Preparation and Characterization of Monoclonal Antibodies Against the Porcine Rotavirus VP6 Protein
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Enzymes and Related Reagents
2.3. Truncated Expression of Recombinant Gene pET-28a(+)-VP6
2.4. Expression and Purification of VP6 Recombinant Protein
2.5. Immunization of Mice
2.6. Cell Fusion and Subcloning
2.7. Identification of Monoclonal Antibody Specificity
2.8. Construction of VP6 Protein Truncation Mutants
2.9. Epitope Identification and Conservation Analysis
3. Results
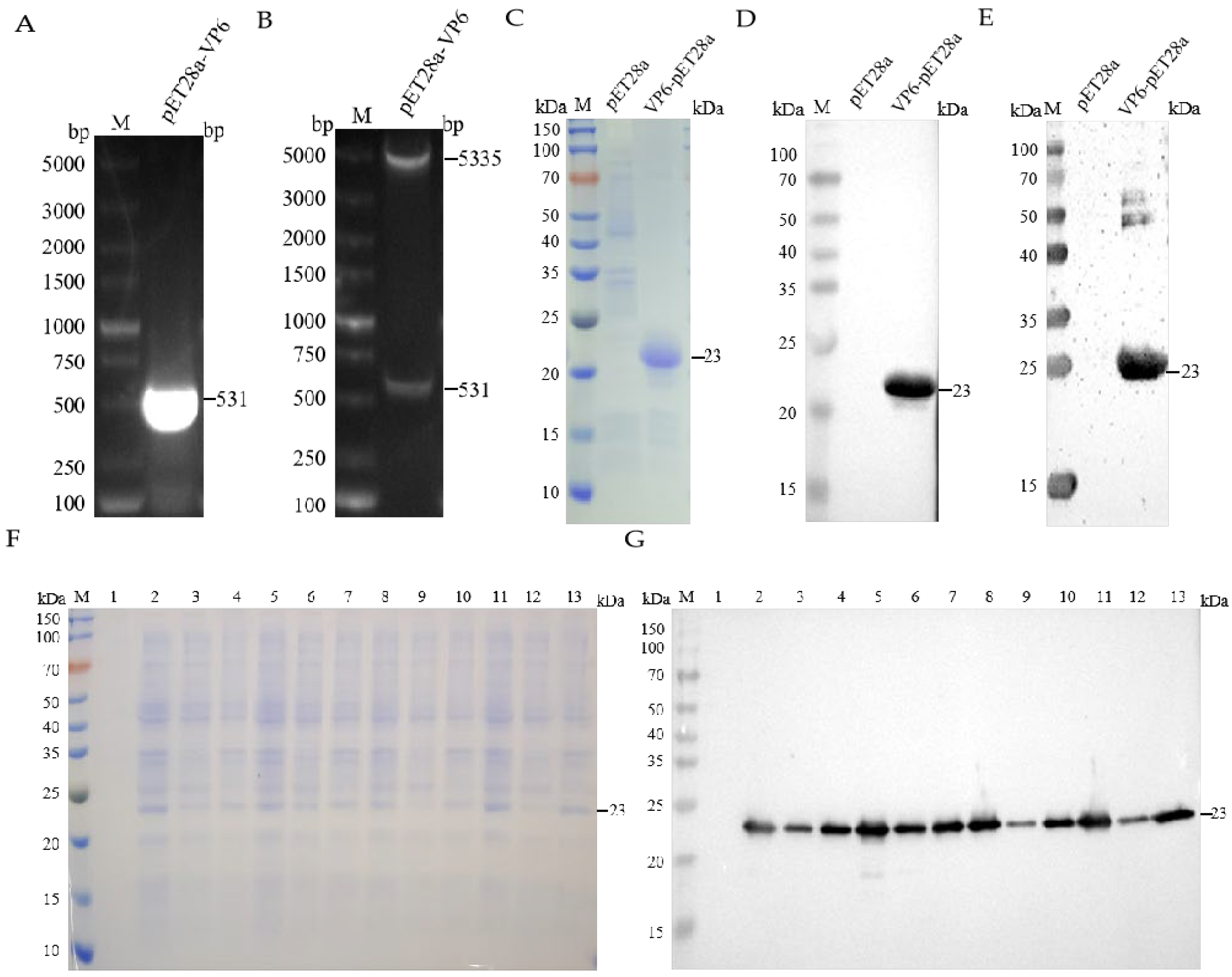
3.1. Construction, Expression, and Purification of Recombinant pET-28a(+)-VP6
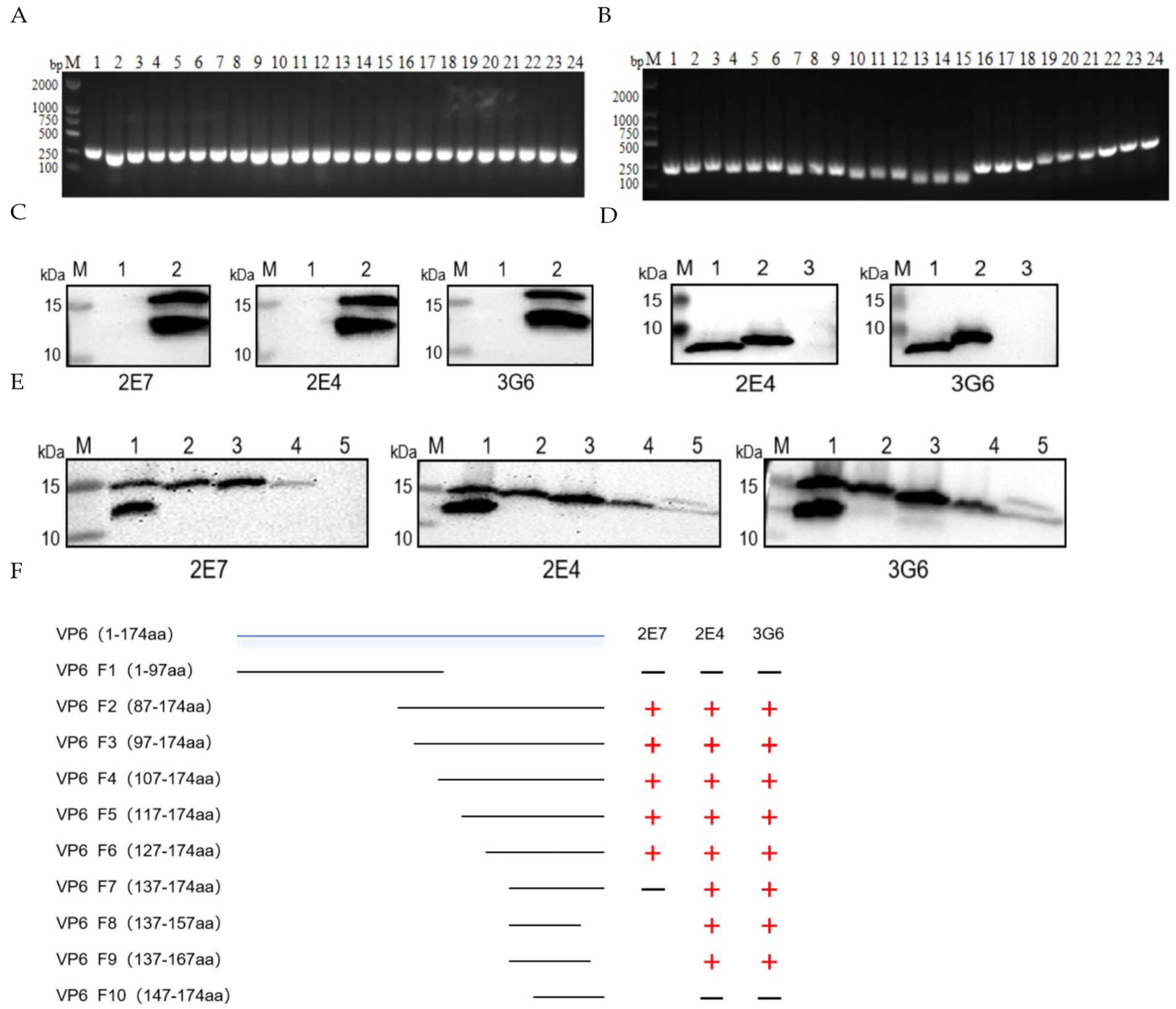
3.2. Generation and Characterization of VP6-Specific Monoclonal Antibodies
3.3. Construction of VP6 Truncation Variants and Epitope Mapping
3.4. Epitope Conservation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mebus, C.A.; Underdahl, N.R.; Rhodes, M.B.; Twiehaus, M.J. Further studies on neonatal calf diarrhea virus. Proc. Annu. Meet. US Anim. Health Assoc. 1969, 73, 97–99. [Google Scholar]
- Woode, G.N.; Bridger, J.; Hall, G.A.; Jones, J.M.; Jackson, G. The isolation of reovirus-like agents (rota-viruses) from acute gastroenteritis of piglets. J. Med. Microbiol. 1976, 9, 203–209. [Google Scholar] [CrossRef]
- Sánchez-Tacuba, L.; Kawagishi, T.; Feng, N.; Jiang, B.; Ding, S.; Greenberg, H.B. The role of the VP4 attachment protein in rotavirus host range restriction in an in vivo suckling mouse model. J. Virol. 2022, 96, e00550-22. [Google Scholar] [CrossRef]
- Mcdonald, S.M.; Nelson, M.I.; Turner, P.E.; Patton, J.T. Reassortment in segmented RNA viruses: Mechanisms and outcomes. Nat. Rev. Microbiol. 2016, 14, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Ciarlet, M.; McDonald, S.M.; Attoui, H.; Banyai, K.; Brister, J.R.; Buesa, J.; Esona, M.D.; Estes, M.K.; Gentsch, J.R.; et al. Uniformity of rotavirus strain nomenclature proposed by the rotavirus classification working group (RCWG). Arch. Virol. 2011, 156, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Labbe, M.; Cohen, J.; Burroughs, M.H.; Zhou, Y.J.; Estes, M.K. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J. Virol. 1994, 68, 5945–5952. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Huang, S.; Zhang, L.; Yang, Q.; Liu, S.; Wang, Z.; Chu, Q.; Tian, M.; Zhao, L.; Sun, Y.; et al. Virus-like particles vaccine based on co-expression of g5 porcine rotavirus VP2-VP6-VP7 induces a powerful immune protective response in mice. Vet. Microbiol. 2024, 298, 110241. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine rotaviruses: Epidemiology, immune responses and control strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef]
- Xue, R.; Tian, Y.; Zhang, Y.; Zhang, M.; Li, Z.; Chen, S.; Liu, Q. Diversity of group a rotavirus of porcine rotavirus in shandong province China. Acta Virol. 2018, 62, 229–234. [Google Scholar] [CrossRef]
- Jacobson, M. On the infectious causes of neonatal piglet diarrhoea—A review. Vet. Sci. 2022, 9, 422. [Google Scholar] [CrossRef]
- Memon, A.M.; Bhuyan, A.A.; Chen, F.; Guo, X.; Menghwar, H.; Zhu, Y.; Ku, X.; Chen, S.; Li, Z.; He, Q. Development and validation of monoclonal antibody-based antigen capture ELISA for detection of group a porcine rotavirus. Viral Immunol. 2017, 30, 264–270. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Li, M.; Zhang, H.; Feng, Z.; Xu, H.; Li, C.; Guo, Z.; Gong, B.; Peng, J.; et al. Development and application of a blocking ELISA based on a n protein monoclonal antibody for the antibody detection against porcine reproductive and respiratory syndrome virus 2. Int. J. Biol. Macromol. 2024, 269, 131842. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, H.; Lin, Z.; Shi, S.; Zhang, B.; Zhang, Y.; Han, S.; He, W.-R.; Wan, B.; Hu, M.; et al. Identification and characterization of a novel B cell epitope of ASFV virulence protein B125R monoclonal antibody. Viruses 2024, 16, 1257. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kuang, Q.; Zheng, X.; Xu, Y.; Feng, Y.; Xiang, Q.; Zhang, G.; Zhou, P. Monoclonal antibody development for early detection of ASFV i73r protein: Identification of a linear antigenic epitope. Virology 2024, 597, 110145. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Z.; You, J.; Zhang, W.; Tang, S.; Gong, L.; Zhang, G. Identification of a novel b cell epitope of ASFV pCP312r recognized using a monoclonal antibody. Vet. Microbiol. 2024, 298, 110247. [Google Scholar] [CrossRef]
- Campanha, J.E.T.; Possatti, F.; Lorenzetti, E.; de Almeida Moraes, D.; Alfieri, A.F.; Alfieri, A.A. Longitudinal study of rotavirus c VP6 genotype i6 in diarrheic piglets up to 1 week old. Braz. J. Microbiol. 2020, 51, 1345–1351. [Google Scholar] [CrossRef]
- Wakuda, M.; Ide, T.; Sasaki, J.; Komoto, S.; Ishii, J.; Sanekata, T.; Taniguchi, K. Porcine rotavirus closely related to novel group of human rotaviruses. Emerg. Infect. Dis. 2011, 17, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Rodger, S.M.; Craven, J.A.; Williams, I. Letter: Demonstration of reovirus-like particles in intestinal contents of piglets with diarrhoea. Aust. Vet. J. 1975, 51, 536. [Google Scholar] [CrossRef] [PubMed]
- Wenske, O.; Ruckner, A.; Piehler, D.; Schwarz, B.; Vahlenkamp, T.W. Epidemiological analysis of porcine rotavirus a genotypes in germany. Vet. Microbiol. 2018, 214, 93–98. [Google Scholar] [CrossRef]
- Abass, G.; Dubal, Z.B.; Rajak, K.K.; Kale, B.M.; Raorane, A.; Dudhe, N.; Malla, B.A.; Desai, D.; Sinha, D.K.; Kumar, O.R.V.; et al. Molecular characterization of porcine rotavirus a from india revealing zooanthroponotic transmission. Anim. Biotechnol. 2022, 33, 1073–1085. [Google Scholar] [CrossRef]
- Shi, H.; Chen, J.; Li, H.; Sun, D.; Wang, C.; Feng, L. Molecular characterization of a rare G9P [23] porcine rotavirus isolate from China. Arch. Virol. 2012, 157, 1897–1903. [Google Scholar] [CrossRef]
- Azevedo, M.P.; Vlasova, A.N.; Saif, L.J. Human rotavirus virus-like particle vaccines evaluated in a neonatal gnotobiotic pig model of human rotavirus disease. Expert Rev. Vaccines 2013, 12, 169–181. [Google Scholar] [CrossRef]
- Li, F.; Mei, Z.; Ju, N.; Sui, L.; Fan, X.; Wang, Z.; Li, J.; Jiang, Y.; Cui, W.; Shan, Z.; et al. Evaluation of the immunogenicity of auxotrophic lactobacillus with CRISPR-cas9d10a system-mediated chromosomal editing to express porcine rotavirus capsid protein VP4. Virulence 2022, 13, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Fischer, D.D.; Kandasamy, S.; Rauf, A.; Langel, S.N.; Wentworth, D.E.; Stucker, K.M.; Halpin, R.A.; Lam, H.C.; Marthaler, D.; et al. Comparative in vitro and in vivo studies of porcine rotavirus G9P [13] and human rotavirus wa G1P [8]. J. Virol. 2016, 90, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Buttery, J.P.; Danchin, M.H.; Lee, K.J.; Carlin, J.B.; McIntyre, P.B.; Elliott, E.J.; Booy, R.; Bines, J.E.; For the PAEDS/APSU Study Group. Intussusception following rotavirus vaccine administration: Post-marketing surveillance in the national immunization program in australia. Vaccine 2011, 29, 3061–3066. [Google Scholar] [CrossRef]
- Chandler-Bostock, R.; Hancox, L.R.; Payne, H.; Iturriza-Gomara, M.; Daly, J.M.; Mellits, K.H. Diversity of group a rotavirus on a UK pig farm. Vet. Microbiol. 2015, 180, 205–211. [Google Scholar] [CrossRef]
- Banos, D.M.; Lopez, S.; Arias, C.F.; Esquivel, F.R. Identification of a t-helper cell epitope on the rotavirus VP6 protein. J. Virol. 1997, 71, 419–426. [Google Scholar] [CrossRef]
- Nagesha, H.S.; Brown, L.E.; Holmes, I.H. Neutralizing monoclonal antibodies against three serotypes of porcine rotavirus. J. Virol. 1989, 63, 3545–3549. [Google Scholar] [CrossRef] [PubMed]
- Afchangi, A.; Jalilvand, S.; Arashkia, A.; Latifi, T.; Farahmand, M.; Shirazi, M.M.A.; Nasab, S.D.M.; Marashi, S.M.; Roohvand, F.; Shoja, Z. Co-administration of rotavirus nanospheres VP6 and NSP4 proteins enhanced the anti-NSP4 humoral responses in immunized mice. Microb. Pathog. 2022, 163, 105405. [Google Scholar] [CrossRef]
- Shoja, Z.; Jalilvand, S.; Latifi, T.; Roohvand, F. Rotavirus VP6: Involvement in immunogenicity, adjuvant activity, and use as a vector for heterologous peptides, drug delivery, and production of nano-biomaterials. Arch. Virol. 2022, 167, 1013–1023. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, X.; Shi, H.; Chen, J.; Han, X.; Wei, P.; Feng, L. The interaction of rotavirus a pig/China/NMTL/2008/G9P [23] VP6 with cellular beta-actin is required for optimal RV replication and infectivity. Vet. Microbiol. 2016, 197, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.M.; Chen, F.; Khan, S.B.; Guo, X.; Khan, R.; Khan, F.A.; Zhu, Y.; He, Q. Development and evaluation of polyclonal antibodies based antigen capture ELISA for detection of porcine rotavirus. Anim. Biotechnol. 2023, 34, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Godinez, O.; Gutierrez-Xicotencatl, L.; Plett-Torres, T.; Pedroza-Saavedra, A.; Gonzalez-Jaimes, A.; Chihu-Amparan, L.; Maldonado-Gama, M.; Espino-Solis, G.; Bonifaz, L.; Esquivel-Guadarrama, F. Targeting of rotavirus VP6 to DEC-205 induces protection against the infection in mice. Vaccine 2015, 33, 4228–4237. [Google Scholar] [CrossRef]
- Kohli, E.; Maurice, L.; Bourgeois, C.; Bour, J.B.; Pothier, P. Epitope mapping of the major inner capsid protein of group A rotavirus using peptide synthesis. Virology 1993, 194, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, J.; Shi, H.; Zhang, X.; Shi, D.; Chi, Y.; Li, C.; Feng, L. Preparation of monoclonal antibodies against VP6 protein of porcine group a rotavirus and identification of its antigenic epitopes. J. Prev. Vet. Med. China 2014, 36, 885–888. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, B.; Mao, D.; Chen, J.; Bi, X.; Zou, L.; Bai, J.; Liu, R.; Hao, P.; Wang, Q.; Zhong, L.; et al. Preparation and Characterization of Monoclonal Antibodies Against the Porcine Rotavirus VP6 Protein. Vet. Sci. 2025, 12, 710. https://doi.org/10.3390/vetsci12080710
Sun B, Mao D, Chen J, Bi X, Zou L, Bai J, Liu R, Hao P, Wang Q, Zhong L, et al. Preparation and Characterization of Monoclonal Antibodies Against the Porcine Rotavirus VP6 Protein. Veterinary Sciences. 2025; 12(8):710. https://doi.org/10.3390/vetsci12080710
Chicago/Turabian StyleSun, Botao, Dingyi Mao, Jing Chen, Xiaoqing Bi, Linke Zou, Jishan Bai, Rongchao Liu, Ping Hao, Qi Wang, Linhan Zhong, and et al. 2025. "Preparation and Characterization of Monoclonal Antibodies Against the Porcine Rotavirus VP6 Protein" Veterinary Sciences 12, no. 8: 710. https://doi.org/10.3390/vetsci12080710
APA StyleSun, B., Mao, D., Chen, J., Bi, X., Zou, L., Bai, J., Liu, R., Hao, P., Wang, Q., Zhong, L., Zhang, P., & Zhou, B. (2025). Preparation and Characterization of Monoclonal Antibodies Against the Porcine Rotavirus VP6 Protein. Veterinary Sciences, 12(8), 710. https://doi.org/10.3390/vetsci12080710







