A Live-Attenuated Chimeric Vaccine Candidate Against the Emerging NADC34-Like PRRSV
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
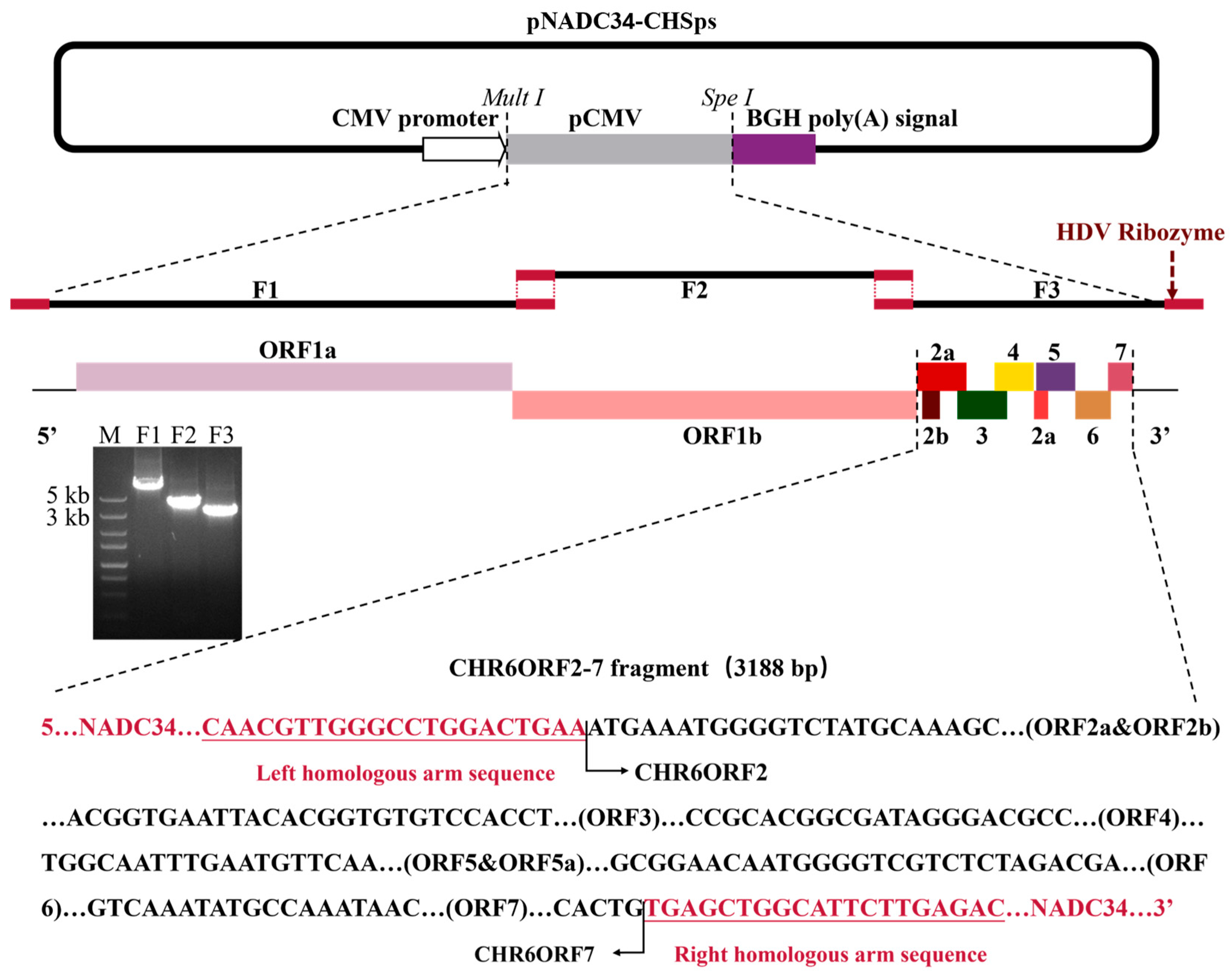
2.2. Construction of Plasmid, Transfection, and Passage
2.3. Characterization of rNADC34-CHSps
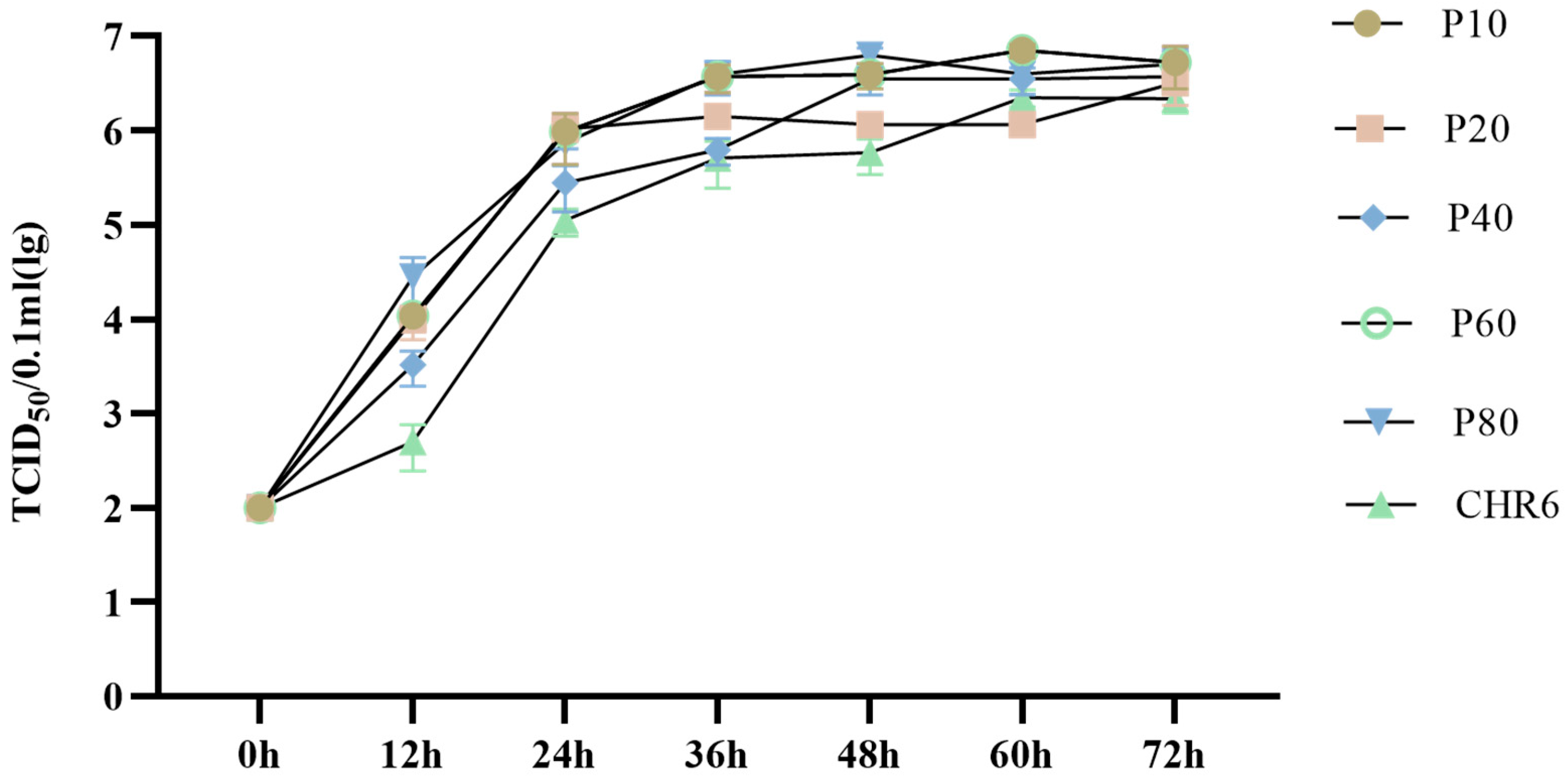
2.4. Characterization of In Vitro Growth Properties in Marc-145 Cells
2.5. Animal Experiment
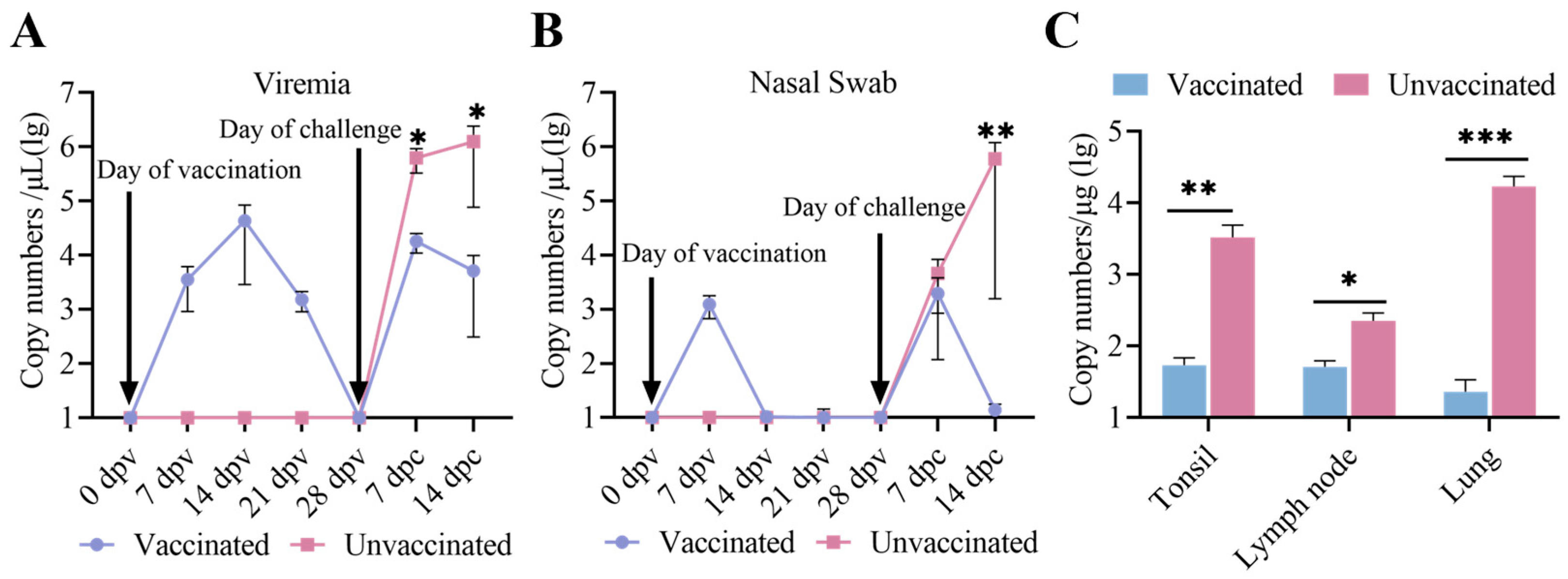
2.6. Viremia and Serological Test
2.7. Histopathology and Immunohistochemistry Staining
2.8. Statistical Analyses
3. Results
3.1. Generation of rNADC34-CHSps Virus
3.2. Growth Kinetics of Different Passages of rNADC34-CHSps Virus
3.3. Clinical Performance After Immunization and Challenge
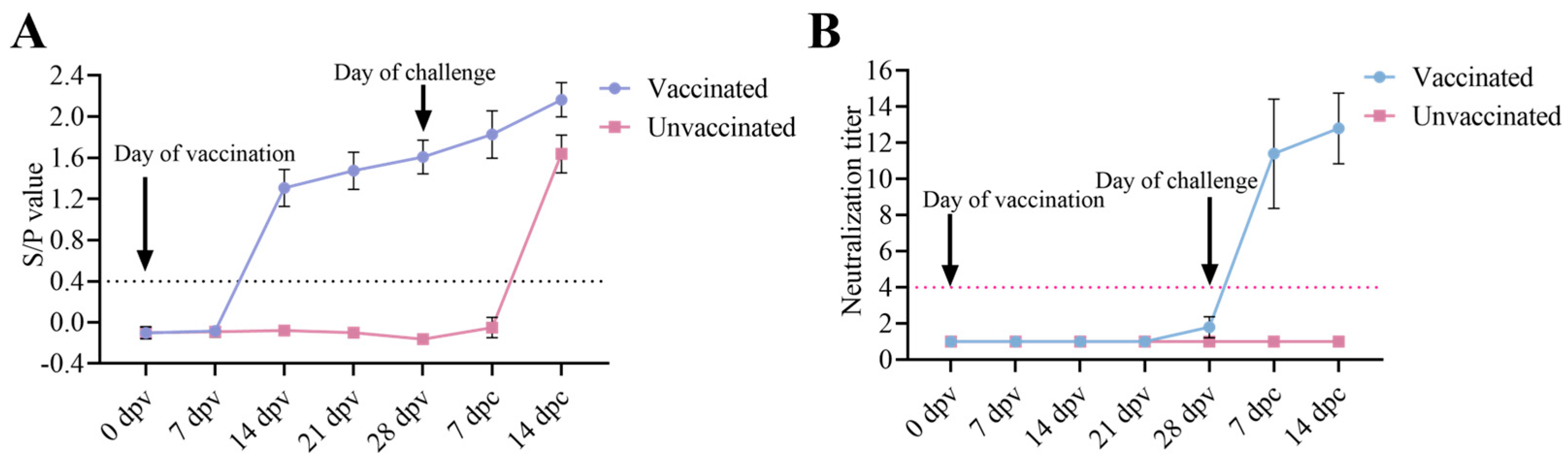
3.4. Antibody Responses of the Piglets
3.5. Viremia, Virus Shedding, and Virus Tissue Distribution Between the Immunized-Challenge Group and the Challenge Group
3.6. Gross Pathological and Histopathological Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhari, J.; Vu, H.L.X. Porcine Reproductive and Respiratory Syndrome Virus Reverse Genetics and the Major Applications. Viruses 2020, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- Balasuriya, U.B.; Carossino, M. Reproductive effects of arteriviruses: Equine arteritis virus and porcine reproductive and respiratory syndrome virus infections. Curr. Opin. Virol. 2017, 27, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Fiers, J.; Cay, A.B.; Maes, D.; Tignon, M. A Comprehensive Review on Porcine Reproductive and Respiratory Syndrome Virus with Emphasis on Immunity. Vaccines 2024, 12, 942. [Google Scholar] [CrossRef]
- Music, N.; Gagnon, C.A. The role of porcine reproductive and respiratory syndrome (PRRS) virus structural and non-structural proteins in virus pathogenesis. Anim. Health Res. Rev. 2010, 11, 135–163. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Guo, X.; Yang, H.C. Genomic characterization of two Chinese isolates of porcine respiratory and reproductive syndrome virus. Arch. Virol. 2004, 149, 1341–1351. [Google Scholar] [CrossRef]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X.; et al. Emergence of fatal PRRSV variants: Unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Ding, Y.; Ge, X.; Guo, X.; Yang, H. Nadc30-like strain of porcine reproductive and respiratory syndrome virus, China. Emerg. Infect. Dis. 2015, 21, 2256–2257. [Google Scholar] [CrossRef]
- Xu, H.; Li, C.; Li, W.; Zhao, J.; Gong, B.; Sun, Q.; Tang, Y.D.; Xiang, L.; Leng, C.; Peng, J.; et al. Novel characteristics of Chinese NADC34-like PRRSV during 2020-2021. Transbound. Emerg. Dis. 2022, 69, e3215–e3224. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Campler, M.R.; Schroeder, D.C.; Yang, M.; Mor, S.K.; Ferreira, J.B.; Arruda, A.G. Detection of Multiple Lineages of PRRSV in Breeding and Growing Swine Farms. Front. Vet. Sci. 2022, 9, 884733. [Google Scholar] [CrossRef]
- Van Geelen, A.G.M.; Anderson, T.K.; Lager, K.M.; Das, P.B.; Otis, N.J.; Montiel, N.A.; Miller, L.C.; Kulshreshtha, V.; Buckley, A.C.; Brockmeier, S.L.; et al. Porcine reproductive and respiratory disease virus: Evolution and recombination yields distinct ORF5 RFLP 1-7-4 viruses with individual pathogenicity. Virology 2018, 513, 168–179. [Google Scholar] [CrossRef]
- Song, S.; Xu, H.; Zhao, J.; Leng, C.; Xiang, L.; Li, C.; Fu, J.; Tang, Y.D.; Peng, J.; Wang, Q.; et al. Pathogenicity of NADC34-like PRRSV HLJDZD32-1901 isolated in China. Vet. Microbiol. 2020, 246, 108727. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.Z.; Ha, Z.; Zhang, H.; Zhang, Y.; Xie, Y.B.; Zhang, H.; Nan, F.L.; Wang, Z.; Zhang, P.; Xu, W.; et al. Pathogenicity of porcine reproductive and respiratory syndrome virus (ORF5 RFLP 1-7-4 viruses) in China. Transbound. Emerg. Dis. 2020, 67, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhu, Z.; Fan, J.; Liu, P.; Li, Y.; Li, Q.; Sun, Z.; Yu, X.; Lee, H.S.; Tian, K.; et al. High Pathogenicity of a Chinese NADC34-like PRRSV on Pigs. Microbiol. Spectr. 2022, 10, e0154122. [Google Scholar] [CrossRef]
- Yuan, L.; Zhu, Z.; Fan, J.; Li, Q.; Liu, P.; Li, X. Efficacy of a commercial PRRSV vaccine on NADC34-like PRRSV challenge. Transbound. Emerg. Dis. 2023, 2023, 4509261. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Xu, Y.; Yang, Y.; Li, J.; Dai, A.; Huang, C.; Luo, M.; Wei, C. Molecular Characteristics and Pathogenicity of a Novel Recombinant Porcine Reproductive and Respiratory Syndrome Virus Strain from NADC30-, NADC34-, and JXA1-Like Strains That Emerged in China. Microbiol. Spectr. 2022, 10, e0266722. [Google Scholar] [CrossRef]
- Wang, H.; Feng, W. Current Status of Porcine Reproductive and Respiratory Syndrome Vaccines. Vaccines 2024, 12, 1387. [Google Scholar] [CrossRef]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction. Vaccine 2015, 33, 4069–4080. [Google Scholar] [CrossRef]
- Vu, H.L.X.; Pattnaik, A.K.; Osorio, F.A. Strategies to broaden the cross-protective efficacy of vaccines against porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2017, 206, 29–34. [Google Scholar] [CrossRef]
- Leng, C.; Zhang, W.; Zhang, H.; Kan, Y.; Yao, L.; Zhai, H.; Li, M.; Li, Z.; Liu, C.; An, T.; et al. ORF1a of highly pathogenic PRRS attenuated vaccine virus plays a key role in neutralizing antibody induction in piglets and virus neutralization in vitro. Virol. J. 2017, 14, 159. [Google Scholar] [CrossRef]
- Kappes, M.A.; Miller, C.L.; Faaberg, K.S. Porcine reproductive and respiratory syndrome virus nonstructural protein 2 (nsp2) topology and selective isoform integration in artificial membranes. Virology 2015, 481, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Wei, Z.; Zevenhoven-Dobbe, J.C.; Liu, R.; Tong, G.; Snijder, E.J.; Yuan, S. Arterivirus minor envelope proteins are a major determinant of viral tropism in cell culture. J. Virol. 2012, 86, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Vereecke, N.; Theuns, S.; Oh, D.; Vanderheijden, N.; Trus, I.; Sauer, J.; Vyt, P.; Bonckaert, C.; Lalonde, C.; et al. Comparison of Primary Virus Isolation in Pulmonary Alveolar Macrophages and Four Different Continuous Cell Lines for Type 1 and Type 2 Porcine Reproductive and Respiratory Syndrome Virus. Vaccines 2021, 9, 594. [Google Scholar] [CrossRef]
- Zhang, H.L.; Tang, Y.D.; Liu, C.X.; Xiang, L.R.; Zhang, W.L.; Leng, C.L.; Wang, Q.; An, T.Q.; Peng, J.M.; Tian, Z.J.; et al. Adaptions of field PRRSVs in Marc-145 cells were determined by variations in the minor envelope proteins GP2a-GP3. Vet. Microbiol. 2018, 222, 46–54. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Gong, W.; Zhang, D.; She, R.; Xu, B.; Ning, Y. Comparative Respiratory Pathogenicity and Dynamic Tissue Distribution of Chinese Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus and its Attenuated Strain in Piglets. J. Comp. Pathol. 2015, 153, 38–49. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, Y.; Han, J.; Burkhart, K.M.; Vaughn, E.M.; Roof, M.B.; Faaberg, K.S. Attenuation of porcine reproductive and respiratory syndrome virus strain MN184 using chimeric construction with vaccine sequence. Virology 2008, 371, 418–429. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Zhang, C.; Tu, Y.; Tong, J.; Liu, Y.; Chang, Y.; Jiang, C.; Wang, S.; Zhou, E.M.; et al. Immune responses to modified live virus vaccines developed from classical or highly pathogenic PRRSV following challenge with a highly pathogenic PRRSV strain. Dev. Comp. Immunol. 2016, 62, 1–7. [Google Scholar] [CrossRef]
- Tian, Z.J.; An, T.Q.; Zhou, Y.J.; Peng, J.M.; Hu, S.P.; Wei, T.C.; Jiang, Y.F.; Xiao, Y.; Tong, G.Z. An attenuated live vaccine based on highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) protects piglets against HP-PRRS. Vet. Microbiol. 2009, 138, 34–40. [Google Scholar] [CrossRef]
- Xie, J.; Trus, I.; Oh, D.; Kvisgaard, L.K.; Rappe, J.C.F.; Ruggli, N.; Vanderheijden, N.; Larsen, L.E.; Lefèvre, F.; Nauwynck, H.J. A Triple Amino Acid Substitution at Position 88/94/95 in Glycoprotein GP2a of Type 1 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV1) Is Responsible for Adaptation to MARC-145 Cells. Viruses 2019, 11, 36. [Google Scholar] [CrossRef]
- Chen, N.; Li, S.; Li, X.; Ye, M.; Xiao, Y.; Yan, X.; Li, X.; Zhu, J. The infectious cDNA clone of commercial HP-PRRS JXA1-R-attenuated vaccine can be a potential effective live vaccine vector. Transbound. Emerg. Dis. 2020, 67, 1820–1827. [Google Scholar] [CrossRef]
- Yu, P.; Wei, R.; Dong, W.; Zhu, Z.; Zhang, X.; Chen, Y.; Liu, X.; Guo, C. CD163ΔSRCR5 MARC-145 Cells Resist PRRSV-2 Infection via Inhibiting Virus Uncoating, Which Requires the Interaction of CD163 With Calpain 1. Front. Microbiol. 2020, 10, 3115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, H.; Zhang, X.; He, S.; Dong, W.; Wang, X.; Chen, Y.; Liu, X.; Guo, C. Lipopolysaccharide Downregulates CD163 Expression to Inhibit PRRSV Infection via TLR4-NF-κB Pathway. Front. Microbiol. 2020, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ye, Z.; Wang, W.; Li, Y.; Sun, Z.; Yu, X.; Tian, K.; Li, X. A rescued virus from the infectious clone of a PRRSV NADC34-like strain exhibits high pathogenicity for nursery pigs. J. Integr. Agric. 2024, in press. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, P.; Yuan, L.; Lian, Z.; Hu, D.; Yao, X.; Li, X. Induction of UPR Promotes Interferon Response to Inhibit PRRSV Replication via PKR and NF-κB Pathway. Front. Microbiol. 2021, 12, 757690. [Google Scholar] [CrossRef]
- Xu, H.; Li, C.; Gong, B.; Li, W.; Guo, Z.; Sun, Q.; Zhao, J.; Xiang, L.; Li, J.; Tang, Y.D.; et al. Live-Attenuated Vaccine Derived from the SD-R Strain against NADC34-like Porcine Reproductive and Respiratory Syndrome Virus. Vaccines 2023, 11, 1349. [Google Scholar] [CrossRef]
- Chen, N.; Ye, M.; Xiao, Y.; Li, S.; Huang, Y.; Li, X.; Tian, K.; Zhu, J. Development of universal and quadruplex real-time RT-PCR assays for simultaneous detection and differentiation of porcine reproductive and respiratory syndrome viruses. Transbound. Emerg. Dis. 2019, 66, 2271–2278. [Google Scholar] [CrossRef]
- Huang, G.; Liu, X.; Tang, X.; Du, L.; Feng, W.; Hu, X.; Zhu, L.; Li, Q.; Suo, X. Increased Neutralizing Antibody Production and Interferon-γ Secretion in Response to Porcine Reproductive and Respiratory Syndrome Virus Immunization in Genetically Modified Pigs. Front. Immunol. 2017, 8, 1110. [Google Scholar] [CrossRef]
- Li, C.; Gong, B.; Sun, Q.; Xu, H.; Zhao, J.; Xiang, L.; Tang, Y.D.; Leng, C.; Li, W.; Guo, Z.; et al. First Detection of NADC34-like PRRSV as a Main Epidemic Strain on a Large Farm in China. Pathogens 2021, 11, 32. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, Y.; Yang, J.; Li, W.Z.; Yu, X.X.; Wang, S.Y.; Li, L.A.; Yu, H. Recombination between NADC34-like and QYYZ-like strain of porcine reproductive and respiratory syndrome virus with high pathogenicity for piglets in China. Transbound. Emerg. Dis. 2022, 69, e3202–e3207. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Wang, F.X.; Han, X.Y.; Guo, H.; Liu, C.Y.; Hou, L.N.; Wang, Y.X.; Zheng, H.; Wang, L.; Wen, Y.J. Recent advances in the study of NADC34-like porcine reproductive and respiratory syndrome virus in China. Front. Microbiol. 2022, 13, 950402. [Google Scholar] [CrossRef]
- Kwon, B.; Ansari, I.H.; Pattnaik, A.K.; Osorio, F.A. Identification of virulence determinants of porcine reproductive and respiratory syndrome virus through construction of chimeric clones. Virology 2008, 380, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Gao, J.C.; Xiong, J.Y.; Guo, J.C.; Yang, Y.B.; Jiang, C.G.; Tang, Y.D.; Tian, Z.J.; Cai, X.H.; Tong, G.Z.; et al. Two Residues in NSP9 Contribute to the Enhanced Replication and Pathogenicity of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus. J. Virol. 2018, 92, e02209-17. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, J.S.; Wang, Y.; Layton, S.; Ciacci-Zanella, J.; Roof, M.B.; Faaberg, K.S. Vaccine efficacy of porcine reproductive and respiratory syndrome virus chimeras. Vaccine 2010, 28, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Loving, C.L.; Osorio, F.A.; Murtaugh, M.P.; Zuckermann, F.A. Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus. Vet. Immunol. Immunopathol. 2015, 167, 1–14. [Google Scholar] [CrossRef]
- Kappes, M.A.; Miller, C.L.; Faaberg, K.S. Highly divergent strains of porcine reproductive and respiratory syndrome virus incorporate multiple isoforms of nonstructural protein 2 into virions. J. Virol. 2013, 87, 13456–13465. [Google Scholar] [CrossRef]

| Primer | Sequence (5′-3′) |
|---|---|
| JS2021NADC34F1-LF | CAGAGCTGCATGCTAATAGCATGACGTATAGGTGTTGGCTCTATG |
| JS2021NADC34F1-LR | TAAGGAGCAGTGTTTAAACTGCTAGC |
| JS2021NADC34F2-LF | GCAGTGTTTAAACTGCTAGCCGCCA |
| JS2021NADC34F2-LR | TTCAGTCCAGGCCCAACGTTGGTTCAAC |
| CHR6F3-LF | AACGTTGGGCCTGGACTGAAATGAAATGGGGTCTATGCAA |
| CHR6F3-LR | GTCTCAAGAATGCCAGCTCATCATGCTGAGGGTGATGC |
| Vec-F | TGAGCTGGCATTCTTGAGACATCCCAGTGCTTG |
| Vec-R | GCTATTAGCATGCAGCTCTGCTTATATAG |
| JS2021NADC34F1B-F | TTATTGGGCGGGGCACGCTACGTCT |
| JS2021NADC34F1A-R | AGACGTAGCGTGCCCCGCCCAATAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Zhang, Z.; Zhu, Z.; Sun, Z.; Tian, K.; Li, X. A Live-Attenuated Chimeric Vaccine Candidate Against the Emerging NADC34-Like PRRSV. Vet. Sci. 2025, 12, 290. https://doi.org/10.3390/vetsci12030290
Ye Z, Zhang Z, Zhu Z, Sun Z, Tian K, Li X. A Live-Attenuated Chimeric Vaccine Candidate Against the Emerging NADC34-Like PRRSV. Veterinary Sciences. 2025; 12(3):290. https://doi.org/10.3390/vetsci12030290
Chicago/Turabian StyleYe, Zhengqin, Zhendong Zhang, Zhenbang Zhu, Zhe Sun, Kegong Tian, and Xiangdong Li. 2025. "A Live-Attenuated Chimeric Vaccine Candidate Against the Emerging NADC34-Like PRRSV" Veterinary Sciences 12, no. 3: 290. https://doi.org/10.3390/vetsci12030290
APA StyleYe, Z., Zhang, Z., Zhu, Z., Sun, Z., Tian, K., & Li, X. (2025). A Live-Attenuated Chimeric Vaccine Candidate Against the Emerging NADC34-Like PRRSV. Veterinary Sciences, 12(3), 290. https://doi.org/10.3390/vetsci12030290








