tumoral Resistance Mechanisms
3.1. Decreased Influx of Chemotherapeutic Drugs
3.2. Increased Efflux of Chemotherapeutic Drugs
3.2.1. Multidrug Resistance Protein 1
3.2.2. MDR-Associated Protein 1
3.2.3. Breast Cancer Resistance Protein
3.2.4. Increased ABC Transporters
3.3. Enhanced DNA Repair
3.4. Metabolic Changes
Increased Drug Inactivation and Reduced Activation of Chemotherapeutic Agents
3.5. Changes in the Drug Targets
3.6. Apoptosis Inhibition
3.6.1. The BCL-2 Family of Proteins
3.6.2. Inhibitor-of-Apoptosis Proteins (IAPs)
3.6.3. Cellular FLICE-Inhibitory Protein (C-FLIP)
3.7. Multidrug Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| BCL-2 | B cell lymphoma-2 |
| BCRP | Breast cancer resistance protein |
| c-FLIP | Cellular FLICE-inhibitory protein |
| CML | Chronic myeloid leukemia |
| CYP | Cytochrome P450 |
| DCK | Deoxycytidine kinase |
| DISC | Death-inducing signaling complex |
| DOX | Doxorubicin |
| ER | Estrogen receptor |
| FPGS | Folylpolyglutamate synthetase |
| GGH | γ-glutamyl-hydrolase |
| GLUT2 | Glucose transporter 2 |
| GST | Glutathione S-transferases |
| HR | Homologous recombination |
| IAPs | Inhibitor-of-apoptosis proteins |
| MDR1 | Multidrug resistance protein 1 |
| MRP1 | MDR-associated protein 1 |
| MRR | Homologous recombination repair |
| NER | Nucleotide excision repair system |
| NSCLC | Non-small-cell lung carcinoma |
| PDGFR | Platelet-derived growth factor receptors |
| PgP | P-glycoprotein |
| RFC-1 | Reduced folate carrier 1 |
| SLC | Solute Carrier Family |
| TLA | Three-letter acronym |
| TS | Thymidylate synthase |
| TYMS | Thymidylate synthase gene |
| UGT | Uridine diphospho-glucuronosyltransferase |
| VEGFR1/2/3 | Vascular endothelial growth factor receptors 1, 2, and 3 |
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; Lleonart, M. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Cho, J.-Y. Comparative oncology: Overcoming human cancer through companion animal studies. Exp. Mol. Med. 2023, 55, 725–734. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140231. [Google Scholar] [CrossRef]
- Kisek, K.; Kinugasa-Katayama, Y.; Takakura, N. Tumor microenvironment for cancer stem cells. Adv. Drug Deliv. Rev. 2016, 99, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Piretto, E.; Delitala, M.; Ferraro, M. Combination therapies and intra-tumoral competition: Insights from mathematical modeling. J. Theor. Biol. 2018, 446, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Dickens, E.; Ahmed, S. Principles of cancer treatment by chemotherapy. Surgery 2018, 36, 134–138. [Google Scholar] [CrossRef]
- Pang, L.Y.; Argyle, D.J. Veterinary oncology: Biology, big data and precision medicine. Vet. J. 2016, 213, 38–45. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Kohn, B.; Gruber, A.D. Mechanisms of tumour resistance against chemotherapeutic agents in veterinary oncology. Vet. J. 2016, 207, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pawłowski, K.M.; Mucha, J.; Majchrzak, K.; Motyl, T.; Król, M. Expression and role of PGP, BCRP, MRP1 and MRP3 in multidrug resistance of canine mammary cancer cells. BMC Vet. Res. 2013, 9, 119. [Google Scholar] [CrossRef]
- Martinez, M.N.; Papich, M.G.; Drusano, G.L. Dosing regimen matters: The importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 2012, 56, 2795–2805. [Google Scholar] [CrossRef]
- Riviere, J.E.; Papich, M.G. Veterinary Pharmacology and Therapeutics, 10th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 123–140. [Google Scholar]
- Court, M.H.; Greenblatt, D.J. Molecular genetic basis for deficient acetaminophen glucuronidation in cats: UGT1A6 is a pseudogene, and UGT1A9 is absent. Pharmacogenet. Genom. 2000, 10, 355–369. [Google Scholar] [CrossRef]
- Zandvliet, M.; Teske, E.; Schrickx, J. Multi-drug resistance in a canine lymphoid cell line due to increased P-glycoprotein expression, a potential model for drug-resistant canine lymphoma. Toxicol. Vitro. 2014, 28, 1498–1506. [Google Scholar] [CrossRef]
- Rocha, C.R.R.; Silva, M.M.; Quinet, A.; Cabral-Neto, J.B.; Menck, C.F.M. DNA repair pathways and cisplatin resistance: An intimate relationship. Clinics 2018, 73 (Suppl. S1), e478s. [Google Scholar] [CrossRef]
- Szabó, I.; Orbán, E.; Schlosser, G.; Hudecz, F.; Bánóczi, Z. Cell-penetrating conjugates of pentaglutamylated methotrexate as potential anticancer drugs against resistant tumor cells. Eur. J. Med. Chem. 2016, 115, 361–368. [Google Scholar] [CrossRef]
- Xavier, J.B.; Young, V.B.; Skufca, J.; Ginty, F.; Testerman, T.; Pearson, A.T.; Macklin, P.; Mitchell, A.; Shmulevich, I.; Xie, L.; et al. The cancer microbiome: Distinguishing direct and indirect effects requires a systemic view. Trends Cancer 2020, 6, 192–204. [Google Scholar] [CrossRef]
- Miguel, R.B. Alvos Intracelulares e Mecanismos de ação de Complexos de cobre (II) ou Zinco (II) Oxindolimínicos Com Atividade Antitumoral. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2018. [Google Scholar]
- De man, F.M.; Goey, A.K.L.; van Schaik, R.H.N.; Mathijssen, R.H.J.; Bins, S. Individualization of irinotecan treatment: A review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin. Pharmacokinet. 2018, 57, 1229–1254. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, S.; Kikuchi, R.; Kusuhara, H.; Imai, S.; Maeda, K.; Sugiyama, Y. DNA methylation profiles of organic anion transporting polypeptide 1B3 in cancer cell lines. Pharm. Res. 2010, 27, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Goldman, I.D. Membrane transport of chemotherapeutics and drug resistance: Beyond the ABC family of exporters to the role of carrier-mediated processes. Clin. Cancer Res. 2002, 8, 4–6. [Google Scholar]
- Schnedl, W.J.; Ferber, S.; Johnson, J.H.; Newgard, C.B. STZ transport and cytotoxicity: Specific enhancement in GLUT2-expressing cells. Diabetes 1994, 43, 1326–1333. [Google Scholar] [CrossRef]
- Fleming, R.A. An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1997, 17, 146S–154S. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.; Monte, M.J.; Blazquez, A.G.; Macias, R.I.; A Serrano, M.; Briz, O. The role of reduced intracellular concentrations of active drugs in the lack of response to anticancer chemotherapy. Acta Pharmacol. Sin. 2013, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Puris, E.; Fricker, G.; Gynther, M. The role of solute carrier transporters in efficient anticancer drug delivery and therapy. Pharmaceutics 2023, 15, 364. [Google Scholar] [CrossRef]
- Zandvliet, M.; Teske, E. Mechanisms of Drug Resistance in Veterinary Oncology—A Review with an Emphasis on Canine Lymphoma. Vet. Sci. 2015, 2, 150–184. [Google Scholar] [CrossRef]
- Lewis, R.S.; Fidel, J.; Dassanayake, S.; Court, M.H.; Burke, N.S.; Mealey, K.L. Comparison of chemotherapeutic drug resistance in cells transfected with canineABCG2 or felineABCG. Vet. Comp. Oncol. 2015, 15, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.D.L.; Remião, F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef]
- Lilienthal, I.; Herold, N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int. J. Mol. Sci. 2020, 21, 6885. [Google Scholar] [CrossRef]
- Tomiyasu, H.; Tsujimoto, H. Comparative Aspects of Molecular Mechanisms of Drug Resistance through ABC Transporters and Other Related Molecules in Canine Lymphoma. Vet. Sci. 2015, 2, 185–205. [Google Scholar] [CrossRef]
- Levi, M.; Salaroli, R.; Parenti, F.; De Maria, R.; Zannoni, A.; Bernardini, C.; Gola, C.; Brocco, A.; Marangio, A.; Benazzi, C.; et al. Doxorubicin treatment modulates chemoresistance and affects the cell cycle in two canine mammary tumour cell lines. BMC Vet. Res. 2021, 17, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Zahreddine, H.; Borden, K.L.B. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.-P. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tiwari, A.K. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011, 278, 3226–3245. [Google Scholar] [CrossRef]
- Mealey, K.L.; Fidel, J. P-glycoprotein-mediated drug interactions in animals and humans: An overview. J. Vet. Pharmacol. Ther. 2015, 38, 444–455. [Google Scholar]
- Castro-López, J.; Teles, M.; Fierro, C.; Allenspach, K.; Planellas, M.; Pastor, J. Pilot study: Duodenal MDR1 and COX2 gene expression. In cats with inflammatory bowel disease and low-grade alimentary lymphoma. J. Feline Med. Surg. 2018, 20, 759–766. [Google Scholar] [CrossRef]
- Kurimchak, A.M.; Herrera-Montávez, C.; Montserrat-Sangrà, S.; Araiza-Olivera, D.; Hu, J.; Neumann-Domer, R.; Kuruvilla, M.; Bellacosa, A.; Testa, J.R.; Jin, J.; et al. The drug efflux pump MDR1 promotes intrinsic and acquired resistance to PROTACs in cancer cells. Sci. Signal. 2022, 15, eabn2707. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multi-drug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Sakaeda, T.; Nakamura, T.; Okumura, K. MDR1 genotype-related pharmacokinetics and pharmacodynamics. Biol. Pharm. Bull. 2002, 25, 1391–1400. [Google Scholar] [CrossRef]
- Conseil, G.; Deeley, R.G.; Cole, S.P.C. Polymorphisms of MRP1 (ABCC1) and related ATP-dependent drug transporters. Pharmacogenet. Genom. 2005, 15, 523–533. [Google Scholar] [CrossRef]
- Munoz, M.; Henderson, M.; Haber, M.; Norris, M. Role of the MRP1/ABCC1 multidrug transporter protein in cancer. IUBMB Life 2007, 59, 752–757. [Google Scholar] [CrossRef]
- Cole, S.P. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J. Biol. Chem. 2014, 289, 30880–30888. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Pokharel, D.; Bebawy, M. MRP1 and its role in anticancer drug resistance. Drug Metab. Rev. 2015, 47, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-D.; Shang, Y.; Wang, C.; Ni, J.; Wang, A.-M.; Li, G.-J.; Su, L.; Chen, S.-Z. c-FLIP promotes drug resistance in non-small-cell lung cancer cells via upregulating FoxM1 expression. Acta Pharmacol. Sin. 2022, 43, 2956–2966. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Ross, D.D. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 31, 73–99. [Google Scholar] [CrossRef]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2014, 17, 65–82. [Google Scholar] [CrossRef]
- Noguchi, M.; Inokuchi, M.; Zen, Y. Complement of peritumoral and subareolar injection in breast cancer sentinel lymph node biopsy. J. Surg. Oncol. 2009, 100, 100–105. [Google Scholar] [CrossRef]
- Chong, T.C.; Wong, I.L.K.; Cui, J.; Law, M.C.; Zhu, X.; Hu, X.; Kan, J.W.Y.; Yan, C.S.W.; Chan, T.H.; Chow, L.M.C. Characterization of a Potent, Selective, and Safe Inhibitor, Ac15(Az8)2, in Reversing Multidrug Resistance Mediated by Breast Cancer Resistance Protein (BCRP/ABCG2). Int. J. Mol. Sci. 2022, 23, 13261. [Google Scholar] [CrossRef]
- Imai, Y.; Nakane, M.; Kage, K.; Tsukahara, S.; Ishikawa, E.; Tsuruo, T.; Miki, Y.; Sugimoto, Y. C421A Polymorphism in the Human Breast Cancer Resistance Protein Gene is Associated with Low Expression of Q141K Protein and Low-Level Drug Resistance. Mol. Cancer Ther. 2002, 1, 611–616. [Google Scholar]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Gustafson, D.L.; Bailey, D.B. Cancer chemotherapy. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D., Thamm, D.H., Liptak, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 181–208. [Google Scholar]
- Arora, S.; Kothandapani, A.; Tillison, K.; Kalman-Maltese, V.; Patrick, S.M. Downregulation of XPF–ERCC1 enhances cisplatin efficacy in cancer cells. DNA Repair 2010, 9, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Mao, P.; Jiang, G.; Liu, L.; Wang, J.; Yang, W.; Owusu, L.; Li, W. Functional characterization of a novel transcript of ERCC1 in chemotherapy resistance of ovarian cancer. Oncotarget 2017, 8, 85759–85771. [Google Scholar] [CrossRef]
- Marchandet, L.; Lallier, M.; Charrier, C.; Baud’huin, M.; Ory, B.; Lamoureux, F. Mechanisms of resistance to conventional therapies for osteosarcoma. Cancers 2021, 13, 683. [Google Scholar] [CrossRef]
- Ludwig, L.; Dobromylskyj, M.; Wood, G.A.; van der Weyden, L. Feline oncogenomics: What do we know about the genetics of cancer in domestic cats? Vet. Sci. 2022, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.M.; Scherl, A.; Nguyen, A.; Man, F.Y.; Weinberg, E.; Zeng, Z.; Saltz, L.; Paty, P.B.; Tavazoie, S.F. Extracellular metabolic energetics can promote cancer progression. Cell 2015, 160, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A. Fine-tuning the lipogenic/lipolytic balance to optimize the metabolic requirements of cancer cell growth: Molecular mechanisms and therapeutic perspectives. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2010, 1801, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Cocetta, V.; Ragazzi, E.; Montopoli, M. Links between cancer metabolism and cisplatin resistance. Int. Rev. Cell Mol. Biol. 2020, 354, 107–164. [Google Scholar] [CrossRef]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef]
- Obre, E.; Rossignol, R. Emerging concepts in bioenergetics and cancer research: Met-abolic flexibility, coupling, sym-biosis, 563 switch, oxidative tumors, metabolic remodeling, signaling and bioenergetic therapy. Int. J. Biochem. Cell Biol. 2015, 59, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Min, H.-Y.; Lee, H.-Y. Cellular dormancy in cancer: Mechanisms and potential targeting strategies. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2023, 55, 720–736. [Google Scholar] [CrossRef] [PubMed]
- Schiliro, C.; Firestein, B.L. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Hu, X.; Xu, B.; Tong, T.; Jing, Y.; Xi, L.; Zhou, W.; Lu, J.; Wang, X.; Yang, X.; et al. Glutathione S-transferase isozyme alpha 1 is predominantly involved in the cisplatin resistance of common types of solid cancer. Oncol. Rep. 2019, 41, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell pro-liferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Lowell, B.B.; Shulman, G.I. Mitochondrial dysfunction and type 2 diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Marin-Valencia, I.; Yang, C.; Mashimo, T.; Cho, S.; Baek, H.; Yang, X.L.; Rajagopalan, K.N.; Maddie, M.; Vemireddy, V.; Zhao, Z.; et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in ge-netically diverse human glioblastomas in the mouse brain. J. Clin. Investig. 2012, 122, 393–401. [Google Scholar]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef]
- Paoloni, M.; Khanna, C. Translation of new cancer treatments from pet dogs to humans. Nat. Rev. Cancer 2008, 8, 147–156. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Pires, I.; Parente, M.; Gregório, H.; Lopes, C.S. COX-2 over-expression correlates with VEGF and tumour angiogenesis in canine mammary cancer. Vet. J. 2011, 189, 77–82. [Google Scholar] [CrossRef]
- Smedley, R.C.; Spangler, W.L.; Esplin, D.G.; Kitchell, B.E.; Bergman, P.J.; Ho, H.Y.; Bergin, I.L.; Kiupel, M. Prognostic markers for canine melanocytic neoplasms: A comparative review of the literature and goals for future investigation. Vet. Pathol. 2011, 48, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.L.; Hsieh, C.M.; Chan, N.L.; Hiasa, H. Topoisomerases as anticancer targets. Biochem. J. 2018, 475, 373–398. [Google Scholar] [CrossRef]
- Gorre, M.E.; Mohammed, M.; Ellwood, K.; Hsu, N.; Paquette, R.; Rao, P.N.; Sawyers, C.L. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001, 293, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Toy, W.; Shen, Y.; Won, H.; Green, B.; Sakr, R.A.; Will, M.; Li, Z.; Gala, K.; Fanning, S.; King, T.A.; et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat. Genet. 2013, 45, 1439–1445. [Google Scholar] [CrossRef]
- Zandvliet, M. Canine lymphoma: A review. Vet. Q. 2016, 36, 76–104. [Google Scholar] [CrossRef]
- London, C.A.; Malpas, P.B.; Wood-Follis, S.L.; Boucher, J.F.; Rusk, A.W.; Rosenberg, M.P.; Henry, C.J.; Mitchener, K.L.; Klein, M.K.; Hintermeister, J.G. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin. Cancer Res. 2009, 15, 3856–3865. [Google Scholar] [CrossRef]
- Martano, M.; Morello, E.; Buracco, P. Feline injection-site sarcoma: Past, present and future perspectives. Vet. J. 2011, 188, 136–141. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac. J. Cancer Prev. 2015, 16, 2129–2144. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- D’aRcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Rebhun, R.B.; Lana, S.E.; Ehrhart, E.J.; Charles, J.B.; Thamm, D.H. Comparative analysis of survivin expression in untreated and re-lapsed canine lymphoma. J. Vet. Intern. Med. 2008, 22, 989–995. [Google Scholar] [CrossRef]
- Opferman, J.T.; Kothari, A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2017, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Straten, P.T.; Andersen, M.H. The anti-apoptotic members of the Bcl-2 family are attractive tumor-associated antigens. Oncotarget 2010, 1, 239–245. [Google Scholar] [CrossRef]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 117. [Google Scholar] [CrossRef]
- Valentini, E.; D’AGuanno, S.; Di Martile, M.; Montesano, C.; Ferraresi, V.; Patsilinakos, A.; Sabatino, M.; Antonini, L.; Chiacchiarini, M.; Valente, S.; et al. Targeting the anti-apoptotic Bcl-2 family proteins: Machine learning virtual screening and biological evaluation of new small molecules. Theranostics 2022, 12, 2427–2444. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2017, 25, 27–36. [Google Scholar] [CrossRef]
- Weyhenmeyer, B.; Murphy, A.C.; Prehn, J.; Murphy, B. Targeting the anti-apoptotic Bcl-2 family members for the treatment of cancer. Exp. Oncol. 2012, 34, 192–199. [Google Scholar] [PubMed]
- Opferman, J.T. Attacking cancer’s Achilles heel: Antagonism of anti-apoptotic BCL-2 family members. FEBS J. 2015, 283, 2661–2675. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Liu, Y.; Han, B. Bivalent SMAC Mimetics for Treating Cancer by Antagonizing Inhibitor of Apoptosis Proteins. ChemMedChem 2019, 14, 1951–1962. [Google Scholar] [CrossRef]
- Bagnoli, M.; Canevari, S.; Mezzanzanica, D. Cellular FLICE-inhibitory protein (c-FLIP) signalling: A key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int. J. Biochem. Cell Biol. 2010, 42, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Shirley, S.; Micheau, O. Targeting c-FLIP in cancer. Cancer Lett. 2013, 332, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska, H.; Piekiełko-Witkowska, A. Splicing factors of SR and hnRNP families as regulators of apoptosis in cancer. Cancer Lett. 2017, 396, 53–65. [Google Scholar] [CrossRef]
- Wu, G.; Diaz, A.K.; Paugh, B.S.; Rankin, S.L.; Ju, B.; Li, Y.; Zhu, X.; Qu, C.; Chen, X.; Zhang, J.; et al. The genomic 618 landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014, 46, 444–450. [Google Scholar]
- Hashemi, M.; Arani, H.Z.; Orouei, S.; Fallah, S.; Ghorbani, A.; Khaledabadi, M.; Kakavand, A.; Tavakolpournegari, A.; Saebfar, H.; Heidari, H.; et al. EMT mechanism in breast cancer metastasis and drug resistance: Revisiting molecular interactions and biological functions. Biomed. Pharmacother. 2022, 155, 113774. [Google Scholar] [CrossRef]
- Duesberg, P.; Stindl, R.; Hehlmann, R. Explaining the high mutation rates of cancer cells to drug and multidrug resistance by chromosome reassortments that are catalyzed by aneuploidy. Proc. Natl. Acad. Sci. USA 2000, 97, 14295–14300. [Google Scholar] [CrossRef]
- Wilson, T.R.; Redmond, K.M.; McLaughlin, K.M.; Crawford, N.; Gately, K.; O’Byrne, K.; Le-Clorrenec, C.; Holohan, C.; A Fennell, D.; Johnston, P.G.; et al. Procaspase 8 overexpression in non-small-cell lung cancer promotes apoptosis induced by FLIP silencing. Cell Death Differ. 2009, 16, 1352–1361. [Google Scholar] [CrossRef]
- Kirschner, K.; Melton, D.W. Multiple roles of the ERCC1-XPF endonuclease in DNA repair and resistance to anticancer drugs. Anticancer. Res. 2010, 30, 3223–3232. [Google Scholar]
- Tanaka, K.; Miyata, H.; Sugimura, K.; Fukuda, S.; Kanemura, T.; Yamashita, K.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; et al. miR-27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer-associated fibroblasts. Carcinogenesis 2015, 36, 894–903. [Google Scholar] [CrossRef]
- Raghunand, N.; Mahoney, B.P.; Gillies, R.J. Tumor acidity, ion trap-ping and chemotherapeutics. II. pH-dependent partition co-ef-cients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem. Pharmacol. 2003, 66, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, T.; Pradeep, S.; McGuire, M.; Wu, S.Y.; Ma, S.; Hatakeyama, H.; Lyons, Y.A.; Hisamatsu, T.; Noh, K.; Villar-Prados, A.; et al. Induction of anti-VEGF therapy resistance by up-regu-lated expression of microseminoprotein (MSMP). Oncogene 2018, 37, 722–731. [Google Scholar] [CrossRef]
- Kim, S.-J.; Uehara, H.; Yazici, S.; Busby, J.E.; Nakamura, T.; He, J.; Maya, M.; Logothetis, C.; Mathew, P.; Wang, X.; et al. Targeting platelet-derived growth factor receptor on endothelial cells of multidrug-resistant prostate cancer. JNCI J. Natl. Cancer Inst. 2006, 98, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Shee, K.; Yang, W.; Hinds, J.W.; Hampsch, R.A.; Varn, F.S.; Traphagen, N.A.; Patel, K.; Cheng, C.; Jenkins, N.P.; Kettenbach, A.N.; et al. Therapeutically targeting tumor microenvironment–mediated drug resistance in estrogen receptor–positive breast cancer. J. Exp. Med. 2018, 215, 895–910. [Google Scholar] [CrossRef]
- Lippolis, C.; Refolo, M.G.; D’alessandro, R.; Carella, N.; Messa, C.; Cavallini, A.; Carr, B.I. Resistance to multikinase inhibitor actions mediated by insulin like growth factor-1. J. Exp. Clin. Cancer Res. 2015, 34, 90. [Google Scholar] [CrossRef] [PubMed]
- Kavallaris, M.; Tait, A.S.; Walsh, B.J.; He, L.; Horwitz, S.B.; Norris, M.D.; Haber, M. Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells. Cancer Res. 2001, 61, 5803–5809. [Google Scholar] [PubMed]
- Chen, S.; Dai, Y.; Harada, H.; Dent, P.; Grant, S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007, 67, 782–791. [Google Scholar] [CrossRef]
- Xu, W.; Wang, S.; Chen, Q.; Zhang, Y.; Ni, P.; Wu, X.; Zhang, J.; Qiang, F.; Li, A.; Røe, O.D.; et al. TXNL1-XRCC1 pathwayregulates cisplatin-induced cell death and contributes to resistance in human gastric cancer. Cell Death Dis. 2014, 5, e1055. [Google Scholar] [CrossRef]
- Tan, G.M.Y.; Poudel, A.; Rad, S.M.A.H.; McLellan, A.D. Anti-Apoptotic c-FLIP Reduces the Anti-Tumour Activity of Chimeric Antigen Receptor T Cells. Cancers 2022, 14, 4854. [Google Scholar] [CrossRef] [PubMed]
- Piro, F.; Leonardi, L.; Rossi, R. Expression of Bcl 2 in spontaneous canine osteosarcoma. Open Vet. J. 2015, 5, 27–29. [Google Scholar] [PubMed]
- Barra, C.N.; Macedo, B.M.; Cadrobbi, K.G.; Pulz, L.H.; Huete, G.C.; Kleeb, S.R.; Xavier, J.G.; Catão-Dias, J.L.; Nishiya, A.T.; Fukumasu, H.; et al. Apoptotic intrinsic pathway proteins predict survival in canine cutaneous mast cell tumours. Vet. Comp. Oncol. 2018, 16, 248–257. [Google Scholar] [CrossRef] [PubMed]
| Resistance Mechanism | Chemotherapeutic Agents | Cancer Type | References |
|---|---|---|---|
| Increased output and decreased input | Vinca alkaloids (vinblastine, vincristine, and catharanthine), anthracyclines (doxorubicin and daunorubicin), taxanes (paclitaxel and docetaxel), epipodophyllotoxins (etoposide and teniposide), camptothecins derivates (topotecan and methotrexate), anthracenes (bisantrene and mitoxantrone), platinum compounds (for example, cisplatin and oxaliplatin), and proteolysis-targeting chimera drugs. | Canine lymphoma; acute lymphoblastic leukemia, non-small-cell lung carcinoma (NSCLC), human ovarian cancer, canine mammary tumor cell, acute myeloid leukemia, human colon carcinoma, human breast carcinoma, human gastric carcinoma, human fibrosarcoma, human glioblastoma, human myeloma, human prostate cancer cell, and human osteosarcoma. | [31,32,35,36,44,45,46,48,49,50,51,70,104] |
| Enhanced DNA repair | Vinca alkaloids (vinblastine, vincristine, and catharanthine), platinum compounds (cisplatin and oxaliplatin), anthracyclines (doxorubicin), and topoisomerase I inhibitors (irinotecan). | Human ovarian cancer, human testicular cancer, sarcoma, canine lymphoma, human osteosarcoma, and human small-cell lung carcinoma. | [16,32,35,56,70,105] |
| Metabolic changes and detoxification | Antimetabolites (5-fluorouracil, cytosine arabinoside, methotrexate, gemcitabine, and cytarabine), vinca alkaloids (vinblastine, vincristine, and catharanthine), and platinum compounds (cisplatin and oxaliplatin). | Human breast cancer, human colorectal cancer, human pancreatic cancer, human gastric cancer, human head and neck cancer, ovarian cancer, canine lymphoma, leukemia, human testicular cancer, human osteosarcoma, and human small-cell lung carcinoma. | [16,32,56,65,106,107] |
| Tumor microenvironment modulation | mTORC1 inhibitors, PI3K, platinum drugs, DOX, VEGFR1/2/3 inhibitors, histone deacetylase inhibitors, phosphatidylinositol3-kinase, anti-VEF drugs, and taxane drugs. | Human esophageal cancer, human prostate cancer, and human breast cancer. | [108,109,110,111,112,113] |
| Cell-targets modification | Camptothecins derivatives (topotecan and methotrexate) and topoisomerase I inhibitors (irinotecan). | Human breast cancer, canine lymphoma, human leukemia, human colorectal cancer, human small-cell lung carcinoma and human chronic myelogenous leukemia, and human osteosarcoma. | [16,32,35,56,104,114] |
| Apoptosis inhibition | Vinca alkaloids (vinblastine, vincristine, and catharanthine), platinum compounds (cisplatin), anthracyclines (doxorubicin), epipodophyllotoxins (etoposide), and topoisomerase I inhibitors (irinotecan). | Human colorectal cancer, human small-cell lung carcinoma, human gastric adenocarcinoma, human anaplastic thyroid cancer, human pancreatic carcinoma, human Kaposi’s sarcoma, human Ewing’s sarcoma, human non-small-cell lung cancer, human breast cancer, canine lymphoma, human leukemia, human myeloma, human osteosarcoma, human glioblastoma, human melanoma, feline mammary carcinoma, canine osteosarcoma, and mast cell tumor. | [29,32,35,50,65,88,95,99,101,111,115,116,117,118,119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cravo, D.L.d.M.; Pimentel, P.A.B.; Garcia, A.P.V.; Junqueira, A.L.d.M.; Soares, F.S.; Giuliano, A.; Almendros, A.; Horta, R.d.S. Comparative Analysis of Chemotherapy Resistance Mechanisms in Humans and Companion Animals. Vet. Sci. 2025, 12, 747. https://doi.org/10.3390/vetsci12080747
Cravo DLdM, Pimentel PAB, Garcia APV, Junqueira ALdM, Soares FS, Giuliano A, Almendros A, Horta RdS. Comparative Analysis of Chemotherapy Resistance Mechanisms in Humans and Companion Animals. Veterinary Sciences. 2025; 12(8):747. https://doi.org/10.3390/vetsci12080747
Chicago/Turabian StyleCravo, Daniel Luiz de Miranda, Pedro Antônio Bronhara Pimentel, Ana Paula Vargas Garcia, André Luiz de Moura Junqueira, Fabiana Sanches Soares, Antonio Giuliano, Angel Almendros, and Rodrigo dos Santos Horta. 2025. "Comparative Analysis of Chemotherapy Resistance Mechanisms in Humans and Companion Animals" Veterinary Sciences 12, no. 8: 747. https://doi.org/10.3390/vetsci12080747
APA StyleCravo, D. L. d. M., Pimentel, P. A. B., Garcia, A. P. V., Junqueira, A. L. d. M., Soares, F. S., Giuliano, A., Almendros, A., & Horta, R. d. S. (2025). Comparative Analysis of Chemotherapy Resistance Mechanisms in Humans and Companion Animals. Veterinary Sciences, 12(8), 747. https://doi.org/10.3390/vetsci12080747






