A Case of Canine Hepatitis with Hepatocellular Attack by Non-Neoplastic Perforin-Laden Lymphocytes
Simple Summary
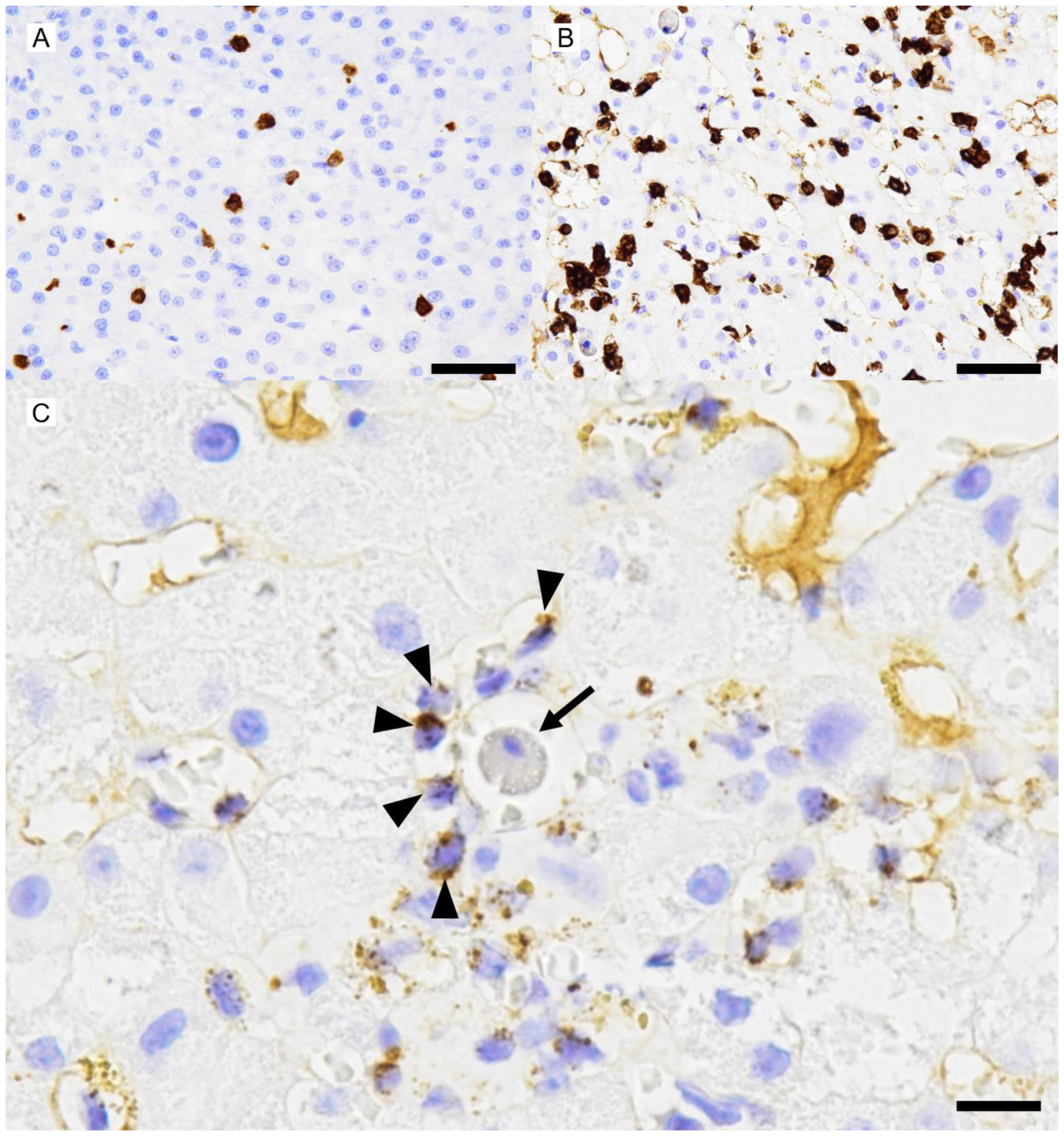
Abstract
1. Introduction
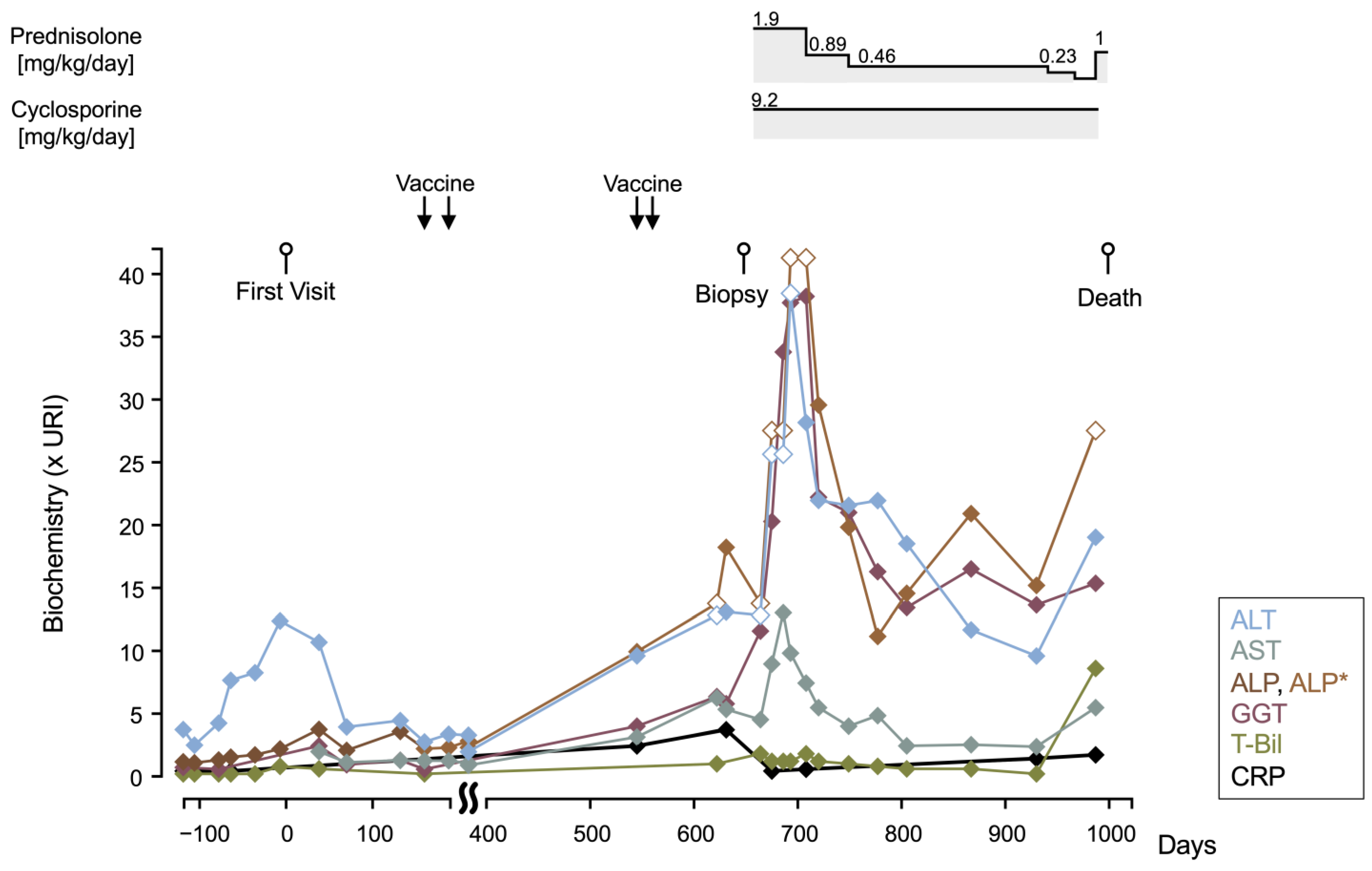
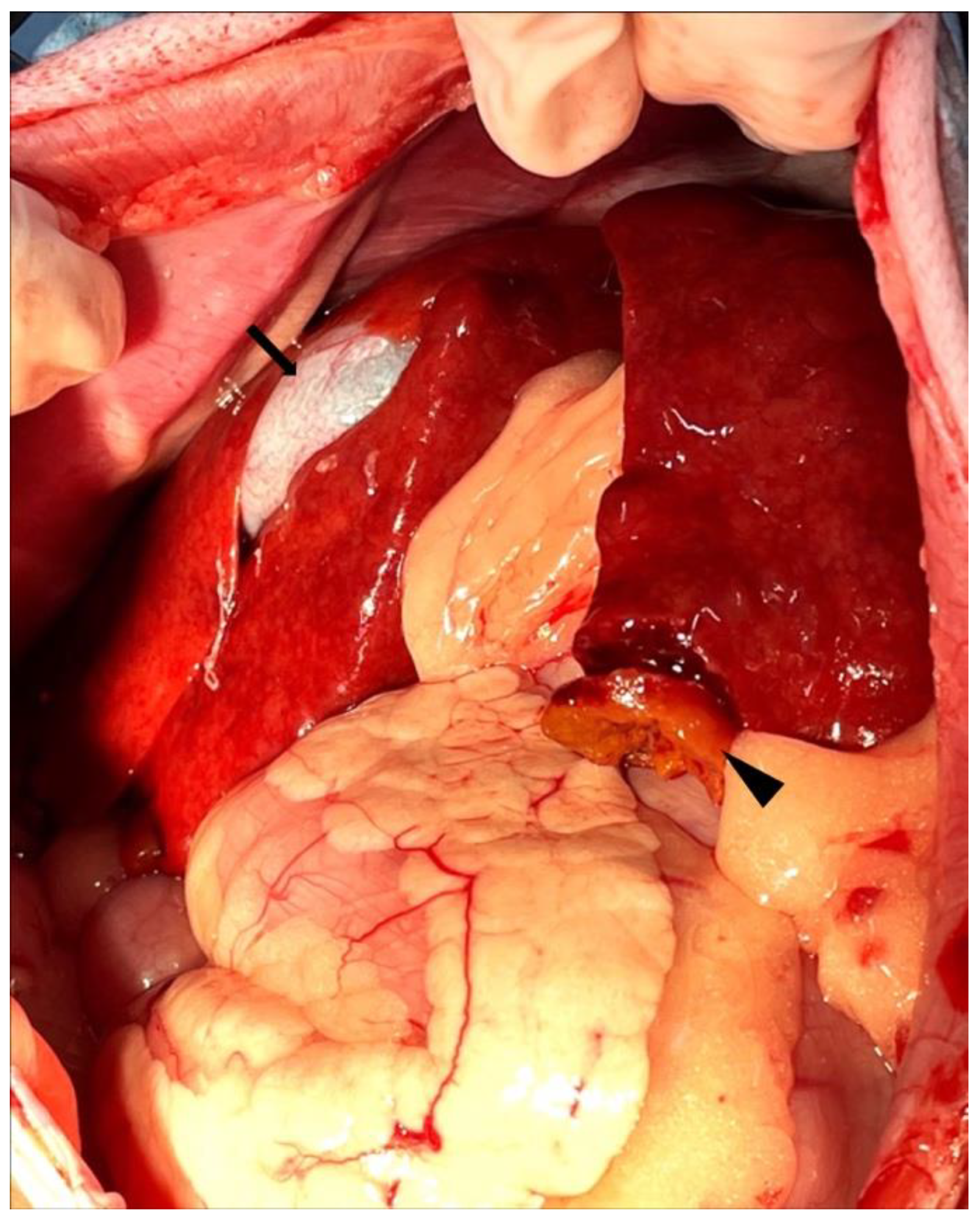
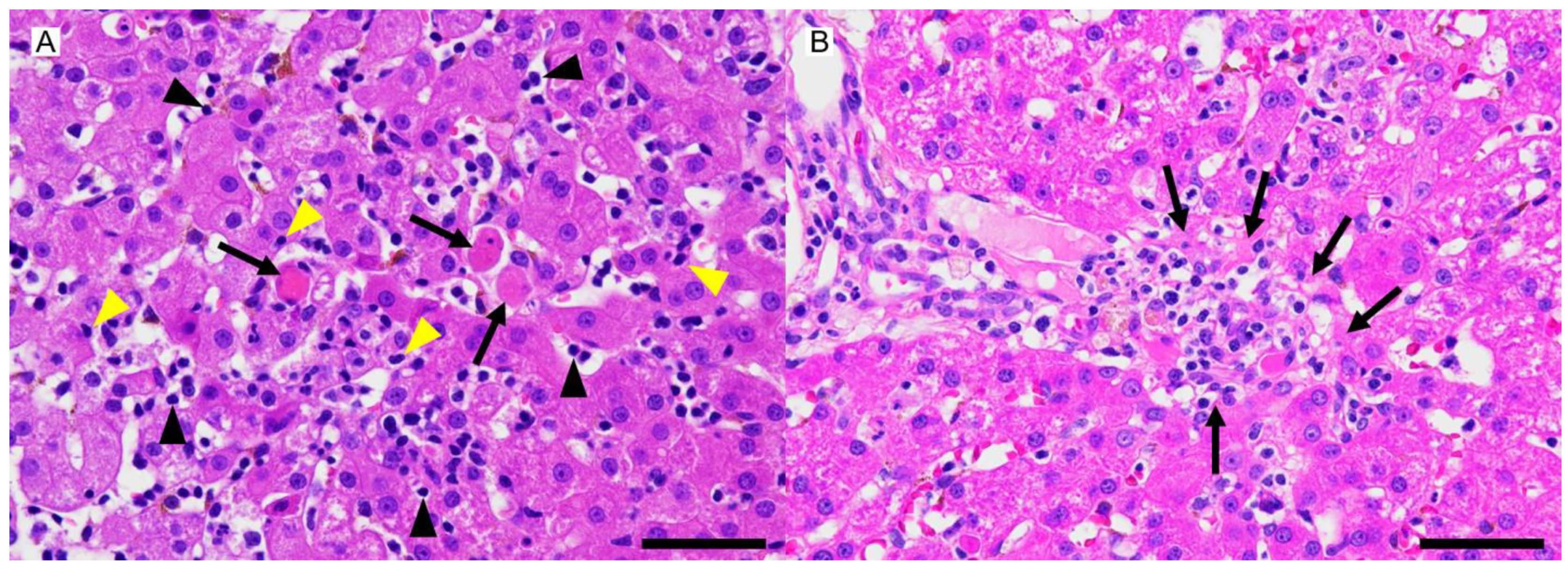
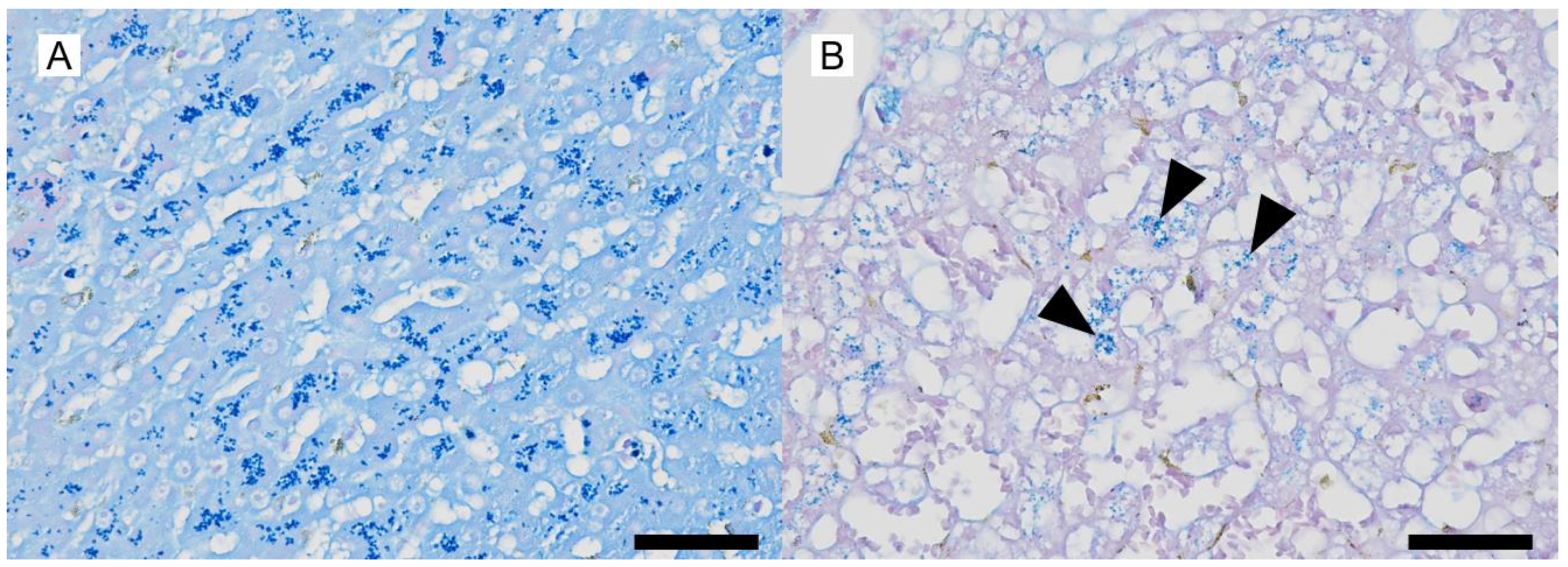
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WSAVA Liver Standardization Group. WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Disease; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Poldervaart, J.H.; Favier, R.P.; Penning, L.C.; Van Den Ingh, T.S.G.A.M.; Rothuizen, J. Primary hepatitis in dogs: A retrospective review (2002–2006). J. Vet. Intern. Med. 2009, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.R.L.; Center, S.A.; Cullen, J.M.; Penninck, D.G.; Richter, K.P.; Twedt, D.C.; Watson, P.J. ACVIM consensus statement on the diagnosis and treatment of chronic hepatitis in dogs. J. Vet. Intern. Med. 2019, 33, 1173–1200. [Google Scholar] [CrossRef] [PubMed]
- Bayton, W.; Watson, P.J.; Bexfield, N.H. Prednisolone therapy for chronic hepatitis in English springer spaniels: A prospective study of 12 cases. Vet. Rec. 2020, 186, e21. [Google Scholar] [CrossRef] [PubMed]
- Favier, R.P.; Poldervaart, J.H.; Ingh, T.S.V.D.; Penning, L.C.; Rothuizen, J. A retrospective study of oral prednisolone treatment in canine chronic hepatitis. Vet. Quart 2013, 33, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Ullal, T.; Ambrosini, Y.; Rao, S.; Webster, C.R.L.; Twedt, D. Retrospective evaluation of cyclosporine in the treatment of presumed idiopathic chronic hepatitis in dogs. J. Vet. Intern. Med. 2019, 33, 2046–2056. [Google Scholar] [CrossRef] [PubMed]
- Dyggve, H.; Meri, S.; Spillmann, T.; Jarva, H.; Speeti, M. Antihistone autoantibodies in Dobermans with hepatitis. J. Vet. Intern. Med. 2017, 31, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, L.P.; Shaw, D.; Dolan, M.; Raisbeck, M.; Crawford, S.; Dennis, G.L.; Olwin, D.B. Hereditary copper toxicosis in West Highland white terriers. Vet. Pathol. 1986, 23, 148–154. [Google Scholar] [CrossRef] [PubMed]
- McCallum, K.E.; Constantino-Casas, F.; Cullen, J.M.; Warland, J.H.; Swales, H.; Linghley, N.; Kortum, A.J.; Sterritt, A.J.; Cogan, T.; Watson, P.J. Hepatic leptospiral infections in dogs without obvious renal involvement. J. Vet. Intern. Med. 2019, 33, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Enzan, H.; Hara, H.; Himeno, H.; Iwamura, S.; Saibara, T.; Onishi, S.; Yamamoto, Y. Immunohistochemical identification of Ito cells and their myofibroblastic transformation in adult human liver. Virchows. Arch. 1994, 424, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ijzer, J.; Roskams, T.; Molenbeek, R.F.; Ultee, T.; Penning, L.C.; Rothuizen, J.; Ingh, T.S.v.D. Morphological characterisation of portal myofibroblasts and hepatic stellate cells in the normal dog liver. Comp. Hepatol. 2006, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.M.; Stalker, M.J. Liver and biliary system. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, pp. 258–352. [Google Scholar]
- Speeti, M.; Eriksson, J.; Saari, S.; Westermarck, E. Lesions of subclinical Doberman hepatitis. Vet. Pathol. 1998, 35, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Bexfield, N.H.; Andres-Abdo, C.; Scase, T.J.; Constantino-Casas, F.; Watson, P.J. Chronic hepatitis in the English springer spaniel: Clinical presentation, histological description and outcome. Vet. Rec. 2011, 169, 415. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J. Diagnosis and management of autoimmune hepatitis: Current status and future directions. Gut. Liver 2016, 10, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Lohse, A.W.; Sebode, M.; Bhathal, P.S.; Clouston, A.D.; Dienes, H.P.; Jain, D.; Gouw, A.S.H.; Guindi, M.; Kakar, S.; Kleiner, D.E.; et al. Consensus recommendations for histological criteria of autoimmune hepatitis from the International AIH Pathology Group. Liver Int. 2022, 42, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Balitzer, D.; Shafizadeh, N.; Peters, M.G.; Ferrell, L.D.; Alshak, N.; Kakar, S. Autoimmune hepatitis: Review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Modern Pathol. 2017, 30, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Behairy, B.E.; El-Araby, H.A.; El Kader, H.H.A.; Ehsan, N.A.; Salem, M.E.; Zakaria, H.M.; Khedr, M.A. Assessment of intrahepatic regulatory T cells in children with autoimmune hepatitis. Ann. Hepatol. 2016, 15, 682–690. [Google Scholar] [PubMed]
- Buitrago-Molina, L.E.; Pietrek, J.; Noyan, F.; Schlue, J.; Manns, M.P.; Wedemeyer, H.; Hardtke-Wolenski, M.; Jaeckel, E. Treg-specific IL-2 therapy can reestablish intrahepatic immune regulation in autoimmune hepatitis. J. Autoimmun. 2021, 117, 102591. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.M.; Vernau, W.; Hodges, J.; Kass, P.H.; Vilches-Moure, J.G.; McElliot, V.; Moore, P.F. Hepatosplenic and hepatocytotropic T-Cell lymphoma. Vet. Pathol. 2013, 50, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; van Oijen, M.G.; Pronk, M.; Drenth, J.P. Treatment options for autoimmune hepatitis: A systematic review of randomized controlled trials. J. Hepatol. 2010, 53, 191–198. [Google Scholar] [CrossRef] [PubMed]
| Antibody To | Host | Type, Clone | Dilution | Source | Catalog Number |
|---|---|---|---|---|---|
| CD3 | Mouse | Monoclonal, LN10 | Ready to use | Leica Biosystems | PA0553 |
| CD20 | Mouse | Monoclonal, L26 | ×200 | Leica Biosystems | NCL-L-CD20-L26 |
| Perforin | Mouse | Monoclonal, 5B10 | Ready to use | Abcam | ab75573 |
| MUM1 | Mouse | Monoclonal, MUM1p | ×100 | Dako | M7259 |
| αSMA | Mouse | Monoclonal, asm-1 | Ready to use | Leica Biosystems | PA0943 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furusato, S.; Kondo, E.; Mitsui, I.; Tsuyama, Y. A Case of Canine Hepatitis with Hepatocellular Attack by Non-Neoplastic Perforin-Laden Lymphocytes. Vet. Sci. 2025, 12, 211. https://doi.org/10.3390/vetsci12030211
Furusato S, Kondo E, Mitsui I, Tsuyama Y. A Case of Canine Hepatitis with Hepatocellular Attack by Non-Neoplastic Perforin-Laden Lymphocytes. Veterinary Sciences. 2025; 12(3):211. https://doi.org/10.3390/vetsci12030211
Chicago/Turabian StyleFurusato, Shimon, Eriko Kondo, Ikki Mitsui, and Yu Tsuyama. 2025. "A Case of Canine Hepatitis with Hepatocellular Attack by Non-Neoplastic Perforin-Laden Lymphocytes" Veterinary Sciences 12, no. 3: 211. https://doi.org/10.3390/vetsci12030211
APA StyleFurusato, S., Kondo, E., Mitsui, I., & Tsuyama, Y. (2025). A Case of Canine Hepatitis with Hepatocellular Attack by Non-Neoplastic Perforin-Laden Lymphocytes. Veterinary Sciences, 12(3), 211. https://doi.org/10.3390/vetsci12030211






