N-Cadherin-Mediated Epithelial–Mesenchymal Transition as a Prognostic Indicator in Canine Melanoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Cases
2.3. Histopathological Evaluation
2.4. Immunohistochemistry
2.5. Immunohistochemistry Assessment
2.6. Survival Time
2.7. Statistical Analysis
3. Results
3.1. Clinical-Histopathological Parameters
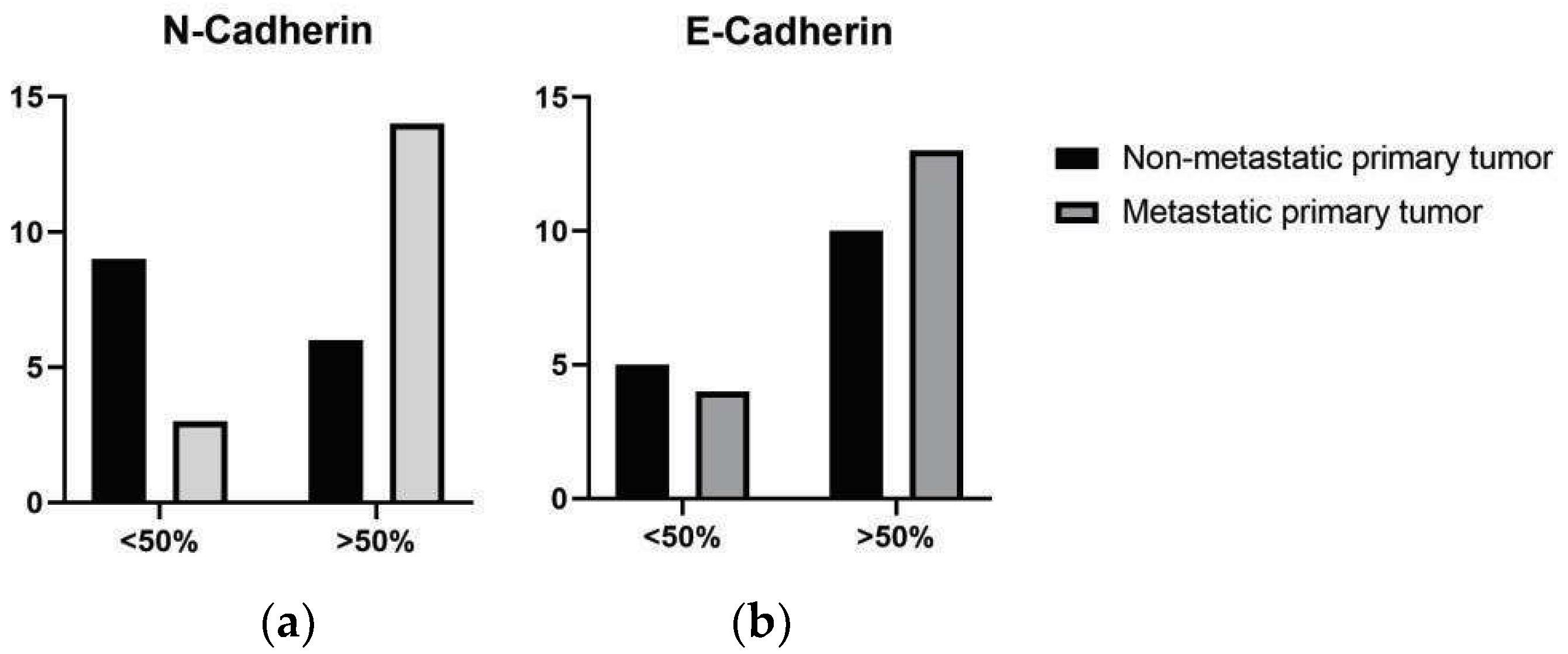
3.2. Immunohistochemical Evaluation
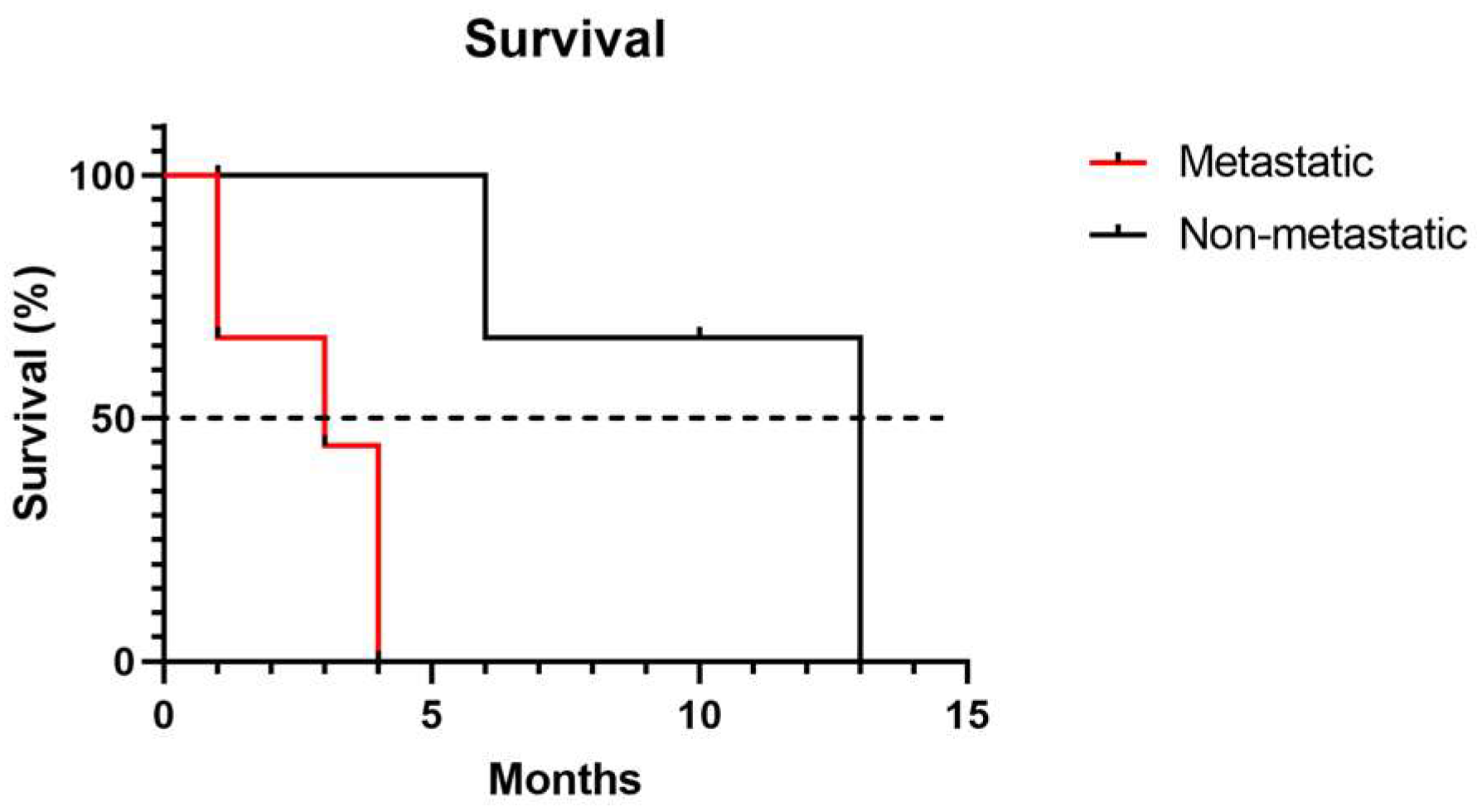
3.3. Survival Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAPES | Coordenação de Aperfeiçoamento de Pessoal de Nível Superior |
| CEUA | Animal Experimentation Ethics Committee |
| CNPQ | Conselho Nacional de Desenvolvimento Científico e Tecnológico |
| DAB | 3,3-Diaminobenzidine |
| EDTA | Ethylenediaminetetraacetic acid |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| FAPEMIG | Fundação de Amparo à Pesquisa de Minas Gerais |
| HE | Hematoxylin-eosin |
References
- Colombo, K.C.; de Lima, D.A.; Rossi, L.A.; Bianchi, M.M.; da Fonseca Sapin, C. Oral cavity melanoma in dogs: Epidemiological, clinical, and pathological characteristics. Res. Soc. Dev. 2022, 11, e230111335332. [Google Scholar] [CrossRef]
- Gillard, M.; Cadieu, E.; De Brito, C.; Abadie, J.; Vergier, B.; Devauchelle, P.; André, C. Naturally occurring melanomas in dogs as models for non-UV pathways of human melanomas. Pigment Cell Melanoma Res. 2013, 27, 90–102. [Google Scholar] [CrossRef]
- Barreto, H.M.; Sá, M.A.F. Oral melanocytic melanoma in dogs—A literature review. Rev. Científica Do UBM 2017, 19, 245–261. [Google Scholar]
- Prouteau, L.; André, C. Canine Melanomas as Models for Human Melanomas: Clinical, Histological, and Genetic Comparison. Genes 2019, 10, 501. [Google Scholar] [CrossRef]
- Miller, A.J.; Mihm, M.C. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef]
- Pedri, D.; Karras, P.; Landeloos, E.; Marine, J.C.; Rambow, F. Epithelial-to-mesenchymal-like transition events in melanoma. FEBS J. 2021, 289, 1352–1368. [Google Scholar] [CrossRef]
- Medeiros, A.S.; Jordão, L.A.A.R.; Figueredo, C.R.L. The role of epithelial-mesenchymal transition in the metastatic potential of malignant tumors. Rev. Saúde Ciência 2018, 7, 82–93. [Google Scholar]
- Cao, Z.Q.; Wang, Z.; Leng, P. Aberrant N-cadherin expression in cancer. Biomed. Pharmacother. 2019, 118, 109320. [Google Scholar] [CrossRef] [PubMed]
- Veloso, E.S.; Gonçalves, I.N.N.; Silveira, T.L.; Santo, J.T.E.; Figueiredo, L.V.; Varaschin, M.S.; Ferreira, E. ZEB and Snail expression indicate epithelial-mesenchymal transition in canine melanoma. Res. Vet. Sci. 2020, 131, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, S.; Porcellato, I.; Mechelli, L.; Menchetti, L.; Iussich, S.; De Maria, R.; Brachelente, C. E-Cadherin Expression in Canine Melanocytic Tumors: Histological, Immunohistochemical, and Survival Analysis. Vet. Pathol. 2020, 57, 608–619. [Google Scholar] [CrossRef]
- Wheelock, M.J.; Shintani, Y.; Maeda, M.; Fukumoto, Y.; Johnson, K.R. Cadherin switching. J. Cell Sci. 2008, 121, 727–735. [Google Scholar] [CrossRef]
- Smedley, R.C.; Spangler, W.L.; Esplin, D.G.; Kitchell, B.E.; Bergman, P.J.; Ho, H.Y.; Kiupel, M. Prognostic Markers for Canine Melanocytic Neoplasms. Vet. Pathol. 2011, 48, 54–72. [Google Scholar] [CrossRef]
- Araki, K.; Shimura, T.; Suzuki, H.; Tsutsumi, S.; Wada, W.; Yajima, T.; Kuwano, H. E/N-cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br. J. Cancer 2011, 105, 1885–1893. [Google Scholar] [CrossRef]
- LI, G.; Satyamoorthy, K.; Herlyn, M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001, 61, 3819–3825. [Google Scholar]
- Yan, S.; Holderness, B.M.; Li, Z.; Seidel, G.D.; Gui, J.; Fisher, J.L.; Ernstoff, M.S. Epithelial–Mesenchymal Expression Phenotype of Primary Melanoma and Matched Metastases and Relationship with Overall Survival. Anticancer Res. 2016, 36, 6449–6456. [Google Scholar] [CrossRef]
- Han, J.I.; Kim, Y.; Kim, D.Y.; Na, K.J. Alteration in E-Cadherin/-Catenin Expression in Canine Melanotic Tumors. Vet. Pathol. 2012, 50, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Wilde, A.; Fouquier d’Hérouël, A.; McIntosh, E.; Ertaylan, G.; Skupin, A.; Kuestner, R.E.; Huang, S. Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS ONE 2015, 10, e0126522. [Google Scholar] [CrossRef]
- Lüönd, F.; Sugiyama, N.; Bill, R.; Bornes, L.; Hager, C.; Tang, F.; Christofori, G. Distinct contributions of partial and full epithelial-mesenchymal transition to breast cancer malignancy. Dev. Cell 2021, 56, 3203–3221.e11. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.K.; Endo, K.; Itoh, Y.; Osada, A.H.; Kimura, Y.; Ueki, K.; Saitoh, M. Ets family proteins regulate the EMT transcription factors Snail and ZEB in cancer cells. FEBS Open Bio 2022, 12, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yang, L.; Chen, C.; Ashrafizadeh, M.; Tian, Y.; Sun, R. Wnt/β-catenin-driven EMT regulation in human cancers. Cell. Mol. Life Sci. 2024, 81, 79. [Google Scholar] [CrossRef]
- Andyck, H.H.; Hillen, L.M.; Bosisio, F.M.; van den Oord, J.; Zur Hausen, A.; Winnepenninckx, V. Rethinking the biology of metastatic melanoma: A holistic approach. Cancer Metastasis Rev. 2021, 40, 603–624. [Google Scholar] [CrossRef]
- Christiansen, J.J.; Rajasekaran, A.K. Reassessing Epithelial to Mesenchymal Transition as a Prerequisite for Carcinoma Invasion and Metastasis. Cancer Res. 2006, 66, 8319–8326. [Google Scholar] [CrossRef] [PubMed]
- Leme, M.B.P.; Waitzberg, Â.F.; Artigiani, R.; Matos, D. The relationship between E-cadherin and the prognosis of colorectal adenocarcinoma. Rev. Colégio Bras. Cir. 2005, 32, 201–204. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Taniguchi, T.; Makino, M.; Kaibara, N. Reduced E-cadherin expression and enlargement of cancer nuclei strongly correlate with hematogenic metastasis in colorectal adenocarcinoma. Scand. J. Gastroenterol. 2000, 35, 839–846. [Google Scholar] [CrossRef]
- Kimura, T.; Tanaka, S.; Haruma, K.; Sumii, K.; Kajiyama, G.; Shimamoto, F.; Kohno, N. Clinical significance of MUC1 and E-cadherin expression, cellular proliferation, and angiogenesis at the deepest invasive portion of colorectal cancer. Int. J. Oncol. 2000, 16, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Nieman, M.T.; Prudoff, R.S.; Johnson, K.R.; Wheelock, M.J. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol. 1999, 147, 631–644. [Google Scholar] [CrossRef]
- Gravdal, K.; Halvorsen, O.J.; Haukaas, S.A.; Akslen, L.A. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin. Cancer Res. 2007, 13, 7003–7011. [Google Scholar] [CrossRef]


| Antibody | Clone | Manufacturer Code | Manufacturer | Dilution | Antigen Retrieval Method | Secondary Antibody | Incubation Time |
|---|---|---|---|---|---|---|---|
| Melan-A | A103 | SC-53537 | Dako | 1:100 | Citrate + pressurized wetheat | Novolink * | 16 h |
| Melanoma Antigen | PNL-2 | SC-59306 | SantaCruz | 1:100 | Citrate + pressurized wetheat | Novolink * | 16 h |
| E-Cadherin | 4A2C7 | 180223 | Invitrogen | 1:50 | EDTA + pressurized wetheat | Novolink * | 16 h |
| N-Cadherin | 6G11 | M3613 | Dako | 1:50 | Citrate + pressurized wetheat | Novolink * | 16 h |
| n = 32 | Metastatic Primary Tumor 17 (100%) | Non-Metastatic Primary Tumor 15 (100%) |
|---|---|---|
| Histological Type | ||
| Epithelioid | 9 (52.94%) | 7 (46.67%) |
| Fusiform | 2 (11.76%) | 2 (13.33%) |
| Mixed | 5 (29.41%) | 6 (40.00%) |
| Balanoid | 1 (5.88%) | 0 (0%) |
| Degree of Pigmentation | ||
| <50% | 10 (31.25%) | 7 (21.87%) |
| >50% | 7 (21.87%) | 8 (25.00%) |
| Necrosis | ||
| Absent | 1 (5.88%) | 4 (26.67%) |
| Present | 16 (94.12%) | 11 (73.33%) |
| Lymphatic embolus | ||
| Absent | 5 (29.41%) | 8 (53.33%) |
| Present | 12 (70.59%) | 7 (46.67%) |
| Atypia | ||
| <20% | 0 (0%) | 0 (0%) |
| >20% | 17 (100%) | 15 (100%) |
| Ulceration | ||
| Absent | 0 (0%) | 3 (20.00%) |
| Present | 17 (100%) | 12 (80.00%) |
| Desmoplasia | ||
| Absent | 4 (23.53%) | 3 (20.00%) |
| Present | 13 (76.47%) | 12 (80.00%) |
| Location | Metastatic Status | Marker | High Expression n (%) * | Lower Expression n (%) * |
|---|---|---|---|---|
| Oral | Non-metastatic | E-Cadherin | 6 (19%) | 3 (9%) |
| N-Cadherin | 4 (13%) | 5 (16%) | ||
| Metastatic | E-Cadherin | 9 (28%) | 1 (3%) | |
| N-Cadherin | 8 (25%) | 2 (6%) | ||
| Cutaneous | Non-metastatic | E-Cadherin | 4 (13%) | 2 (6%) |
| N-Cadherin | 2 (6%) | 4 (13%) | ||
| Cutaneous | Metastatic | E-Cadherin | 4 (13%) | 3 (13%) |
| N-Cadherin | 6 (19%) | 1 (3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, T.B.G.; Taborda, D.Y.O.; Fenelon, C.S.; de Oliveira Duarte, N.; Nakagaki, K.Y.R.; Abreu, C.C.; Flecher, M.C.; Del Puerto, H.L.; Ferreira, E. N-Cadherin-Mediated Epithelial–Mesenchymal Transition as a Prognostic Indicator in Canine Melanoma. Vet. Sci. 2025, 12, 1023. https://doi.org/10.3390/vetsci12111023
Lopes TBG, Taborda DYO, Fenelon CS, de Oliveira Duarte N, Nakagaki KYR, Abreu CC, Flecher MC, Del Puerto HL, Ferreira E. N-Cadherin-Mediated Epithelial–Mesenchymal Transition as a Prognostic Indicator in Canine Melanoma. Veterinary Sciences. 2025; 12(11):1023. https://doi.org/10.3390/vetsci12111023
Chicago/Turabian StyleLopes, Thacyana Beatriz Guimarães, Daiana Yively Osorio Taborda, Clarice Soares Fenelon, Nayara de Oliveira Duarte, Karen Yumi Ribeiro Nakagaki, Camila Costa Abreu, Mayra Cunha Flecher, Helen Lima Del Puerto, and Enio Ferreira. 2025. "N-Cadherin-Mediated Epithelial–Mesenchymal Transition as a Prognostic Indicator in Canine Melanoma" Veterinary Sciences 12, no. 11: 1023. https://doi.org/10.3390/vetsci12111023
APA StyleLopes, T. B. G., Taborda, D. Y. O., Fenelon, C. S., de Oliveira Duarte, N., Nakagaki, K. Y. R., Abreu, C. C., Flecher, M. C., Del Puerto, H. L., & Ferreira, E. (2025). N-Cadherin-Mediated Epithelial–Mesenchymal Transition as a Prognostic Indicator in Canine Melanoma. Veterinary Sciences, 12(11), 1023. https://doi.org/10.3390/vetsci12111023





