1. Introduction
The initial stages of postnatal piglet development are critical for the establishment of the gastrointestinal tract’s structure, the maturation of digestive enzymes, and the colonization of intestinal microbiota [
1,
2]. The early weeks of a piglet’s life represent a pivotal “window of opportunity” that facilitates the intestinal adaptation processes, which in turn confer lifelong growth advantages [
3]. Higher weaning weights in piglets are correlated with enhanced average daily gains during the subsequent growing-finishing phases and earlier attainment of slaughter weights [
4]. On large, modern pig farms, neonatal piglets face numerous stressors, including insufficient milk supply, pathogen exposure, and suboptimal management practices. These challenges can lead to compromised intestinal development, diminished immune responses, alterations in the intestinal microbiota, reduced growth performance, and increased mortality rates and incidence of diarrhea [
5,
6]. However, current nutritional interventions often show variable efficacy or fail to comprehensively address the multifactorial nature of these stressors. In practical pig farming, this often manifests as a high incidence of pre-weaning diarrhea, impaired immune function, and increased mortality, particularly in piglets facing early-life stressors [
7,
8,
9]. Therefore, improving intestinal health and development during the suckling period is crucial for the long-term wellbeing of pigs.
Nutritional interventions early in life are imperative to modify the gut microbiota composition of piglets and bolster their immune systems [
10,
11]. Current interventions include oral supplementation (such as prebiotics and functional amino acids), enhanced milk provision, and the administration of peristaltic feeds before weaning. Research has shown that supplementation with an alkaline mineral complex in drinking water can enhance intestinal morphology, mitigate inflammatory responses, and fortify intestinal barrier function, thereby accelerating the growth performance of weaned piglets [
12]. The Na
+/K
+ ratio plays an important role in maintaining intestinal ionic homeostasis and facilitating the transport of nutrients by small intestinal epithelial cells, such as glucose [
13,
14]. Studies have shown that early supplementation of isotonic protein solutions can improve the intestinal microbiota of piglets [
15]. Despite these advances, there remains a need for integrated nutritional strategies that simultaneously target multiple aspects of gut health (microbiota, barrier, immunity) in the critical pre-weaning period. Consequently, we have designed an isotonic protein solution nutritional supplement designed to enhance the gut health and growth performance of pre-weaning piglets, mainly containing whey protein, Na
+/K
+, and glucose. This study aims to evaluate the effects of this synergistic formulation on gut microbiota composition, intestinal barrier integrity and morphology, immune markers, and growth performance in pre-weaning piglets, hypothesizing that it will enhance intestinal resilience and provide a foundation for improved lifelong productivity.
2. Materials and Methods
2.1. Animals
All experiments were conducted in strict accordance with the principles and guidelines of the Southwest University Institutional Animal Care and Use Committee (Approval No. GB14925-2010, 2019). The study adhered to the ARRIVE guidelines to ensure ethical and transparent reporting of animal research. The piglets used in the experiment, along with their feeding and management, were sourced from and handled at Guangyuan Futai Pig Farm, a commercial and standardized pig breeding facility located in Sichuan, China.
2.2. Study Design and Animal Diets
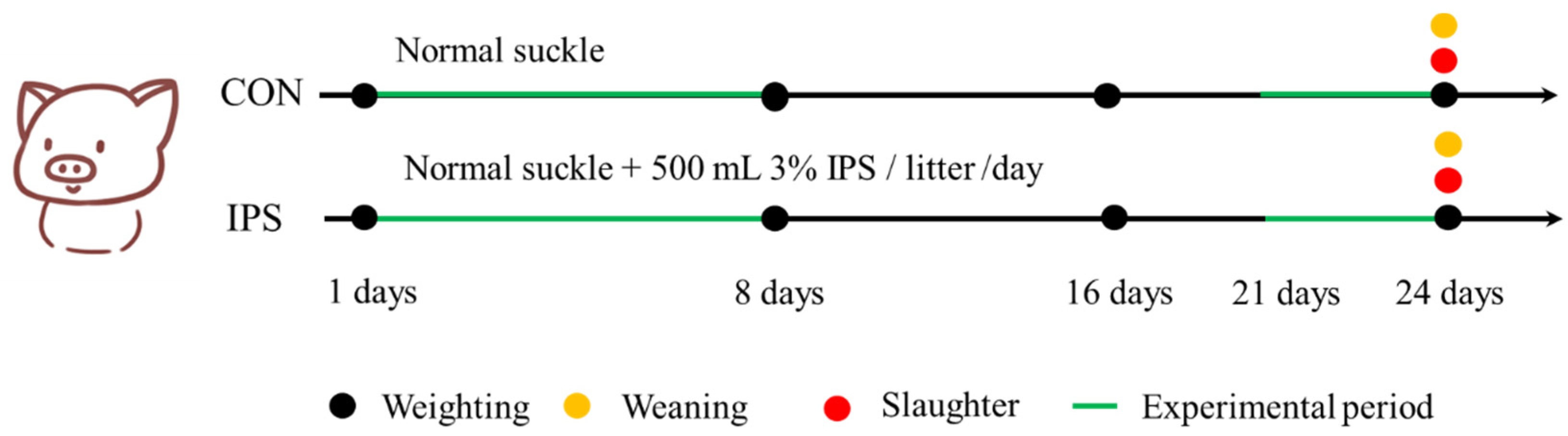
In this trial, 16 pregnant sows with average parities of 2–3 were selected. The sows did not have diarrhea or any other digestive disease and were not subjected to antibiotic treatment prior to the study. The sows were transferred to the farrowing room about a week before the expected farrowing date. At farrowing, the 16 sows produced over 160 newborn piglets (Duroc-Landrace-Yorkshire). Each group comprised 8 litters of piglets (10 piglets per litter). All individual piglets were measured for growth performance indicators, including body weight. However, statistical analyses were conducted at the litter level by calculating the average body weight per litter. Group differences were evaluated based on the mean values from 8 litters per group (
n = 8 litters/group). For slaughter-related measurements (blood and intestinal tissue analyses), 6 litters were randomly selected from each group (1 piglet per selected litter), with piglets chosen to represent their respective litters. Specifically, only piglets whose body weights deviated by no more than 5% from the litter average were selected for sampling. The resulting data were treated as litter-level measurements, and intergroup comparisons were performed using litters as the statistical unit (
n = 6 litters/group). No significant differences in initial body weight were observed among litters (
p > 0.05), and the gender ratio was evenly distributed. Both sows and their litters were housed within the same farrowing crates, with the average temperature in the production area maintained at 25–27 °C. The NRC criteria for diet formulation for lactating sows were adhered to in our trial, and the nutrient content is detailed in
Table 1. Sows were fed individually throughout the trial period and had free access to water and feed.
Within 24 h after farrowing, all piglets were individually marked and weighed, with live birth records documented. Litter size was standardized to 10 piglets per sow through cross-fostering, ensuring no significant inter-group difference in initial body weight and a balanced sex ratio across all litters. The piglets in the control group received no additional supplements before weaning. From days 2 to 8 of age, piglets in the IPS group were supplemented once daily with 500 mL of 3% IPS solution provided in open-top containers. Three days pre-weaning, IPS piglets received a porridge mixture consisting of creep feed and 3% IPS solution (1.5 kg solution: 1 kg dry feed). To control for feed physical form, CON piglets received an isovolumetric porridge mixture of creep feed and water (1.5 kg water: 1 kg dry feed) during the same pre-weaning period. The experimental design is summarized in
Figure 1, and the nutritional composition of IPS is shown in
Table 2. The 3% concentration was determined based on the osmotic pressure of mammalian plasma, which typically ranges from 290 to 310 mOsm/L. This concentration ensures an isotonic solution with an osmotic pressure of approximately 300 mOsm/L. All animals remained clinically healthy throughout the trial. Daily intake of IPS solution, porridge mixtures, and dry feed was recorded per litter.
2.3. Growth Performance
At 1, 8, 16 and 24 days of age, the weight of all piglets was measured, and the weighing time was consistent at each time point. The average daily gain (ADG) was calculated according to the following formula: ADG = total weight gain/(number of test days × number of tests).
2.4. Sample Collection
At 24 days of age, 6 piglets were randomly selected from each group. Total 12 piglets were exsanguinated after being injected with sodium pentobarbital (50 mg/kg). Whole blood samples were collected and centrifuged at 3000× g for 15 min in EDTA tubes and separated plasma samples were stored at −80 °C for biochemical profile analysis. The gastrointestinal tracts were dissected to extract a sample specifically from the intestine. The middle segment of the jejunum and ileum (approximately 2 cm) was fixed in 4% paraformaldehyde for intestinal morphological measurement. The intestinal mucosa from the middle segment of the jejunum was gently scraped, and both the jejunum mucosa samples and the contents of the ileocecal region were stored at −80 °C for subsequent microbiota analysis.
2.5. Immune and Plasma Biochemical Profiling
Plasma biochemical parameters, including urea nitrogen (BUN), glucose (GLU), total cholesterol (TC), total protein (TP), albumin (ALB), globulin (GLB), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured by using an automated biochemistry analyzer with the commercial kits (SMT-120VP, Seamaty Technology, Chengdu, China).
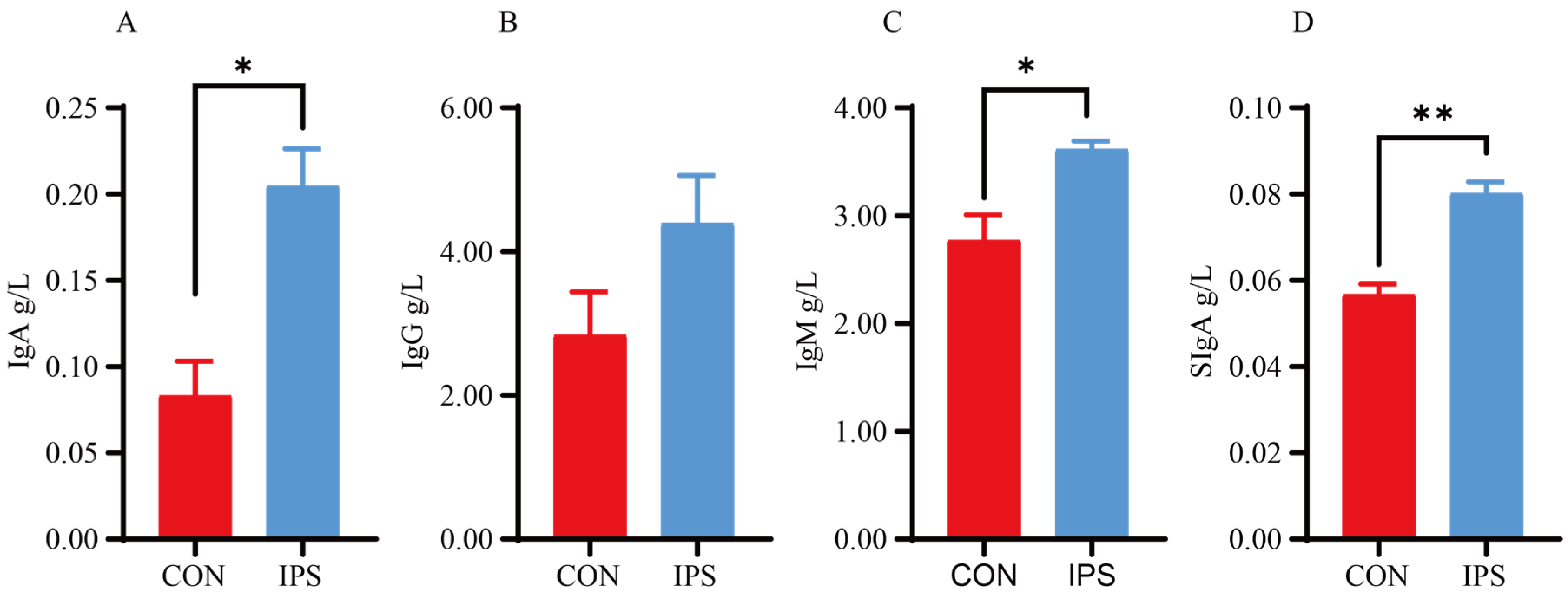
Plasma immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM) and jejunal mucosal secretory immunoglobulin A (SIgA) were measured by commercial porcine ELISA kits (RX500986P, RX500984P, RX500977P, RX501029P, Ruixin Biological Technology, Quanzhou, China). In addition, the content of secreted SIgA was analyzed with the supernatant that was obtained from the jejunal mucosal homogenate after centrifugation (3000× g, 10 min, 4 °C).
2.6. Intestinal Morphology Analysis
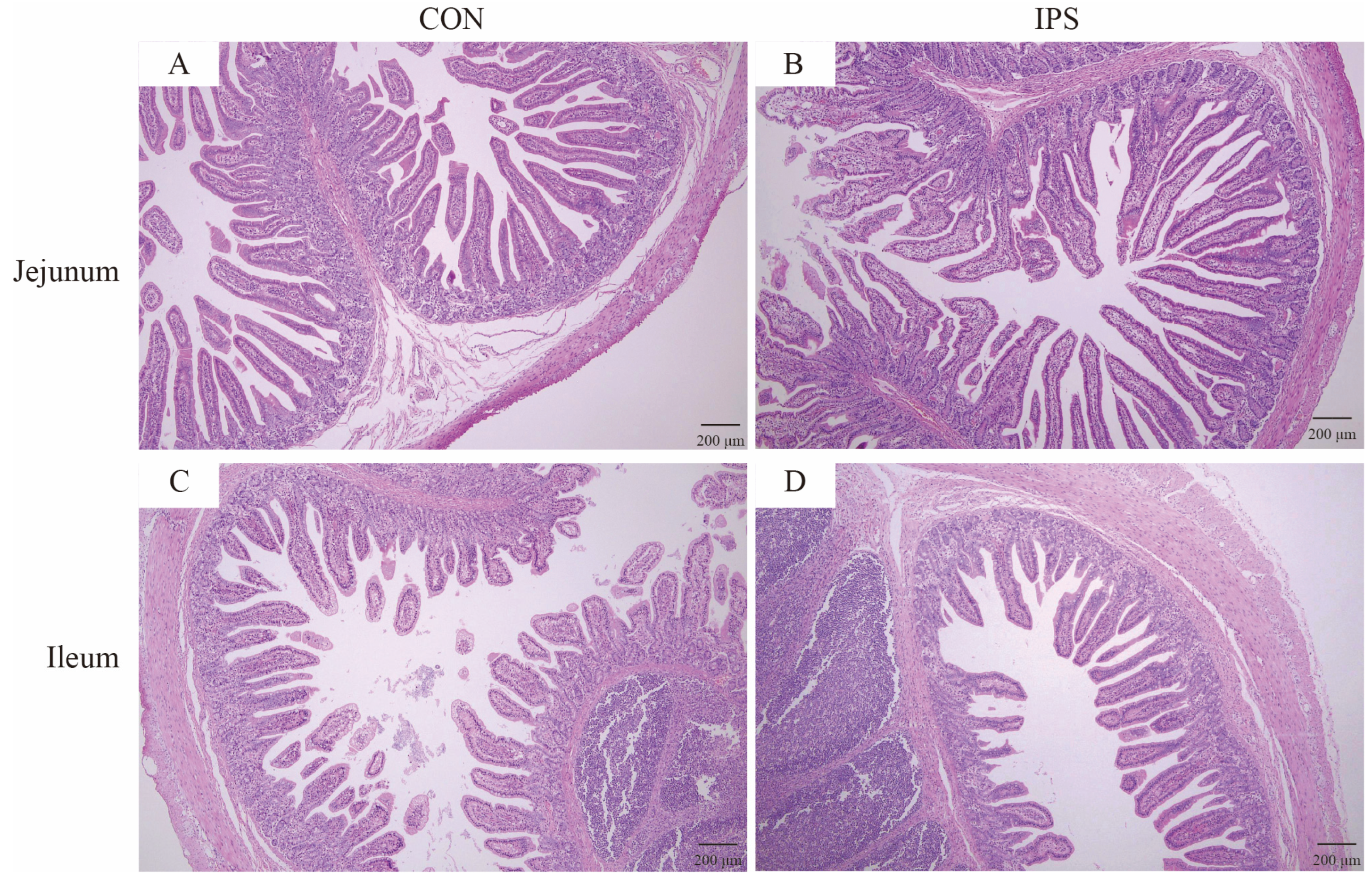
Jejunal and ileal intestinal samples were fixed with 4% paraformaldehyde, embedded with paraffin, and stained with hematoxylin and eosin. Tissue section (6 μm thickness) images were captured by using a digital trinocular camera microscope (BA210 LED Digital, Motic Microscopes, Xiamen, China). Measurements of intraseptal intestinal villi height (VH) and crypt depth (CD) were analyzed using Motic Images Advanced 3.2 software, allowing for the calculation of the V/C. Each sample from each experimental group was examined for at least 10 well-oriented intact villi and their associated crypts.
2.7. RNA Extraction and RT-qPCR Analysis
Total RNA was extracted from the jejunal by the TRIZOL method. The concentration and purity of RNA were determined by Nanodrop™ One spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The mRNA was then reverse-transcribed to cDNA using the PrimeScript™ RT kit and gDNA Eraser (RR047A, TAKARA, Beijing, China). The extracted mRNA was spectrophotometrically quantified (OD
260/OD
280: 1.8–2.2). The reverse-transcribed cDNA was then stored in a refrigerator at −20 °C for later use. The relative expression of mRNA was measured using TB Green Premix Ex Taq™ II (RR820A, TAKARA) with the CFX96 Touch™ Real-time PCR Detection system (Bio-Rad, Hercules, CA, USA). The RT-PCR thermal cycling conditions were 95 °C for 30 s; 95 °C for 5 s; 60 °C for 30 s for 40 cycles. The relative level of mRNA expression was calculated by using the 2
−ΔΔCt method after normalization with
β-actin as a housekeeping gene. The primers used in this study are listed in
Table S1.
2.8. Microbiome Analysis
The microbial communities in the jejunum and cecum of piglets were analyzed using high-throughput 16S rDNA sequencing technology (Gene Denovo, Guangzhou, China). Genomic DNA was extracted from the contents of jejunum and cecum samples, followed by amplification of the V3–V4 hypervariable region of the 16S rDNA using barcode-specific primers. The primer sequences used were 341F: CCTACGGGNGGCWGCAG and 806R: GGACTACHVGGGTATCTAAT. The purified amplicons were ligated to sequencing adapters to construct a paired-end sequencing library, which was then sequenced on an Illumina NovaSeq 6000 platform (PE250, Illumina, San Diego, CA, USA). Finally, the generated 16S rDNA sequencing data were processed and analyzed using the Omicsmart online analysis tool.
2.9. Statistical Analysis
All data were analyzed using GraphPad Prism 10.4 software (GraphPad Software, San Diego, CA, USA). Independent sample t-tests were performed to assess inter-group differences in mean values. Prior to conducting t-tests, the Shapiro–Wilk procedure was employed to evaluate data normality and homogeneity of variance. When the assumption of normality was not met, non-parametric alternatives, such as the Mann–Whitney U test, were applied. Welch’s t-test and the Wilcoxon rank test were used to compare α diversity indices (Chao1, Shannon, Simpson, and ACE) across groups. All data are presented as mean values, the standard error of mean (SEM) represented variation in data, and a value of p < 0.05 was considered statistically significant.
4. Discussion
This study investigated the effects of IPS as a supplement on various aspects of suckling piglets’ health and development. This research identifies the beneficial effects of IPS on growth performance, immune functionality, intestinal development and health, integrity of the intestinal barrier, and the structure of the microbiota. Nutritional supplements, particularly prebiotics and lactoferrin, have attracted considerable scientific interest for their potential to enhance both growth performance and gastrointestinal health in suckling piglets [
16]. In this investigation, IPS composed of whey protein, glucose, and Na
+/K
+ ions, was shown to improve the growth metrics of suckling piglets, as evidenced by increases in weaning weight and average daily gain. Crucially, the observed enhancements in growth performance were concomitant with advancements in intestinal development and health, thereby elucidating a potential mechanism through which IPS may exert its beneficial effects on piglet health and growth.
Weaning weight serves as a critical metric for assessing the robustness of suckling piglets and is directly correlated with the economic productivity of swine operations [
17]. Our findings indicate that IPS elevated both the weaning weight and the daily gain of the piglets. Previous research has demonstrated that the inclusion of whey protein in diets can enhance the growth metrics of preterm piglets and foster improvements in intestinal development and immune function [
18]. Additionally, previous research has reported that glyco-electrolyte supplements administered to weaned piglets not only improved growth performance but also improved intestinal integrity. Higher weaning weights have a significant impact on pig lifetime growth and slaughter performance [
19]. Although the control lacked an iso-energetic substitute, the negligible caloric contribution of IPS (1.5 g/day/piglet) indicates observed effects stem primarily from bioactive nutrients. In summary, our study found that IPS supplementation positively affected growth performance and weaning weight in suckling piglets.
Blood biochemical indices reflect an animal’s growth performance and metabolic activity [
20]. Results of this study demonstrated a significant decrease of 18.79% in blood urea nitrogen concentrations following IPS supplementation. BUN levels are recognized as a biomarker for protein utilization in animals, with an inverse relationship observed between BUN concentrations and both protein utilization efficiency and average daily gain [
21,
22,
23]. These findings are consistent with the outcomes of our experiments, suggesting that IPS supplementation potentially enhances protein absorption and utilization efficiency in piglets, thereby improving their overall growth performance.
Gut morphology serves as a pivotal indicator of intestinal health [
24], with the first week post-partum being a critical developmental window for piglet intestines [
25]. Small intestinal villi, essential for nutrient digestion and absorption [
23], undergo rapid maturation immediately after birth [
26]. Within the small intestine, parameters such as VH, CD, and the V/C are indicative of the health and functional absorption efficiency of the intestine. An increase in the V/C is associated with enhanced nutrient and fluid absorption [
27,
28], while increased VH directly improves digestive capacity and growth performance [
26]. In this study, IPS supplementation significantly increased jejunal VH and V/C ratio in piglets. Furthermore, other studies have documented the beneficial effects of whey protein and ionized water supplementation on the morphology of the small intestine in piglets [
29,
30]. Notably, the increased VH, V/C ratio directly correlates with the 15.7% ADG gain (Days 16–24), indicating enhanced nutrient absorption efficiency.
The structural development of the gastrointestinal tract is governed by several key developmental genes, notably
IGF-1,
IGF-1R, and
GLP-2, which are significant markers of cell differentiation and intestinal development [
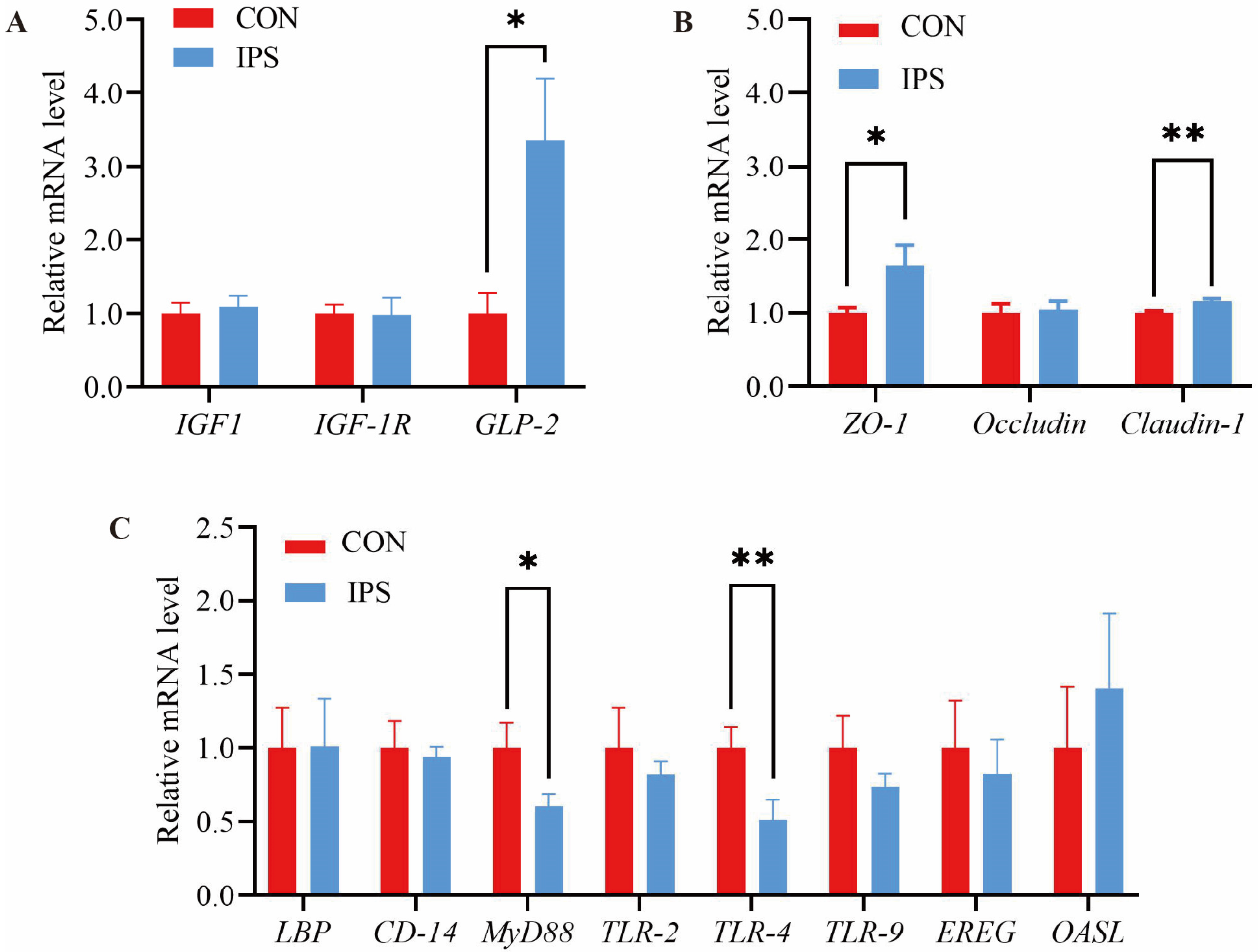
31]. Our investigation observed a marked upregulation of
GLP-2 expression in the jejunal region following IPS supplementation. As an intestinal peptide hormone, elevated
GLP-2 levels can induce a range of beneficial effects on gut health and functionality [
32].
GLP-2 is known to promote mucosal growth and cellular proliferation within the intestine [
33]. This proliferation and the associated increase in intestinal mucosal cell count contribute to the expansion of the intestinal surface area, elevation of VH, and facilitation of nutrient absorption [
34]. Concurrently, our experiments observed an increase in VH within the jejunum of piglets. These observations suggest that IPS supplementation may induce modifications in intestinal mucosal morphology, thereby promoting intestinal development, enhancing nutrient absorption, and ultimately augmenting piglet growth performance.
The intestinal barrier serves as a critical protector of intestinal health, safeguarding against deleterious agents such as bacteria, endotoxins, and antigens [
35]. Particularly during the early stages of piglet development, the intestinal barrier undergoes maturation, characterized by a relatively thin mucus layer and a concurrent gradual maturation of the immune system, coinciding with the rapid growth of the intestinal tract [
36,
37]. Tight junctions are integral components of the intestinal barrier, forming its physical barricade [
35,
38]. Crucial elements of the intestinal mucosal epithelial barrier, including
ZO-1,
Claudin-1, and
Occludin, are intimately associated with intestinal permeability [
39,
40]. This study demonstrated an increase in the mRNA expression of
ZO-1 and
Claudin-1 genes in the jejunum of piglets subsequent to IPS supplementation. This finding is consistent with the research by Chen et al., who reported that the addition of an AMC to drinking water could enhance the expression levels of small intestinal tight junction factors in weaned piglets, thereby improving intestinal barrier function and overall intestinal health. Thus, our results indicate that IPS supplementation may strengthen jejunal barrier function in piglets, potentially contributing to enhanced intestinal health and functionality.
The susceptibility of the gastrointestinal tract in suckling piglets to pathogenic bacterial infections underscores the importance of enhancing their immune defenses to mitigate the incidence of diarrhea and improve survival rates [
41]. Immunoglobulins serve as critical markers of immune competence, playing an essential role in bolstering immunity [
42,
43]. Guo et al. have highlighted the significance of IgA, IgG, and IgM in serum as primary components of humoral immunity, bolstering monocyte macrophage phagocytosis and impeding the proliferation of pathogenic microorganisms [
44,
45,
46]. IgA, with its predominant presence in mucous membranes and serum, plays a crucial role in defending against pathogenic invasions [
47]. Within the intestinal mucosa, IgA exists as a dimer, termed SIgA, which serves as the primary defender in intestinal antigen-specific immunity [
48,
49]. In our study, the supplementation of IPS notably elevated the serum levels of IgA, IgM, and SIgA in suckling piglets.
Toll-like receptors are pivotal components of innate immunity, essential for recognizing pathogens and initiating immune responses. These receptors act as a bridge between innate and adaptive immunity and play a regulatory role in various immune cells and factors [
50]. This study evaluated the impact of IPS supplementation on the expression of TLR pathway-related genes within the jejunal mucosa of piglets. The results revealed that IPS supplementation induced a downregulation of gene expression associated with the
TLR-4 and
MyD88 pathways in the jejunum of suckling piglets. The downregulation of TLR-4/MyD88 signaling may reduce pro-inflammatory cytokine production. Coupled with elevated plasma IgA/IgM, this suggests that IPS optimizes immune balance by mitigating excessive inflammation.
The gut microbiome plays an integral role in maintaining intestinal health, facilitating essential functions such as nutrient absorption, metabolic processes, resistance against pathogenic invasion, and the reinforcement of the intestinal immune barrier [
51,
52,
53]. To validate the functional association between physiological improvements and IPS-induced microbiota restructuring, we performed 16S rDNA sequencing analysis on the contents of both the jejunum and cecum. This study documented a significant alteration at the phylum level in the gut microbiome following IPS supplementation, characterized by decreased relative abundance of
Firmicutes and
Euryarchaeota, alongside an increased prevalence of
Bacteroidota.
Firmicutes and
Bacteroidota represent two dominant phyla within the intestinal microbiota of animals [
54],
Bacteroides as a core genus within the phylum
Bacteroidota, serving as a key player in the breakdown of dietary carbohydrates and polysaccharides. This metabolic function not only enhances nutrient utilization but also promotes intestinal mucosal development, facilitates immune system maturation, strengthens host immune defenses, and contributes to the maintenance of intestinal microecological homeostasis [
55,
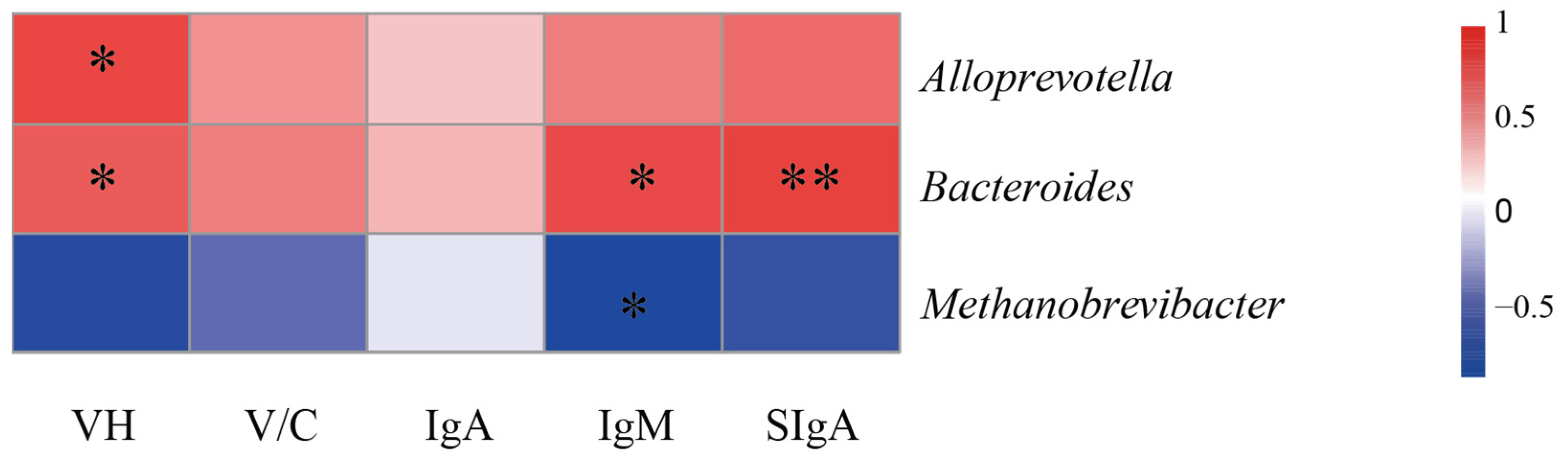
56]. Furthermore, these findings indicate a positive correlation between the presence of
Bacteroides and various indicators of intestinal health, including jejunal VH, plasma IgM, and SIgA concentrations in the jejunal mucosa. Recent studies emphasize the essential role of intestinal
Bacteroides, a gram-negative bacterial genus that dominates the gut microbiota, in sustaining mucosal immune function. These bacteria produce butyrate, which not only activates T cell-mediated immune responses and restricts pathogen colonization but also promotes the expression of plasma cell-derived SIgA in the intestinal mucosa [
57,
58].
At the genus level, IPS supplementation significantly decreased
Methanobrevibacter, a methanogenic archaeon involved in gut microbial balance but paradoxically associated with inflammatory initiation [
59,
60], while increasing the abundance of
Alloprevotella and
Bacteroides. These microbial shifts are functionally linked to enhanced butyrate and propionate production, which activate
FFAR2 receptors and subsequently upregulate tight junction proteins such as
ZO-1 and
Claudin-1 [
61,
62]. These changes correlate directly with physiological improvements;
Alloprevotella shows a positive association with jejunal VH, while
Bacteroides correlates with VH, plasma IgM, and mucosal SIgA levels [
63,
64,
65]. In contrast,
Rikenellaceae_RC9_gut_group, which is enriched in pigs with low feed efficiency and high-fat diet models, appears to exert detrimental effects on growth performance [
66]. Meanwhile, the reduced abundance of
Christensenellaceae_R-7_group aligns with lower fiber intake and iron absorption requirements during lactation [
67,
68], a nutrient essential for intestinal villi development [
69]. Collectively,
Alloprevotella (butyrate producer) and
Bacteroides (propionate producer) act synergistically to reinforce intestinal barrier integrity and immune function, suggesting that IPS enhances piglet growth by modulating gut microbiota composition.