A Comparative Analysis of Clinical Presentation, Prognosis and Outcomes in Paralytic Dogs with a Compressive and a Contusive Intervertebral Disc Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
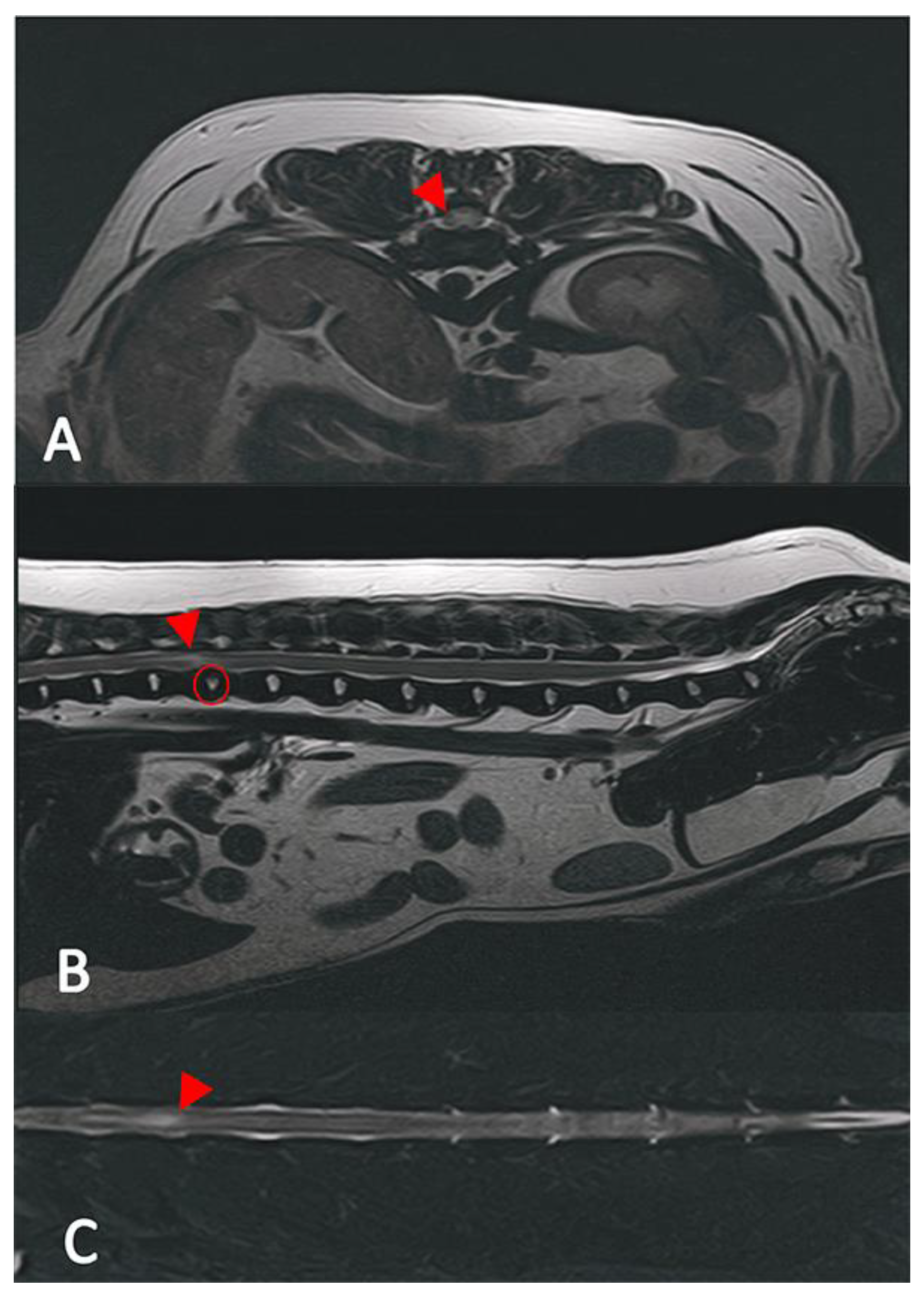
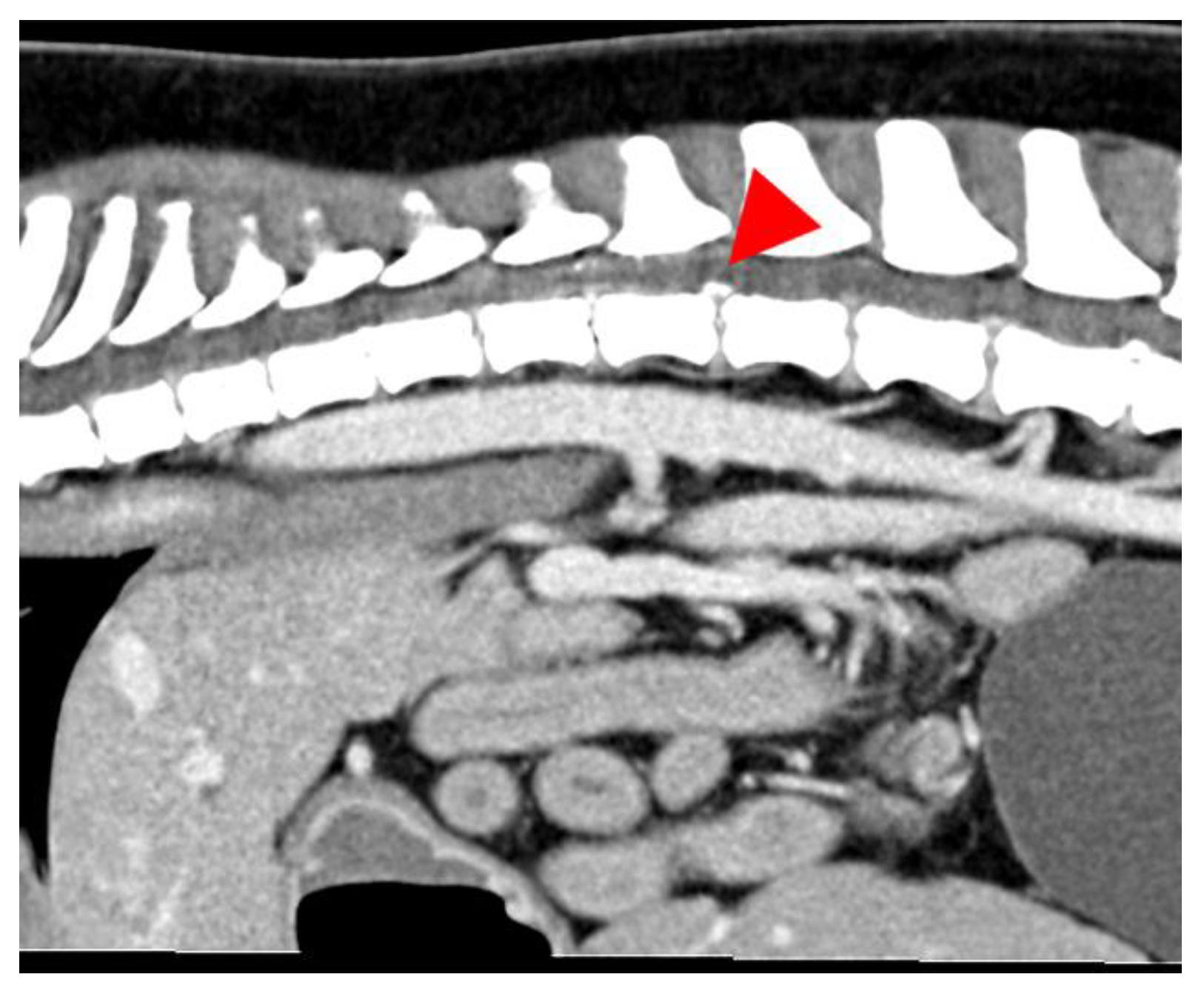
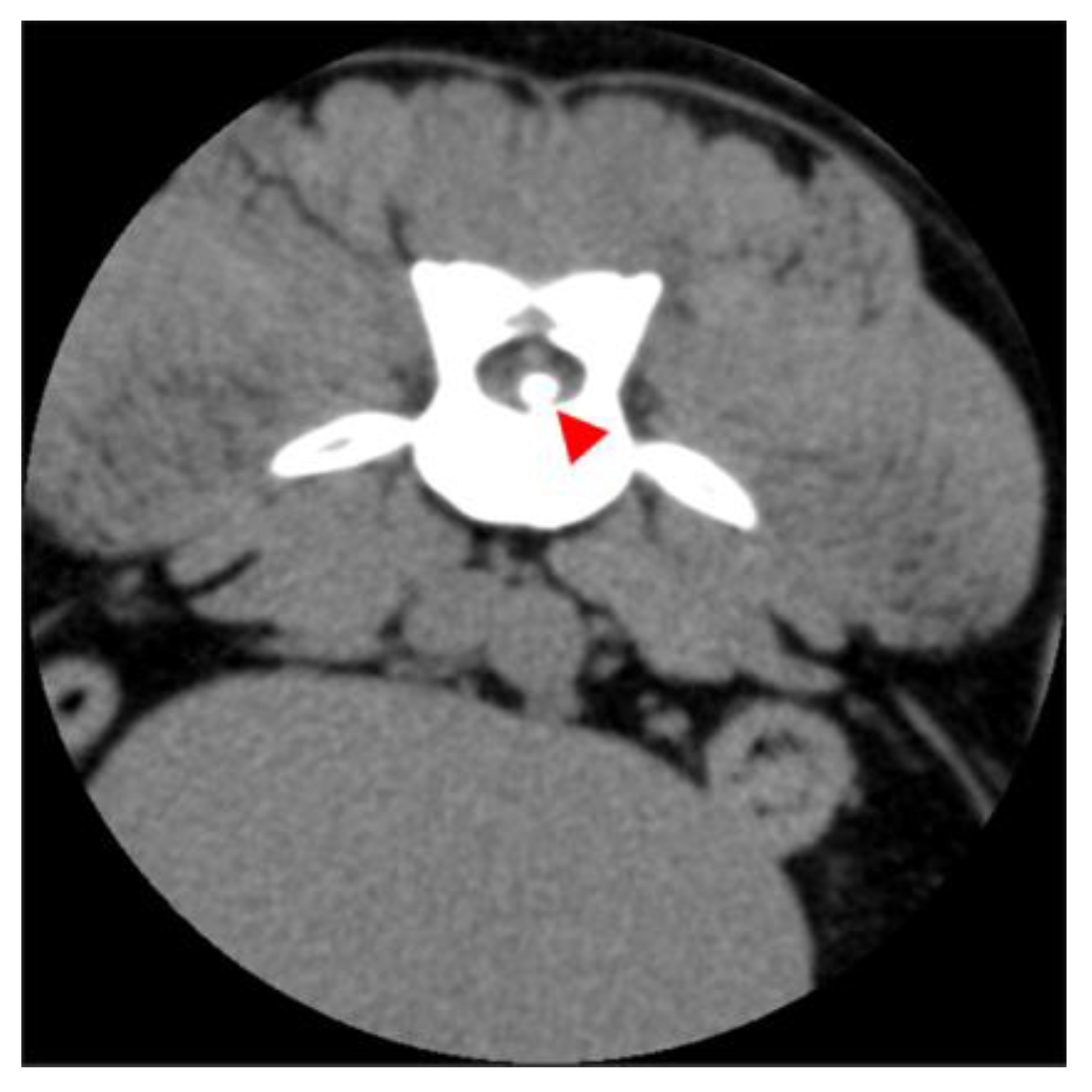
2.2. Diagnostic Imaging (CT and MRI)
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients
3.2. Clinical Signs and Diagnostic Findings
3.3. Short-Term and Long-Term Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IVDE | Intervertebral disc extrusion |
| ANNPE | Acute non-compressive nucleus pulposus extrusion |
| CT | Computer tomography |
| MRI | Magnetic resonance imaging |
References
- Sharp, N.; Wheeler, S. Small Animal Spinal Disorders; Elsevier: Amsterdam, The Netherlands, 2005; pp. 363–379. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780723432098500200 (accessed on 13 September 2022).
- Hansen, H.-J. A Pathologic-Anatomical Study on Disc Degeneration in Dog: With Special Reference to the So-Called Enchondrosis Intervertebralis. Acta Orthop. Scand. 1952, 23, 1–130. Available online: http://www.tandfonline.com/doi/full/10.3109/ort.1952.23.suppl-11.01 (accessed on 13 September 2022). [CrossRef] [PubMed]
- Botsoglou, R.; Sarpekidou, E.; Patsikas, M.; Kazakos, G. Acute non-compressive nucleus pulposus extrusion in dogs and cats: An overview. J. Hell. Vet. Med. Soc. 2021, 72, 2809–2816. [Google Scholar]
- Besalti, O.; Ozak, A.; Pekcan, Z.; Tong, S.; Eminaga, S.; Tacal, T. The role of extruded disk material in thoracolumbar intervertebral disk disease: A retrospective study in 40 dogs. Can. Vet. J. 2005, 46, 814. [Google Scholar]
- Brisson, B.A. Intervertebral disc disease in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 829–858. [Google Scholar] [CrossRef]
- Bergknut, N.; Meij, B.P.; Hagman, R.; De Nies, K.S.; Rutges, J.P.; Smolders, L.A.; Creemers, L.B.; Lagerstedt, A.S.; Hazewinkel, H.A.W.; Grinwis, G.C.M. Intervertebral disc disease in dogs—Part 1: A new histological grading scheme for classification of intervertebral disc degeneration in dogs. Vet. J. 2013, 195, 156–163. [Google Scholar] [CrossRef]
- Priester, W.A. Canine intervertebral disc disease—Occurrence by age, breed, and sex among 8117 cases. Theriogenology 1976, 6, 293–303. Available online: https://linkinghub.elsevier.com/retrieve/pii/0093691X76900212 (accessed on 15 September 2022). [CrossRef]
- Ghosh, P.; Taylor, T.K.F.; Braund, K.G.; Larsen, L.H. A Comparative Chemical and Histochemical Study of the Chondrodystrophoid and Nonchondrodystrophoid Canine Intervertebral Disc. Vet. Pathol. 1976, 13, 414–427. Available online: http://journals.sagepub.com/doi/10.1177/030098587601300603 (accessed on 15 September 2022). [CrossRef]
- Bergknut, N.; Egenvall, A.; Hagman, R.; Gustås, P.; Hazewinkel, H.A.W.; Meij, B.P.; Lagerstedt, A.S. Incidence of intervertebral disk degeneration–related diseases and associated mortality rates in dogs. J. Am. Vet. Med. Assoc. 2012, 240, 1300–1309. Available online: https://avmajournals.avma.org/view/journals/javma/240/11/javma.240.11.1300.xml (accessed on 5 October 2022). [CrossRef]
- Coates, J.R. Intervertebral disk disease. Vet. Clin. N. Am. Small Anim. Pract. 2000, 30, 77–110. [Google Scholar] [CrossRef]
- Moore, S.A.; Tipold, A.; Olby, N.J.; Stein, V.; Granger, N. Current Approaches to the Management of Acute Thoracolumbar Disc Extrusion in Dogs. Front. Vet. Sci. 2020, 7, 610. [Google Scholar] [CrossRef]
- Levine, J.M.; Levine, G.J.; Johnson, S.I.; Kerwin, S.C.; Hettlich, B.F.; Fosgate, G.T. Evaluation of the success of medical management for presumptive cervical intervertebral disk herniation in dogs. Vet. Surg. 2007, 36, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Fujita, H.; Kanazono, S.; Shibata, M.; Yoshigae, Y. Long-term neurologic outcome of hemilaminectomy and disk fenestration for treatment of dogs with thoracolumbar intervertebral disk herniation: 831 cases (2000–2007). J. Am. Vet. Med. Assoc. 2012, 241, 1617–1626. Available online: https://avmajournals.avma.org/view/journals/javma/241/12/javma.241.12.1617.xml (accessed on 3 October 2022). [CrossRef] [PubMed]
- Langerhuus, L.; Miles, J. Proportion recovery and times to ambulation for non-ambulatory dogs with thoracolumbar disc extrusions treated with hemilaminectomy or conservative treatment: A systematic review and meta-analysis of case-series studies. Vet. J. 2017, 220, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.V.; Sharp, N.J.H. A comparison of conservative treatment and fenestration for thoracolumbar intervertebral disc disease in the dog. J. Small Anim. Pract. 1983, 24, 721–729. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1748-5827.1983.tb00360.x (accessed on 6 October 2022). [CrossRef]
- Funkquist, B. Investigations of the Therapeutig and Prophylactic Effects of Disc Evacuation in Cases of Thoraco-Lumbar Herniated Discs in Dogs. Acta Vet. Scand. 1978, 19, 441–457. Available online: https://actavetscand.biomedcentral.com/articles/10.1186/BF03547613 (accessed on 10 December 2022). [CrossRef]
- Baumhardt, R.; Ripplinger, A.; Aiello, G.; Schwab, M.L.; Ferrarin, D.A.; Wrzesinski, M.R.; Rauber, J.; Mazzanti, A. Clinical management of dogs with presumptive diagnosis of thoracolumbar intervertebral disc disease: 164 cases (2006–2017). Pesqui. Vet. Bras. 2020, 40, 55–60. [Google Scholar] [CrossRef]
- Mann, F.A.; Wagner-Mann, C.C.; Dunphy, E.D.; Ruben, D.S.; Rochat, M.C.; Bartels, K.E. Recurrence rate of presumed thoracolumbar intervertebral disc disease in ambulatory dogs with spinal hyperpathia treated with anti-inflammatory drugs: 78 Cases (1997–2000): Retrospective study. J. Vet. Emerg. Crit. Care 2007, 17, 53–60. [Google Scholar] [CrossRef]
- De Decker, S.; Fenn, J. Acute Herniation of Nondegenerate Nucleus Pulposus: Acute Noncompressive Nucleus Pulposus Extrusion and Compressive Hydrated Nucleus Pulposus Extrusion. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 95–109. [Google Scholar] [CrossRef]
- De Risio, L. A review of fibrocartilaginous embolic myelopathy and different types of peracute non-compressive intervertebral disk extrusions in dogs and cats. Front. Vet. Sci. 2015, 2, 24. [Google Scholar] [CrossRef]
- Drum, M.G. Physical Rehabilitation of the Canine Neurologic Patient. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 181–193. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0195561609001478 (accessed on 10 December 2022). [CrossRef]
- De Risio, L.; Adams, V.; Dennis, R.; McConnell, F.J. Association of clinical and magnetic resonance imaging findings with outcome in dogs with presumptive acute noncompressive nucleus pulposus extrusion: 42 cases (2000–2007). J. Am. Vet. Med. Assoc. 2009, 234, 495–504. Available online: https://avmajournals.avma.org/view/journals/javma/234/4/javma.234.4.495.xml (accessed on 10 December 2022). [CrossRef] [PubMed]
- McKee, W.M.; Downes, C.J.; Pink, J.J.; Gemmill, T.J. Presumptive exercise-associated peracute thoracolumbar disc extrusion in 48 dogs. Vet. Rec. 2010, 166, 523–528. [Google Scholar] [CrossRef]
- Fenn, J.; Drees, R.; Volk, H.A.; De Decker, S. Comparison of clinical signs and outcomes between dogs with presumptive ischemic myelopathy and dogs with acute noncompressive nucleus pulposus extrusion. J. Am. Vet. Med. Assoc. 2016, 249, 767–775. Available online: https://avmajournals.avma.org/view/journals/javma/249/7/javma.249.7.767.xml (accessed on 10 December 2022). [CrossRef]
- Chang, Y.; Dennis, R.; Platt, S.R.; Penderis, J. Magnetic resonance imaging of traumatic intervertebral disc extension in dogs. Vet. Rec. 2007, 160, 795–799. Available online: https://bvajournals.onlinelibrary.wiley.com/doi/10.1136/vr.160.23.795 (accessed on 10 December 2022). [CrossRef]
- Mari, L.; Behr, S.; Shea, A.; Dominguez, E.; Johnson, P.J.; Ekiri, A.; De Risio, L. Outcome comparison in dogs with a presumptive diagnosis of thoracolumbar fibrocartilaginous embolic myelopathy and acute non-compressive nucleus pulposus extrusion. Vet. Rec. 2017, 181, 293. [Google Scholar] [CrossRef]
- da Costa, R.C.; De Decker, S.; Lewis, M.J.; Volk, H. Diagnostic Imaging in Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 588338. [Google Scholar] [CrossRef]
- Fenn, J.; Drees, R.; Volk, H.A.; De Decker, S. Inter- and intraobserver agreement for diagnosing presumptive ischemic myelopathy and acute noncompressive nucleus pulposus extrusion in dogs using magnetic resonance imaging. Vet. Radiol. Ultrasound 2016, 57, 33–40. [Google Scholar] [CrossRef]
- Olby, N.J.; Murana, K.R.; Sharp, N.J.H.; Thrall, D.E. The Computed Tomographic Appearance of Acute Thoracolumbar Intervertebral Disc Herniations in Dogs. Vet. Radiol. Ultrasound 2000, 41, 396–402. [Google Scholar] [CrossRef]
- Lim, C.; Kweon, O.K.; Choi, M.C.; Choi, J.; Yoon, J. Computed tomographic characteristics of acute thoracolumbar intervertebral disc disease in dogs. J. Vet. Sci. 2010, 11, 73–79. [Google Scholar] [CrossRef]
- Da Costa, R.C.; Echandi, R.L.; Beauchamp, D. Computed tomography myelographic findings in dogs with cervical spondylomyelopathy. Vet. Radiol. Ultrasound 2012, 53, 64–70. [Google Scholar] [CrossRef]
- Braund, K.G.; Ghosh, P.; Taylor, T.K.F.; Larsen, L.H. Morphological studies of the canine intervertebral disc. The assignment of the beagle to the achondroplastic classification. Res. Vet. Sci. 1975, 19, 167–172. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0034528818335276 (accessed on 10 December 2022). [CrossRef] [PubMed]
- Griffiths, I. A syndrome produced by dorso-lateral “explosions” of the cervical intervertebral discs. Vet. Rec. 1970, 87, 737–741. Available online: https://pubmed.ncbi.nlm.nih.gov/5531244/ (accessed on 10 December 2022). [CrossRef] [PubMed]
- Brown, E.A.; Dickinson, P.J.; Mansour, T.; Sturges, B.K.; Aguilar, M.; Young, A.E.; Korff, C.; Lind, J.; Ettinger, C.L.; Varon, S.; et al. FGF4 retrogene on CFA12 is responsible for chondrodystrophy and intervertebral disc disease in dogs. Proc. Natl. Acad. Sci. USA 2017, 114, 11476–11481. [Google Scholar] [CrossRef]
- Smolders, L.A.; Bergknut, N.; Grinwis, G.C.M.; Hagman, R.; Lagerstedt, A.S.; Hazewinkel, H.A.W.; Tryfonidou, M.A.; Meij, B.P. Intervertebral disc degeneration in the dog. Part 2: Chondrodystrophic and non-chondrodystrophic breeds. Vet. J. 2013, 195, 292–299. [Google Scholar] [CrossRef]
- Mayousse, V.; Desquilbet, L.; Jeandel, A.; Blot, S. Prevalence of neurological disorders in French bulldog: A retrospective study of 343 cases (2002–2016). BMC Vet. Res. 2017, 13, 212. [Google Scholar] [CrossRef]
- Gillett, N.A.; Gerlach, R.; Cassidy, J.J.; Brown, S.A. Age-related changes in the beagle spine. Acta Orthop. Scand. 1988, 59, 503–507. Available online: http://www.tandfonline.com/doi/full/10.3109/17453678809148772 (accessed on 23 January 2022). [CrossRef]
- Leif Kopernik, Verband für das Deutsche Hundewesen (VDH) e.V. VDH Welpenstatistik [Internet]. 2022. Available online: https://www.vdh.de/ueber-den-vdh/welpenstatistik/ (accessed on 6 September 2023).
- Beltran, E. Acute Hydrated Non-Compressive Nucleus Pulposus Extrusion: What Do We Know So Far? [Internet]. 2017. Available online: https://bvajournals.onlinelibrary.wiley.com/doi/10.1136/vr.j5494 (accessed on 23 January 2022).
- Mari, L.; Behr, S.; Shea, A.; Dominguez, E.; Ricco, C.; Alcoverro, E.; Ekiri, A.; Sanchez-Masian, D.; De Risio, L. Predictors of urinary or fecal incontinence in dogs with thoracolumbar acute non-compressive nucleus pulposus extrusion. J. Vet. Intern. Med. 2019, 33, 2693–2700. [Google Scholar] [CrossRef]
- Hoerlein, B.F. Canine Neurology, Diagnosis and Treatment, 3rd ed.; W. B. Saunders Company: Philadelphia, PA, USA, 1978. [Google Scholar]
- Shores, A. Fractures and luxations of the vertebral column. Vet. Clin. N. Am. Small Anim. Pract. 1992, 22, 171–180. [Google Scholar] [CrossRef]
- Fenn, J.; Olby, N.J. Classification of Intervertebral Disc Disease. Front. Vet. Sci. 2020, 7, 579025. [Google Scholar] [CrossRef]
- Griffiths, I.R. The extensive myelopathy of intervertebral disc protrusions in dogs (‘the ascending syndrome’). J. Small Anim. Pract. 1972, 13, 425–437. [Google Scholar] [CrossRef]
- Cordle, K.J.; Seiler, G.S.; Barnes, D.; Olby, N.J. MRI features can help to confirm a diagnosis of progressive myelomalacia, but may not be accurate in dogs lacking characteristic clinical signs at the time of imaging. Vet. Radiol. Ultrasound 2023, 64, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.; Mckee, M. Laminectomy for 34 dogs with thoracolumbar intervertebral disc and loss of deep pain perception. J. Small Anim. Pract. 1999, 40, 417–422. [Google Scholar] [CrossRef]
- Nakamoto, Y.; Uemura, T.; Hasegawa, H.; Nakamoto, M.; Ozawa, T. Outcomes of dogs with progressive myelomalacia treated with hemilaminectomy or with extensive hemilaminectomy and durotomy. Vet. Surg. 2021, 50, 81–88. [Google Scholar] [CrossRef]
- Balducci, F.; Canal, S.; Contiero, B.; Bernardini, M. Prevalence and Risk Factors for Presumptive Ascending/Descending Myelomalacia in Dogs after Thoracolumbar Intervertebral Disk Herniation. J. Vet. Intern. Med. 2017, 31, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Castel, A.; Olby, N.J.; Ru, H.; Mariani, C.L.; Muñana, K.R.; Early, P.J. Risk factors associated with progressive myelomalacia in dogs with complete sensorimotor loss following intervertebral disc extrusion: A retrospective case-control study. BMC Vet. Res. 2019, 15, 433. [Google Scholar] [CrossRef] [PubMed]
| Sharp and Wheeler | |
|---|---|
| Grade | Description |
| 1 | Pain only |
| 2 | Ambulatory paresis |
| 3 | Non-ambulatory paresis |
| 4 | Paralysis with deep pain sensation |
| 5 | Paralysis with loss of deep pain sensation |
| ANNPE | IVDE | p-Value | |
|---|---|---|---|
| Age (years) | 7 (0.2–13) | 5.5 (3–15) | 0.97 |
| Sex | 0.98 | ||
| female | 26 (58%) | 29 (58%) | |
| male | 19 (42%) | 21 (42%) | |
| Body weight (kg) | 23.6 ± 10.7 | 11.5 ± 7.6 | <0.001 |
| Duration of clinical signs before examination (hours) | 3 (1–12) | 16 (1–96) | |
| Time frame from the onset of clinical signs to presentation | <0.001 | ||
| <12 h | 45 (100%) | 17 (34%) | |
| 12–48 h | 0 (0%) | 29 (58%) | |
| >48 h | 0 (0%) | 4 (8%) | |
| Event prior to the onset of clinical signs | <0.001 | ||
| Trauma | 23 (50%) | 2 (4%) | |
| Unknown | 19 (42%) | 43 (86%) | |
| Exercise | 4 (8%) | 5 (10%) | |
| Vocalization at the onset of clinical signs | <0.001 | ||
| Yes | 32 (71%) | 4 (8%) | |
| No | 13 (29%) | 46 (92%) | |
| Sharp and Wheeler grade at the time of examination | 0.16 | ||
| Grade 4 | 33 (73%) | 31 (62%) | |
| Grade 5 | 12 (27%) | 19 (38%) | |
| Deterioration of clinical signs before referral | <0.001 | ||
| No | 39 (87%) | 16 (32%) | |
| Yes | 6 (13%) | 34 (68%) | |
| Medication received before referral | |||
| None | 18 (40%) | 15 (30%) | |
| NSAIDs | 13 (28%) | 24 (48%) | |
| Opioids | 3 (7%) | 5 (10%) | |
| Gabapentin/ Pregabalin | 0 (0%) | 5 (10%) | |
| Corticosteroids | 12 (27%) | 16 (32%) | |
| Unknown | 8 (18%) | 7 (14%) | |
| Others | 11 (24%) | 9 (18%) | |
| Neuroanatomical localization of the lesion | 0.88 | ||
| C1-C5 | 1 (2%) | 1 (2%) | |
| C6-T2 | 0 (0%) | 0 (0%) | |
| T3-L3 | 38 (84%) | 44 (88%) | |
| L4-S3 | 6 (13%) | 5 (10%) | |
| Severity of the lesion | <0.001 | ||
| Mild | 22 (49%) | 6 (12%) | |
| Moderate | 17 (38%) | 33 (66%) | |
| Severe | 6 (13%) | 11 (22%) | |
| Long-term outcome | 0.79 | ||
| Successful | 32 (71%) | 40 (80%) | |
| Unsuccessful | 3 (8%) | 3 (7%) | |
| Duration of hospitalization (days) | 2.5 (0–6) | 3.7 (0–9) | <0.001 |
| Ambulatory at discharge | 5 (11%) | 12 (24%) | 0.10 |
| Time from onset to ambulation | 0.32 | ||
| Within 14 days | 14 (31%) | 24 (48%) | |
| Within 2 months (first 14 days excluded) | 12 (27%) | 9 (18%) | |
| Within 3–8 months | 6 (13%) | 7 (14%) | |
| Euthanized dogs | 10 (22%) | 7 (14%) | 0.3 |
| Due to the owners’ request | 6 (13%) | 1 (2%) | |
| Failure of improvement after 14 days | 1 (2%) | 3 (6%) | |
| Suspected myelomalacia | 3 (6%) | 3 (6%) | |
| Supportive treatment after diagnosis | |||
| Urinary catheter | 20 (44%) | 20 (40%) | |
| NSAIDs | 39 (87%) | 41 (82%) | |
| Opioids | 25 (56%) | 37 (74%) | |
| Gabapentin/Pregabalin | 28 (62%) | 39 (78%) | |
| Vitamin B | 35 (78%) | 29 (58%) | |
| Alpha1 Antagonists | 25 (56%) | 39 (78%) | |
| Parasympathomimetics | 9 (20%) | 27 (54%) | |
| Corticosteroids | 2 (4%) | 0 (0%) | |
| Physiotherapy | 31 (69%) | 28 (56%) | |
| Immobilization | 21 (47%) | 23 (46%) | |
| Others | 8 (18%) | 11 (22%) |
| NA Lokalisation | ANNPE S/W 4 | ANNPE S/W 5 | IVDE S/W 4 | IVDE S/W 5 |
|---|---|---|---|---|
| C1-C5 | 1 | - | 1 | - |
| C6-T2 | - | - | - | - |
| T3-L3 | 29 | 9 | 28 | 16 |
| L4-S3 | 3 | 3 | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurtscheidt, A.; Rupp, S.; Müller, U.; Forterre, F. A Comparative Analysis of Clinical Presentation, Prognosis and Outcomes in Paralytic Dogs with a Compressive and a Contusive Intervertebral Disc Disease. Vet. Sci. 2025, 12, 287. https://doi.org/10.3390/vetsci12030287
Kurtscheidt A, Rupp S, Müller U, Forterre F. A Comparative Analysis of Clinical Presentation, Prognosis and Outcomes in Paralytic Dogs with a Compressive and a Contusive Intervertebral Disc Disease. Veterinary Sciences. 2025; 12(3):287. https://doi.org/10.3390/vetsci12030287
Chicago/Turabian StyleKurtscheidt, Anna, Stefan Rupp, Ute Müller, and Franck Forterre. 2025. "A Comparative Analysis of Clinical Presentation, Prognosis and Outcomes in Paralytic Dogs with a Compressive and a Contusive Intervertebral Disc Disease" Veterinary Sciences 12, no. 3: 287. https://doi.org/10.3390/vetsci12030287
APA StyleKurtscheidt, A., Rupp, S., Müller, U., & Forterre, F. (2025). A Comparative Analysis of Clinical Presentation, Prognosis and Outcomes in Paralytic Dogs with a Compressive and a Contusive Intervertebral Disc Disease. Veterinary Sciences, 12(3), 287. https://doi.org/10.3390/vetsci12030287





