Development and Validation of a Droplet Digital PCR Assay for Detection of Feline Herpesvirus Type-1
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strains and Samples Analysis
2.2. Primer and TaqMan Probe Design
2.3. Nucleic Acid Extraction
2.4. Construction of Standard Plasmids
2.5. qPCR Assay
2.6. Droplet Digital PCR (ddPCR) Assay
2.7. Analytical Sensitivity and Repeatability of the ddPCR and qPCR
2.8. Specificity of the ddPCR Assay
2.9. Clinical Samples Test
3. Results
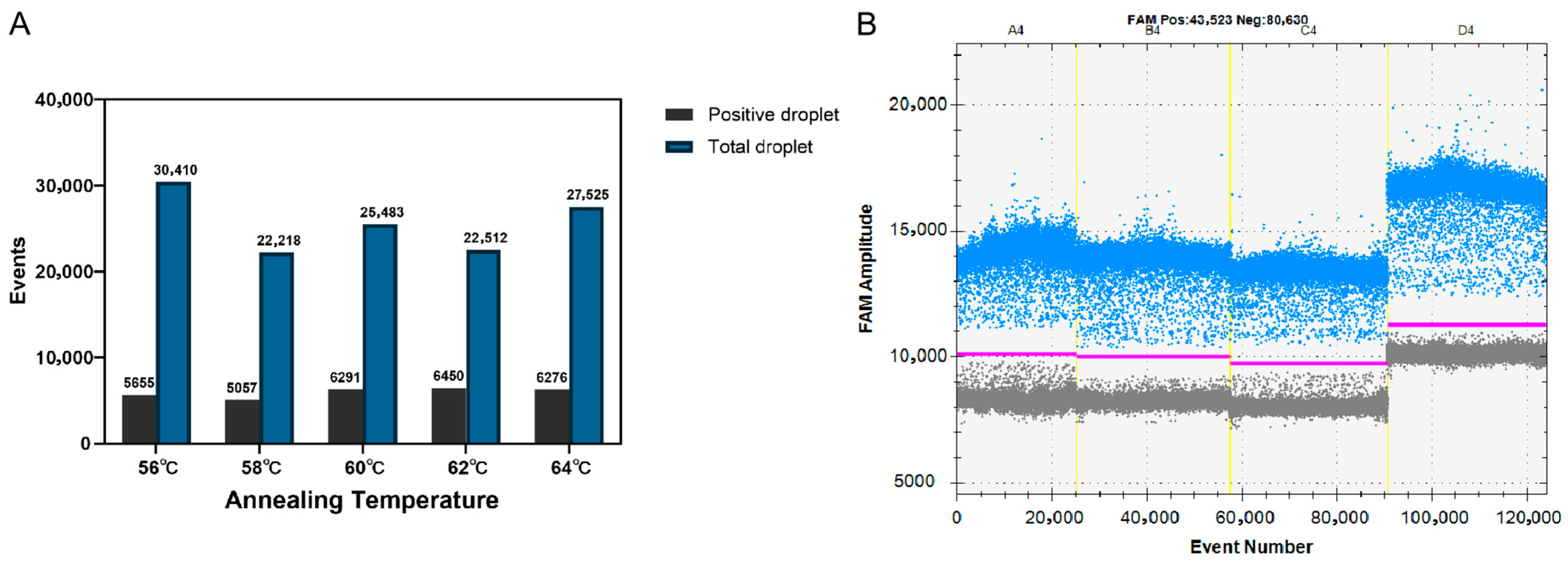
3.1. Development of FHV-1 ddPCR Assay
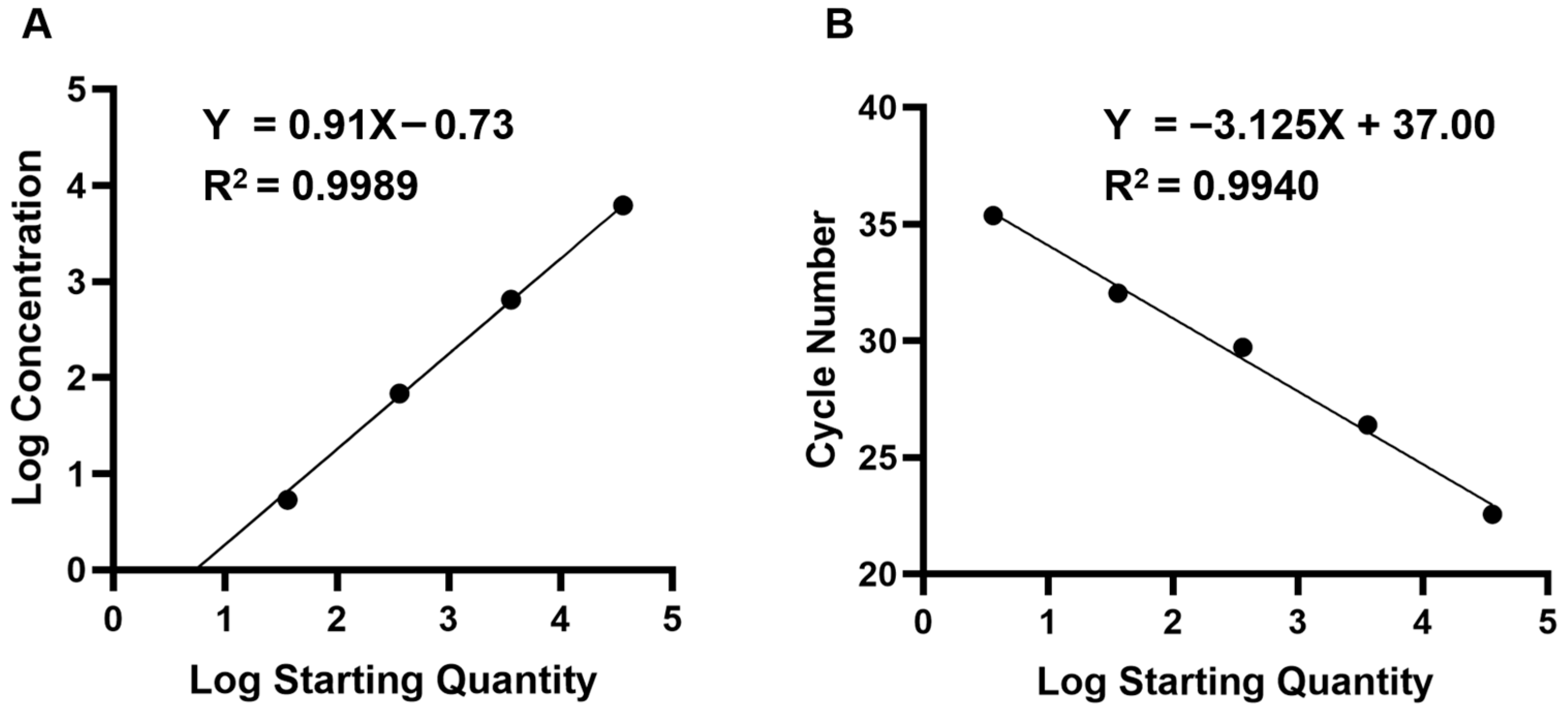
3.2. Detection Limits and Repeatability of the ddPCR and qPCR Assays
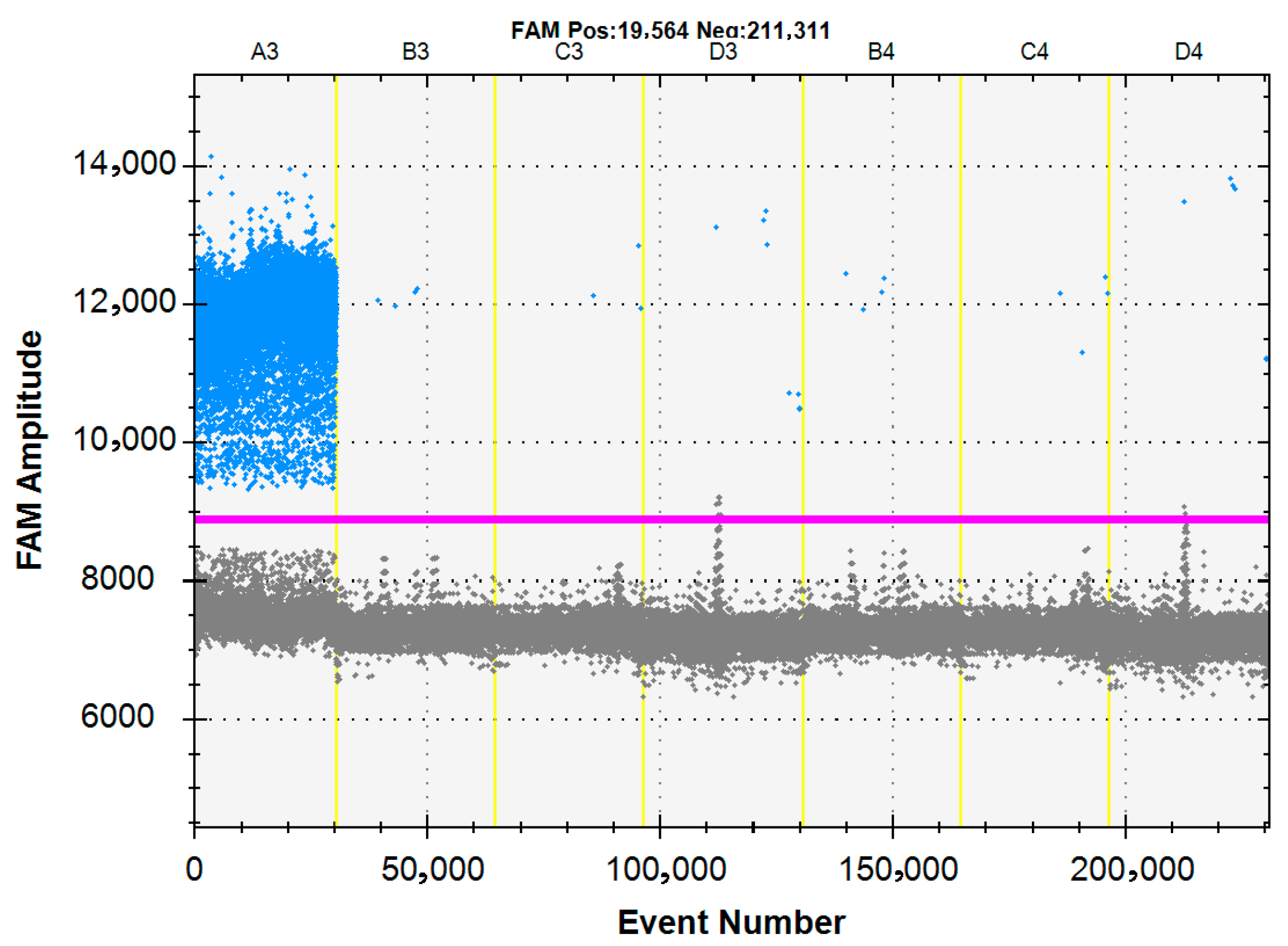
3.3. Specificity of the ddPCR Assay
3.4. Clinical Samples Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ju, H.; Yang, D.; Jin, J.; Wang, J.; Li, X.; Yang, X.; Ge, J.; Zhu, J.; Shen, H.; Lu, J.; et al. Spectrum Detection and Analysis of the Epidemiological Characteristics of Infectious Pathogens in the Feline Respiratory Tract. Arch. Virol. 2024, 169, 177. [Google Scholar] [CrossRef] [PubMed]
- Klose, S.M.; De Souza, D.P.; Devlin, J.M.; Bushell, R.; Browning, G.F.; Vaz, P.K. A “plus One” Strategy Impacts Replication of Felid Alphaherpesvirus 1, Mycoplasma and Chlamydia, and the Metabolism of Coinfected Feline Cells. mSystems 2024, 9, e0085224. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, H.; He, S.; Liu, Y.; Chen, Y.; Qi, X.; Gu, X.; Wen, Y.; Jin, X.; Jin, Y.; et al. Feline Herpesvirus Infection and Pathology in Captive Snow Leopard. Sci. Rep. 2022, 12, 4989. [Google Scholar] [CrossRef]
- Deng, M.; Liang, H.; Xu, Y.; Shi, Q.; Bao, F.; Mei, C.; Dai, Z.; Huang, X. Identification, Genetic Characterization, and Pathogenicity of Three Feline Herpesvirus Type 1 Isolates from Domestic Cats in China. Vet. Sci. 2024, 11, 285. [Google Scholar] [CrossRef]
- Song, M.; Hwang, Y.; Park, J.; Cha, E.; Jeong, H.; Kim, M.; Kim, J.; Baek, S.; Kwon, E.; Park, S.; et al. Quantitative Differential Analysis of Norovirus Outbreak Samples Using RT-ddPCR. Lett. Appl. Microbiol. 2022, 75, 29–35. [Google Scholar] [CrossRef]
- Gibellini, L.; Pecorini, S.; De Biasi, S.; Pinti, M.; Bianchini, E.; De Gaetano, A.; Digaetano, M.; Pullano, R.; Lo Tartaro, D.; Iannone, A.; et al. Exploring Viral Reservoir: The Combining Approach of Cell Sorting and Droplet Digital PCR. Methods 2018, 134–135, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef]
- Quan, P.-L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef]
- Li, H.; Bai, R.; Zhao, Z.; Tao, L.; Ma, M.; Ji, Z.; Jian, M.; Ding, Z.; Dai, X.; Bao, F.; et al. Application of Droplet Digital PCR to Detect the Pathogens of Infectious Diseases. Biosci. Rep. 2018, 38, BSR20181170. [Google Scholar] [CrossRef]
- Kuypers, J.; Jerome, K.R. Applications of Digital PCR for Clinical Microbiology. J. Clin. Microbiol. 2017, 55, 1621–1628. [Google Scholar] [CrossRef]
- Nyendak, M.R.; Lewinsohn, D.A.; Lewinsohn, D.M. New Diagnostic Methods for Tuberculosis. Curr. Opin. Infect. Dis. 2009, 22, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. Digital PCR. Proc. Natl. Acad. Sci. USA 1999, 96, 9236–9241. [Google Scholar] [CrossRef] [PubMed]
- Miotto, E.; Saccenti, E.; Lupini, L.; Callegari, E.; Negrini, M.; Ferracin, M. Quantification of Circulating miRNAs by Droplet Digital PCR: Comparison of EvaGreen- and TaqMan-Based Chemistries. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2638–2642. [Google Scholar] [CrossRef]
- Pinheiro, L.B.; Coleman, V.A.; Hindson, C.M.; Herrmann, J.; Hindson, B.J.; Bhat, S.; Emslie, K.R. Evaluation of a Droplet Digital Polymerase Chain Reaction Format for DNA Copy Number Quantification. Anal. Chem. 2012, 84, 1003–1011. [Google Scholar] [CrossRef]
- White, R.A.; Quake, S.R.; Curr, K. Digital PCR Provides Absolute Quantitation of Viral Load for an Occult RNA Virus. J. Virol. Methods 2012, 179, 45–50. [Google Scholar] [CrossRef]
- Ruelle, J.; Yfantis, V.; Duquenne, A.; Goubau, P. Validation of an Ultrasensitive Digital Droplet PCR Assay for HIV-2 Plasma RNA Quantification. J. Int. AIDS Soc. 2014, 17, 19675. [Google Scholar] [CrossRef]
- Sanders, R.; Huggett, J.F.; Bushell, C.A.; Cowen, S.; Scott, D.J.; Foy, C.A. Evaluation of Digital PCR for Absolute DNA Quantification. Anal. Chem. 2011, 83, 6474–6484. [Google Scholar] [CrossRef]
- Bharuthram, A.; Paximadis, M.; Picton, A.C.P.; Tiemessen, C.T. Comparison of a Quantitative Real-Time PCR Assay and Droplet Digital PCR for Copy Number Analysis of the CCL4L Genes. Infect. Genet. Evol. 2014, 25, 28–35. [Google Scholar] [CrossRef]
- Nshimyimana, J.P.; Cruz, M.C.; Wuertz, S.; Thompson, J.R. Variably Improved Microbial Source Tracking with Digital Droplet PCR. Water Res. 2019, 159, 192–202. [Google Scholar] [CrossRef]
- Belmonte, F.R.; Martin, J.L.; Frescura, K.; Damas, J.; Pereira, F.; Tarnopolsky, M.A.; Kaufman, B.A. Digital PCR Methods Improve Detection Sensitivity and Measurement Precision of Low Abundance mtDNA Deletions. Sci. Rep. 2016, 6, 25186. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Chemama, M.; Eggersdorfer, M.; Arriaga, L.R.; Brenner, M.P.; Weitz, D.A. Robust Scalable High Throughput Production of Monodisperse Drops. Lab. Chip 2016, 16, 4163–4172. [Google Scholar] [CrossRef] [PubMed]
| Primer/Probe | Sequence (5′→3′) | Amplicon Size |
|---|---|---|
| gD-F | TGAGTGGTGGTGTGTGGCATAT | 1125 bp |
| gD-R | ACCGCCCACTTCATATCTTCCC | |
| dd/qPCR-gD-F | GAACCCCCAGTAAGATTCCA | 80 bp |
| dd/qPCR-gD-R | GACCGTTTCGAATCCTCACT | |
| dd/qPCR-gD-Probe | FAM-CCATTCGATATGATCGTCCCGCC-BHQ1 |
| Concentration of Standard Plasmid (Copies/μL) | qPCR (Mean Cq * Value) | ddPCR (Mean Copies/μL ± SD #) |
|---|---|---|
| 3.6 × 105 | 21.08 | Overload ## |
| 3.6 × 104 | 24.25 | 6128.63 ± 69.66 |
| 3.6 × 103 | 27.49 | 644.46 ± 9.23 |
| 3.6 × 102 | 30.40 | 68.56 ± 1.35 |
| 3.6 × 101 | 32.84 | 5.37 ± 0.18 |
| 3.6 × 100 | 33.12 | 0.75 ± 0.03 |
| 3.6 × 10−1 | 33.80 | 0.18 ± 0.01 |
| NTC ** | ND *** | ND *** |
| Concentration of Standard Plasmid (Copies/μL) | Intra-Assay Variation (Robustness) | Inter-Assay Variation (Reproducibility) | ||||
|---|---|---|---|---|---|---|
| Mean * (Copies/μL) | SD # | CV † (%) | Mean * (Copies/μL) | SD # | CV † (%) | |
| 3.6 × 104 | 6128.63 | 74.93 | 1.20 | 6175.40 | 68.01 | 1.10 |
| 3.6 × 103 | 644.46 | 16.27 | 2.52 | 642.93 | 2.83 | 0.44 |
| 3.6 × 102 | 68.56 | 5.04 | 7.35 | 68.93 | 0.93 | 1.35 |
| qPCR | ddPCR | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 12 | 0 | 12 |
| Negative | 11 | 95 | 106 |
| Total | 23 | 95 | 118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wu, D.; Tang, M.; Ye, Z.; Feng, E.; Zhang, H.; Luo, G.; Wang, Z.; Wang, C.; Liu, L.; et al. Development and Validation of a Droplet Digital PCR Assay for Detection of Feline Herpesvirus Type-1. Vet. Sci. 2025, 12, 1107. https://doi.org/10.3390/vetsci12111107
Zhou Y, Wu D, Tang M, Ye Z, Feng E, Zhang H, Luo G, Wang Z, Wang C, Liu L, et al. Development and Validation of a Droplet Digital PCR Assay for Detection of Feline Herpesvirus Type-1. Veterinary Sciences. 2025; 12(11):1107. https://doi.org/10.3390/vetsci12111107
Chicago/Turabian StyleZhou, Yaxi, Danni Wu, Mengle Tang, Zihan Ye, Erkai Feng, Haili Zhang, Guoliang Luo, Zhenjun Wang, Chunxia Wang, Lina Liu, and et al. 2025. "Development and Validation of a Droplet Digital PCR Assay for Detection of Feline Herpesvirus Type-1" Veterinary Sciences 12, no. 11: 1107. https://doi.org/10.3390/vetsci12111107
APA StyleZhou, Y., Wu, D., Tang, M., Ye, Z., Feng, E., Zhang, H., Luo, G., Wang, Z., Wang, C., Liu, L., & Cheng, Y. (2025). Development and Validation of a Droplet Digital PCR Assay for Detection of Feline Herpesvirus Type-1. Veterinary Sciences, 12(11), 1107. https://doi.org/10.3390/vetsci12111107






