Glycerol Monolaurate Complex Improved Antioxidant, Anti-Inflammation, and Gut Microbiota Composition of Offspring in a Sow–Piglet Model
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Management
2.3. Growth Performance
2.4. Samples Collection
2.5. Determination
2.5.1. Determination of Antioxidant Capacity and Cytokines
2.5.2. Determination of Milk Composition
2.5.3. Analysis of Fecal Microbial Diversity
3. Statistical Analysis
4. Results
4.1. Effects of GML on Back Fat and Fecal Scores of Sows
4.2. Effects of GML on Sow Reproductive Performances
4.3. Effects of GML on Sow Feed Intake During Lactation
4.4. Effects of GML on Composition of Colostrum and Normal Milk of Sows
4.5. Effects of GML on Antioxidant Capacity of Sows and Piglets
4.6. Effects of GML on Cytokine Secretion of Sows and Piglets
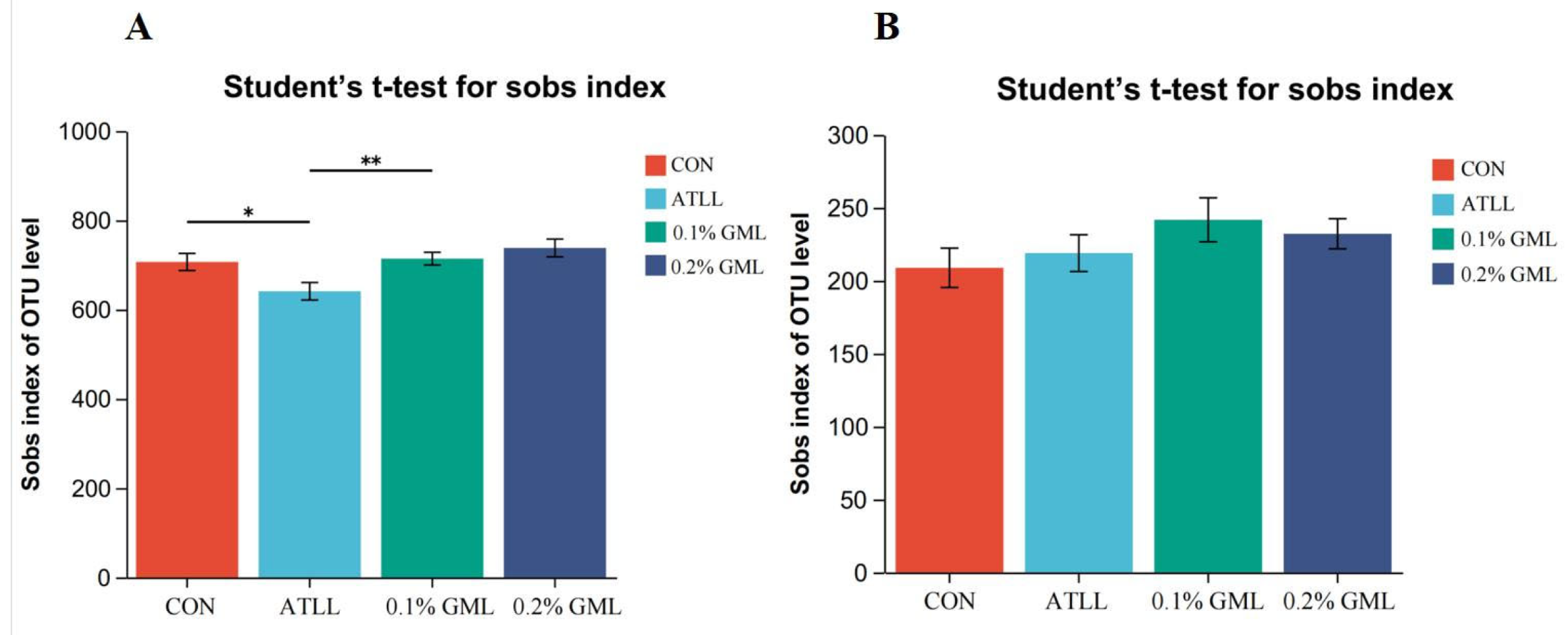
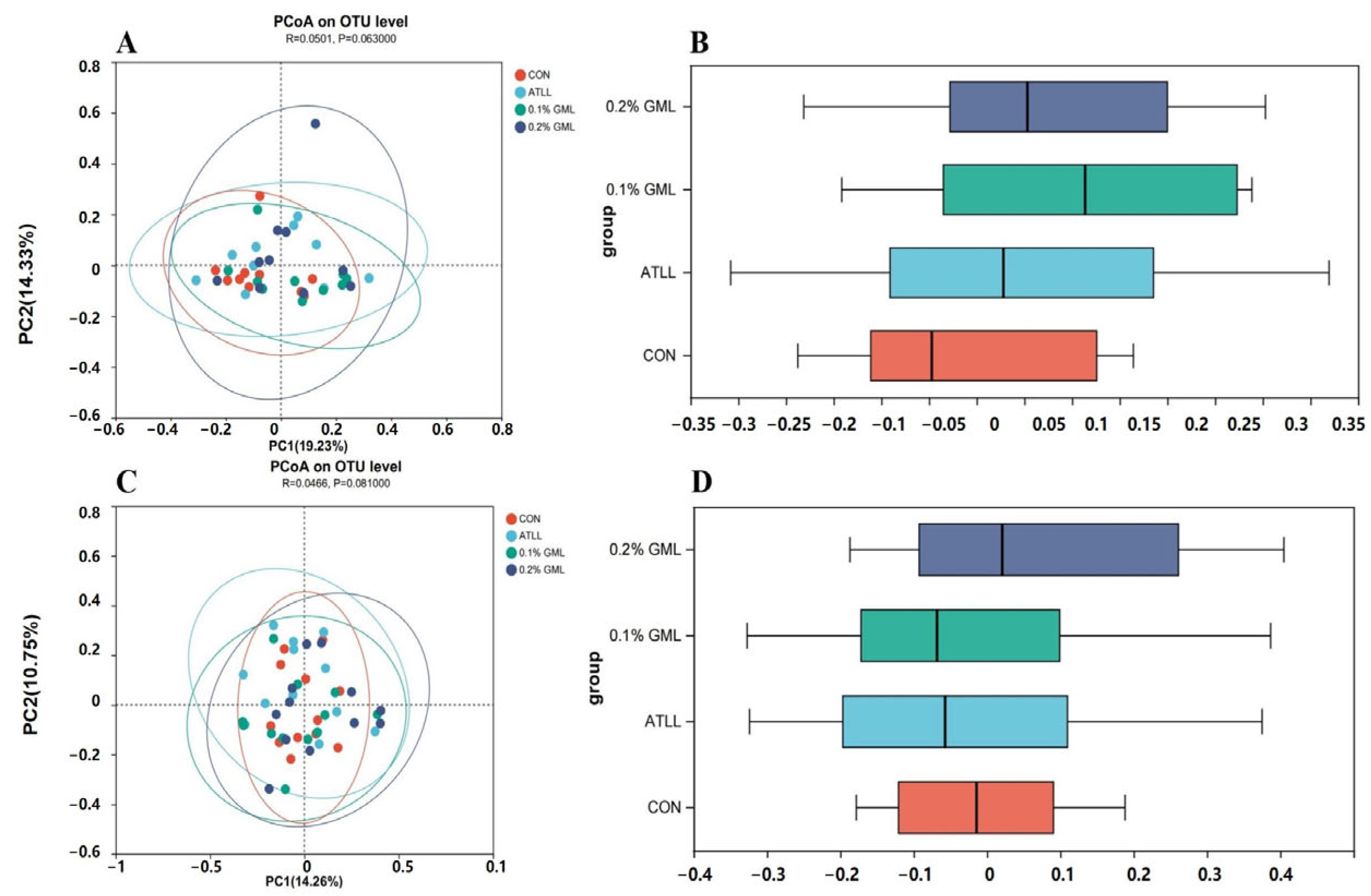
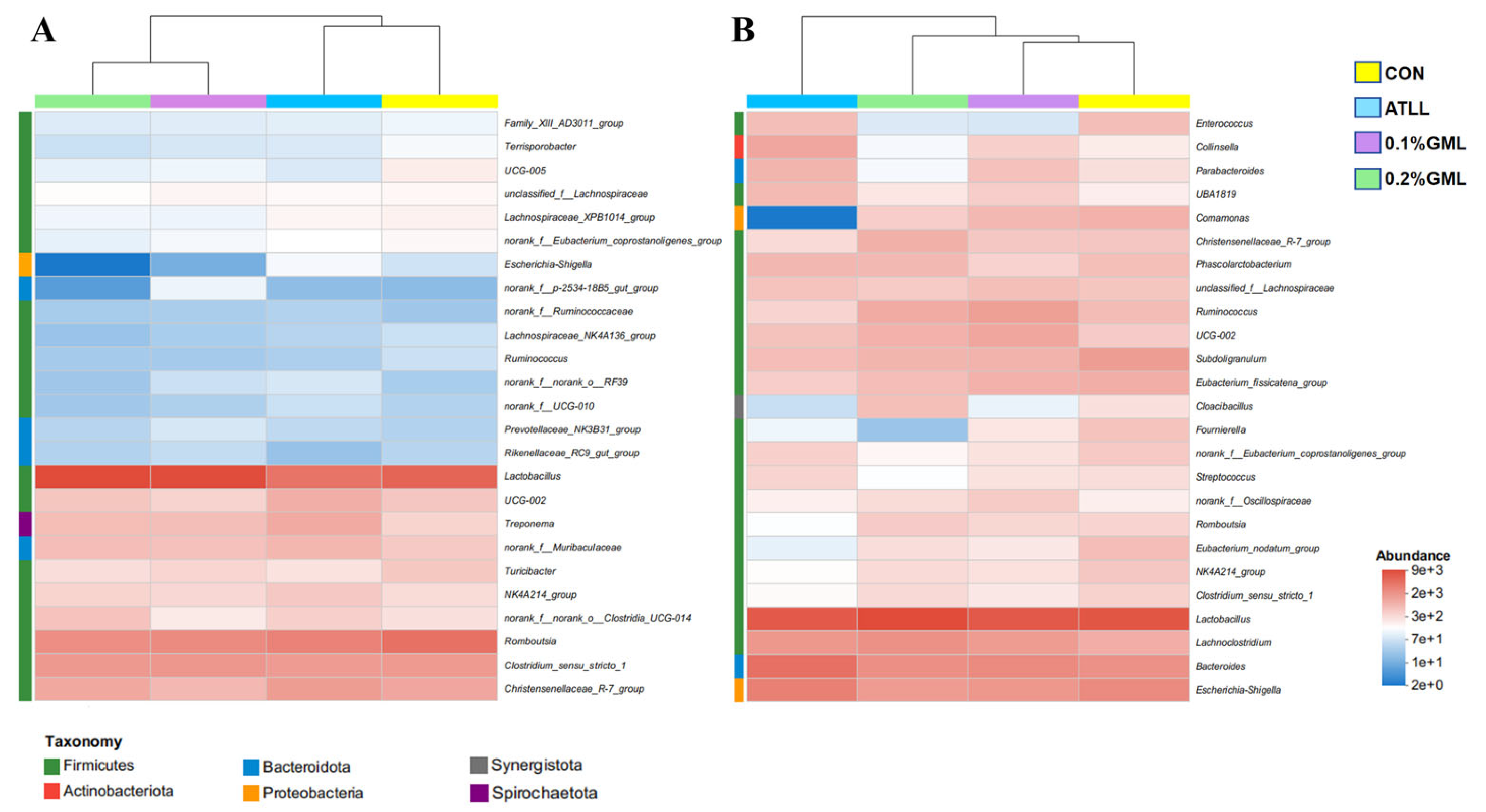
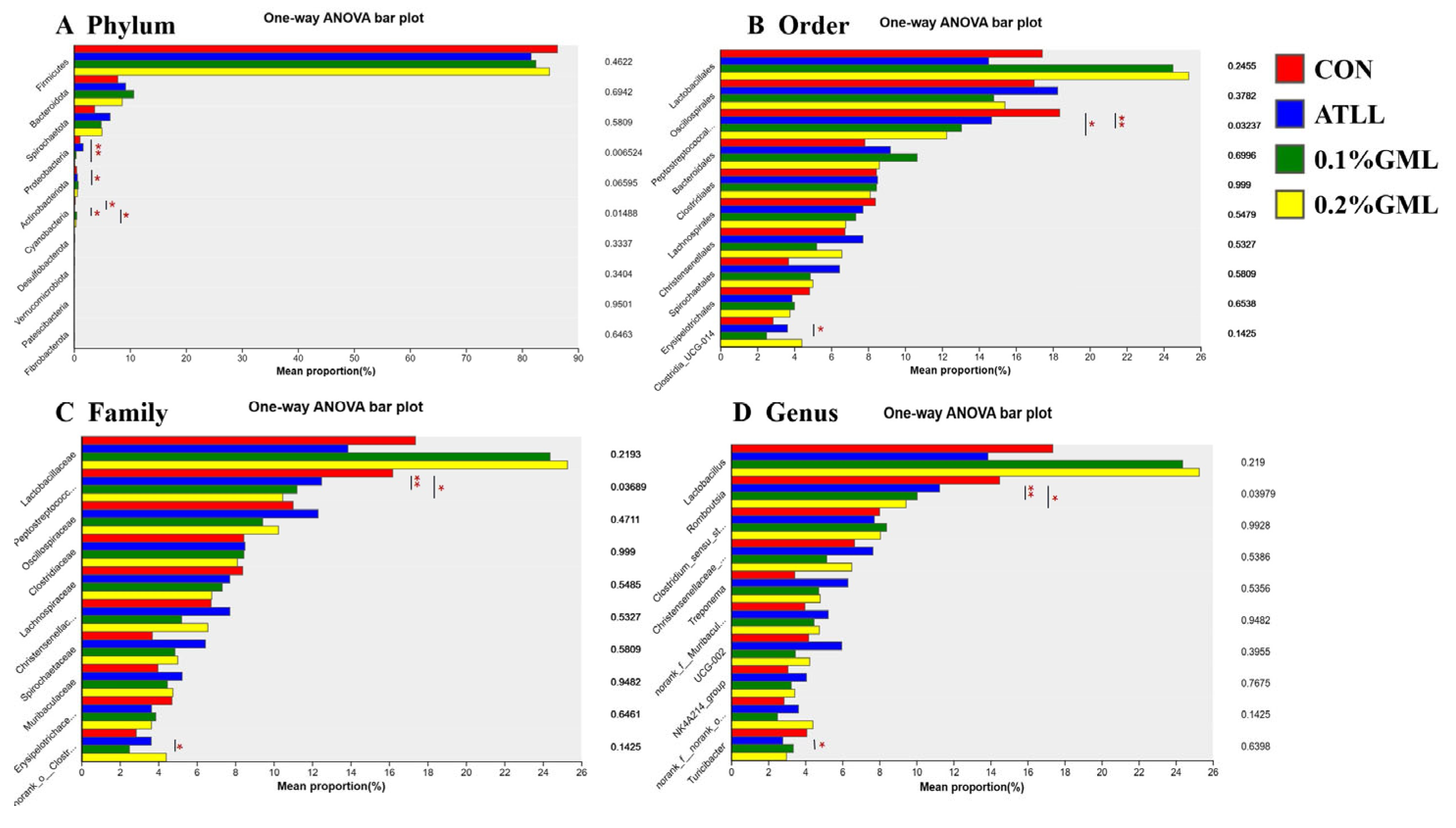
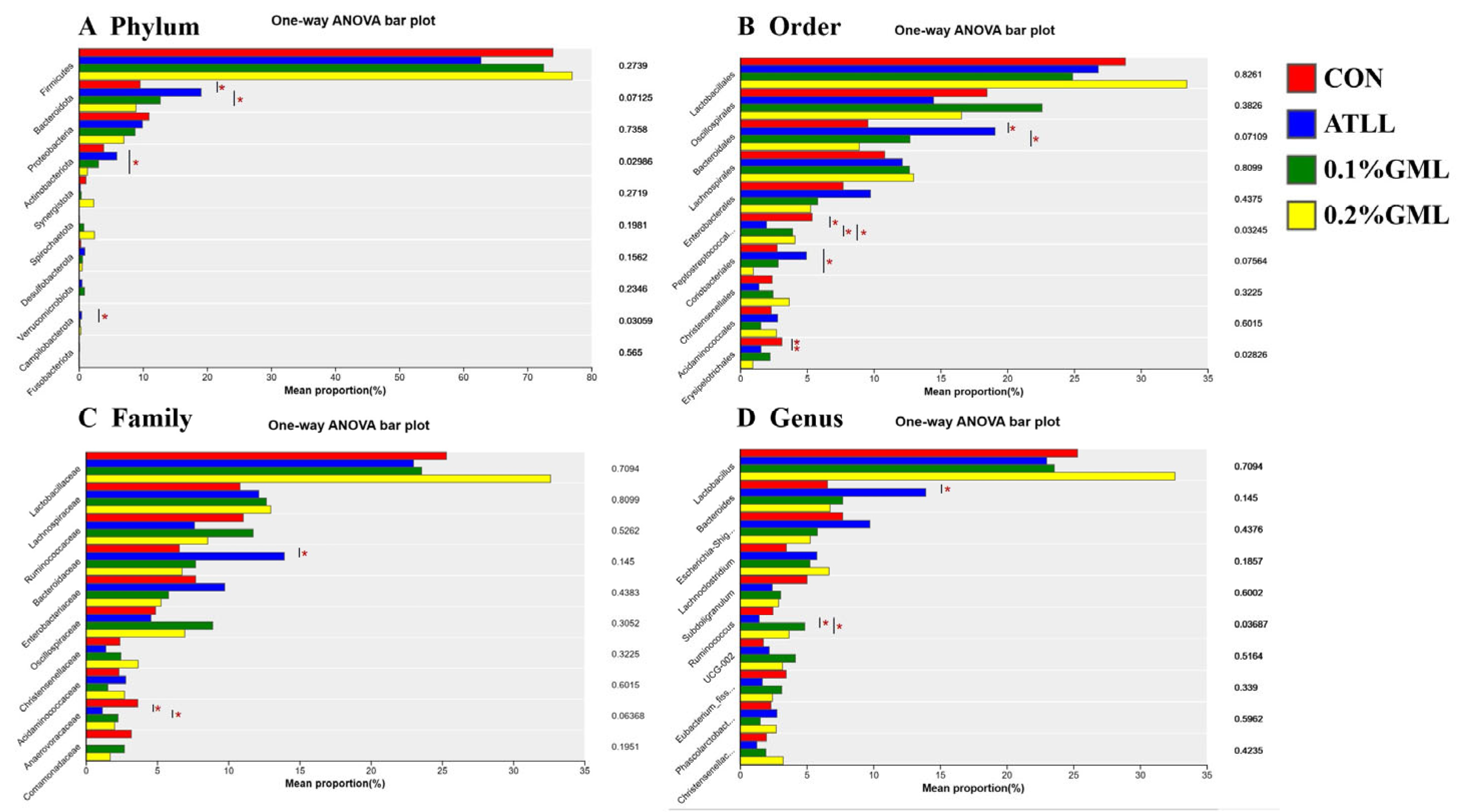
4.7. Effects of GML on Gut Microbial Composition of Sows and Piglets
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Zhang, J.; Olasege, B.S.; Ma, P.; Qiu, X.; Gao, H.; Wang, C.; Wang, Y.; Zhang, Q.; Yang, H.; et al. Estimation of Genetic Parameters for Reproductive Traits in Connectedness Groups of Duroc, Landrace and Yorkshire Pigs in China. J. Anim. Breed. Genet. 2020, 137, 211–222. [Google Scholar] [CrossRef]
- Gu, X.L.; Song, Z.H.; Li, H.; Wu, S.; Wu, S.S.; Ding, Y.N.; He, X.; Yin, Y.L.; Fan, Z.Y. Effects of Dietary Isomaltooligosaccharide and Bacillus Spp. Supplementation During Perinatal Period on Lactational Performance, Blood Metabolites, and Milk Composition of Sows. J. Sci. Food Agric. 2019, 99, 5646–5653. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African Swine Fever: A Re-Emerging Viral Disease Threatening the Global Pig Industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic Pollution in Surface Fresh Waters: Occurrence and Effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Tolba, S.A.; AlSadek, D.M.M.; Abdel Fattah, D.M.; Hassan, A.M.; Metwally, A.E. Effect of Supplemental Glycerol Monolaurate and Oregano Essential Oil Blend on the Growth Performance, Intestinal Morphology, and Amino Acid Digestibility of Broiler Chickens. BMC Vet. Res. 2021, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Wang, Y.; Diarra, M.S.; Alexander, T.; Stanford, K. Challenges of a One-Health Approach to the Development of Alternatives to Antibiotics. Anim. Front. 2018, 8, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A. WS14 The EU Ban of Antibiotics as Feed Additives (2006): Alternatives and Consumer Safety. J. Vet. Pharmacol. Ther. 2006, 29, 41–44. [Google Scholar] [CrossRef]
- Kogut, M.H. Issues and Consequences of Using Nutrition to Modulate the Avian Immune Response. J. Appl. Poult. Res. 2017, 26, 605–612. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Kilgore, S.H.; Seo, K.S.; Leung, D.Y.M. Glycerol Monolaurate Contributes to the Antimicrobial and Anti-inflammatory Activity of Human Milk. Sci. Rep. 2019, 9, 14550. [Google Scholar] [CrossRef]
- Thormar, H.; Hilmarsson, H.; Bergsson, G. Stable Concentrated Emulsions of the 1-Monoglyceride of Capric Acid (Monocaprin) with Microbicidal Activities Against the Food-Borne Bacteria Campylobacter jejuni, Salmonella spp., and Escherichia coli. Appl. Environ. Microbiol. 2006, 72, 522–526. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; Dos Reis, J.H.; Gebert, R.R.; Barreta, M.; Griss, L.G.; Casagrande, R.A.; de Cristo, T.G.; Santiani, F.; Campigotto, G.; Rampazzo, L.; et al. Glycerol Monolaurate in the Diet of Broiler Chickens Replacing Conventional Antimicrobials: Impact on health, Performance and Meat Quality. Microb. Pathog. 2019, 129, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.; Lovett, D.K.; Monahan, F.J.; Callan, J.; Flynn, B.; O’Mara, F.P. Effect of Refined Coconut Oil or Copra Meal on Methane Output and on Intake and Performance of Beef Heifers. J. Anim. Sci. 2006, 84, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Vaddella, V.; Zhou, D. Effects of Chestnut Tannins and Coconut Oil on Growth Performance, Methane Emission, Ruminal Fermentation, and Microbial Populations in Sheep. J. Dairy Sci. 2011, 94, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.A.; Dierick, N.A. The Combined Use of Triacylglycerols Containing Medium-Chain Fatty Acids and Exogenous Lipolytic Enzymes as an Alternative to in-Feed Antibiotics in Piglets: Concept, Possibilities and Limitations. An Overview. Nutr. Res. Rev. 2003, 16, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.S.; Sandouk, A.; Houtman, J.C. Glycerol Monolaurate (GML) Inhibits Human T Cell Signaling and Function by Disrupting Lipid Dynamics. Sci. Rep. 2016, 6, 30225. [Google Scholar]
- Kong, L.; Wang, Z.; Xiao, C.; Zhu, Q.; Song, Z. Glycerol Monolaurate Ameliorated Intestinal Barrier and Immunity in Broilers by Regulating Intestinal Inflammation, Antioxidant Balance, and Intestinal Microbiota. Front. Immunol. 2021, 12, 713485. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Fu, A.; Deng, L.; Zhao, M.; Li, Y.; Zhang, H.; Feng, F. High-dose Glycerol Monolaurate Up-Regulated Beneficial Indigenous Microbiota without Inducing Metabolic Dysfunction and Systemic Inflammation: New Insights into Its Antimicrobial Potential. Nutrients 2019, 11, 1981. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Martín-Orúe, S.M.; Roca, M.; Manzanilla, E.G.; Badiola, I.; Perez, J.F.; Gasa, J. The Response of Gastrointestinal Microbiota to Avilamycin, Butyrate, and Plant Extracts in Early-Weaned Pigs. J. Anim. Sci. 2006, 84, 2725–2734. [Google Scholar] [CrossRef]
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Khaksar, V. Effects of Carboxy Methyl Cellulose and Thymol + Carvacrol on Performance, Digesta Viscosity and Some Blood Metabolites of Broilers. J. Anim. Physiol. Anim. Nutr. 2014, 98, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Hashemipour, H.; Khaksar, V.; Rubio, L.A.; Veldkamp, T.; van Krimpen, M.M. Effect of Feed Supplementation with a Thymol Plus Carvacrol Mixture, in Combination or Not with an NSP-Degrading Enzyme, on Productive and Physiological Parameters of Broilers Fed on Wheat-Based Diets. Anim. Feed Sci. Technol. 2016, 211, 117–131. [Google Scholar] [CrossRef]
- Yin, L.; Chen, J.; Wang, K.; Geng, Y.; Lai, W.; Huang, X.; Chen, D.; Guo, H.; Fang, J.; Chen, Z.; et al. Study the Antibacterial Mechanism of Cinnamaldehyde Against Drug-Resistant Aeromonas Hydrophila In Vitro. Microb. Pathog. 2020, 145, 104208. [Google Scholar] [CrossRef]
- Farrokhfall, K.; Khoshbaten, A.; Zahediasl, S.; Mehrani, H.; Karbalaei, N. Improved Islet Function is Associated with Anti-inflammatory, Antioxidant and Hypoglycemic Potential of Cinnamaldehyde on Metabolic Syndrome Induced by High Tail Fat in Rats. J. Funct. Foods 2014, 10, 397–406. [Google Scholar] [CrossRef]
- Tiihonen, K.; Kettunen, H.; Bento, M.H.; Saarinen, M.; Lahtinen, S.; Ouwehand, A.C.; Schulze, H.; Rautonen, N. The Effect of Feeding Essential Oils on Broiler Performance and Gut Microbiota. Br. Poult. Sci. 2010, 51, 381–392. [Google Scholar] [CrossRef]
- Valentini, J.; Da Silva, A.S.; Fortuoso, B.F.; Reis, J.H.; Gebert, R.R.; Griss, L.G.; Boiago, M.M.; Lopes, L.Q.S.; Santos, R.C.V.; Wagner, R.; et al. Chemical Composition, Lipid Peroxidation, and Fatty Acid Profile in Meat of Broilers Fed with Glycerol Monolaurate Additive. Food Chem. 2020, 330, 127187. [Google Scholar] [CrossRef] [PubMed]
- Kroismayr, A.; Sehm, J.; Pfaffl, M.W.; Schedle, K.; Plitzner, C.; Windisch, W. Effects of Avilamycin and Essential Oils on mRNA Expression of Apoptotic and Inflammatory Markers and Gut Morphology of Piglets. Czech J. Anim. Sci. 2008, 53, 377–387. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, H.; Mi, Y.; Xue, Y.; Li, J.; Ma, Y. Effects of Essential Oil Coated with Glycerol Monolaurate on Growth Performance, Intestinal Morphology, and Serum Profiles in Weaned Piglets. Anim. Biosci. 2023, 36, 753–760. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, M.; Xiong, L.; Lin, T.; Zhang, S.; Yue, X.; Liu, X.; Chen, F.; Zhang, S.; Guan, W. Maternal Supplementation with Glycerol Monolaurate Improves the Intestinal Health of Suckling Piglets by Inhibiting the NF-κB/MAPK Pathways and Improving Oxidative Stability. Food Funct. 2023, 14, 3290–3303. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Hu, J.; Park, J.H.; Kim, I.H. Effect of Dietary Supplementation with Lactobacillus Plantarum on Growth Performance, Fecal Score, Fecal Microbial Counts, Gas Emission and Nutrient Digestibility in Growing Pigs. Anim. Feed. Sci. Technol. 2022, 290, 115295. [Google Scholar] [CrossRef]
- Dai, F.; Lin, T.; Cheng, L.; Wang, J.; Zuo, J.; Feng, D. Effects of Micronized Bamboo Powder on Growth Performance, Serum Biochemical Indexes, Cecal Chyme Microflora and Metabolism of Broilers Aged 1–22 days. Trop. Anim. Health Prod. 2022, 54, 166. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Achard, C.S.; Eugenio, F.A.; Apper, E.; Combes, S.; Quesnel, H. Effect of Live Yeast Supplementation in Sow Diet During Gestation and Lactation on Sow and Piglet Fecal Microbiota, Health, and Performance. J. Anim. Sci. 2022, 100, skac209. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, P.; Plush, K. Parturition and Its Relationship with Stillbirths and Asphyxiated Piglets. Animals 2019, 9, 885. [Google Scholar] [CrossRef]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal Piglet Survival: Impact of Sow Nutrition Around Parturition on Fetal Glycogen Deposition and Production and Composition of Colostrum and Transient Milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Zhang, N.; Zhang, T.; Ma, Y. Effects of α-Glycerol Monolaurate on Intestinal Morphology, Nutrient Digestibility, Serum Profiles, and Gut Microbiota in Weaned Piglets. J. Anim. Sci. 2022, 100, skac046. [Google Scholar] [CrossRef]
- Risley, C.; Lopez, J.; Cordts, C.; von Brandestein, F. PSV-17 Glycerol Monolaurate Improves Litter Performance and Modifies Milk Composition when Fed to Lactating Sows. J. Anim. Sci. 2024, 102 (Suppl. S2), 314–315. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, X.; Liao, S.; Qi, M.; Zha, A.; Zuo, G.; Liao, P.; Chen, Y.; Guo, C.; Tan, B. Effects of Medium-Chain Fatty Acid Glycerides on Nutrient Metabolism and Energy Utilization in Weaned Piglets. Front. Vet. Sci. 2022, 9, 938888. [Google Scholar] [CrossRef]
- Swanson, A.J.; Hesse, A.; Levesque, C.L.; Pierre, B.S. PSVII-7 The Effects on Supplementation of Medium Chain Fatty Acids on Piglet Health and Immune Markers. J. Anim. Sci. 2022, 100 (Suppl. S2), 174–175. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L. Oxidative Stress Status of Highly Prolific Sows During Gestation and Lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef]
- Prater, M.R.; Laudermilch, C.L.; Liang, C.; Holladay, S.D. Placental Oxidative Stress Alters Expression of Murine Osteogenic Genes and Impairs Fetal Skeletal Formation. Placenta 2008, 29, 802–808. [Google Scholar] [CrossRef]
- Ren, W.; Yin, Y.; Liu, G.; Yu, X.; Li, Y.; Yang, G.; Li, T.; Wu, G. Effect of Dietary Arginine Supplementation on Reproductive Performance of Mice with Porcine Circovirus Type 2 Infection. Amino Acids 2012, 42, 2089–2094. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and Antioxidants in Disease: Oxidative Stress in Farm Animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; Galli, G.M.; Griss, L.G.; Armanini, E.H.; Silva, A.D.; Fracasso, M.; Mostardeiro, V.; Morsch, V.M.; Lopes, L.Q.S.; Santos, R.C.V.; et al. Effects of Glycerol Monolaurate on Growth and Physiology of Chicks Consuming Diet Containing Fumonisin. Microb. Pathog. 2020, 147, 104261. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.A.; Benedek, G.; Nguyen, H.; Gerstner, G.; Zhang, Y.; Kent, G.; Vandenbark, A.A.; Bernhagen, J.; Offner, H. Antibiotics Protect Against EAE by Increasing Regulatory and Anti-Inflammatory Cells. Metab. Brain Dis. 2018, 33, 1599–1607. [Google Scholar] [CrossRef]
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal Dysbiosis and Probiotic Applications in Autoimmune Diseases. Immunology 2017, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hurtado, I.; Gimenez, P.; García, I.; Zapater, P.; Francés, R.; González-Navajas, J.M.; Manichanh, C.; Ramos, J.M.; Bellot, P.; Guarner, F.; et al. Norfloxacin is More Effective than Rifaximin in Avoiding Bacterial Translocation in an Animal Model of Cirrhosis. Liver Int. 2018, 38, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiang, Q.; Gu, S.; Gu, Y.; Yao, M.; Huang, W.; Gao, W.; Tang, L.L. Short- and Long-Term Effects of Different Antibiotics on the Gut Microbiota and Cytokines Level in Mice. Infect. Drug Resist. 2022, 15, 6785–6797. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.T.; Rakasz, E.; Schultz-Darken, N.; Nephew, K.; Weisgrau, K.L.; Reilly, C.S.; Li, Q.; Southern, P.J.; Rothenberger, M.; Peterson, M.L.; et al. Glycerol Monolaurate Microbicide Protection against Repeat High-Dose SIV Vaginal Challenge. PLoS ONE 2015, 10, e0129465. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Yasukawa, K.; Harada, H.; Taga, T.; Watanabe, Y.; Matsuda, T.; Kashiwamura, S.; Nakajima, K.; Koyama, K.; Iwamatsu, A.; et al. Complementary DNA for a Novel Human Interleukin (BSF-2) that Induces B Lymphocytes to Produce Immunoglobulin. Nature 1986, 324, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut Microbiome Lipid Metabolism and Its Impact on Host Physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of Gut Microbiota on LPS-Induced Acute Lung Injury by Regulating the TLR4/NF-kB Signaling Pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Xavier, R.J. Conditioning of the Immune System by the Microbiome. Trends Immunol. 2023, 44, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, X.; Zhen, W.; Zeng, D.; Qu, L.; Wang, Z.; Ning, Z. Yeast Culture Improves Egg Quality and Reproductive Performance of Aged Breeder Layers by Regulating Gut Microbes. Front. Microbiol. 2021, 12, 633276. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe 2018, 24, 133–145.e135. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Bessegatto, J.A.; Alfieri, A.A.; Weese, J.S.; Filho, J.A.; Oba, A. Different Antibiotic Growth Promoters Induce Specific Changes in the Cecal Microbiota Membership of Broiler Chicken. PLoS ONE 2017, 12, e0171642. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Wang, H.; Zhang, W.; Zhang, C.; Xie, J.; Ma, X. High-Fibre Diets Regulate Antioxidative Capacity and Promote Intestinal Health by Regulating Bacterial Microbiota in Growing Pigs. J. Anim. Physiol. Anim. Nutr. 2024, 108, 357–365. [Google Scholar] [CrossRef]
- Cao, X.; Tang, L.; Zeng, Z.; Wang, B.; Zhou, Y.; Wang, Q.; Zou, P.; Li, W. Effects of Probiotics BaSC06 on Intestinal Digestion and Absorption, Antioxidant Capacity, Microbiota Composition, and Macrophage Polarization in Pigs for Fattening. Front. Vet. Sci. 2020, 7, 570593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Brinkman, C.C.; Hittle, L.E.; Fricke, W.F.; Mongodin, E.F.; Bromberg, J.S. Gut Microbiota Determines Graft Survival Outcome Through Regulation of Anti-Inflammatory and Pro-Inflammatory Responses. Transplantation 2018, 102, S333. [Google Scholar] [CrossRef]
- Yu, X.; Cui, Z.; Qin, S.; Zhang, R.; Wu, Y.; Liu, J.; Yang, C. Effects of Bacillus licheniformis on Growth Performance, Diarrhea Incidence, Antioxidant Capacity, Immune Function, and Fecal Microflora in Weaned Piglets. Animals 2022, 12, 1609. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Pu, Q.; Zhao, Q.; Zhou, Y.; Jiang, X.; Han, T. Effects of Fucoidan Isolated from Laminaria japonica on Immune Response and Gut Microbiota in Cyclophosphamide-Treated Mice. Front. Immunol. 2022, 13, 916618. [Google Scholar] [CrossRef]
- He, B.; Liu, Y.; Hoang, T.K.; Tian, X.; Taylor, C.M.; Luo, M.; Tran, D.Q.; Tatevian, N.; Rhoads, J.M. Antibiotic-Modulated Microbiome Suppresses Lethal Inflammation and Prolongs Lifespan in Treg-Deficient Mice. Microbiome 2019, 7, 145. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, G.; Yu, J.; Lee, K.; Lee, K.; Si, J.; You, H.J.; Ko, G. Distinct Changes in Microbiota-Mediated Intestinal Metabolites and Immune Responses Induced by Different Antibiotics. Antibiotics 2022, 11, 1762. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, J.; Liu, T.; Wang, J.; Cai, H.; Zhang, X.; Quay Huai Xia, D.; Feng, F.; Tang, J. Differential Modulations of Lauric Acid and Its Glycerides on High Fat Diet-Induced Metabolic Disorders and Gut Microbiota Dysbiosis. Food Res. Int. 2022, 157, 111437. [Google Scholar] [CrossRef]
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-Balance Studies Reveal Associations Between Gut Microbes, Caloric Load, and Nutrient Absorption in Humans. Am. J. Clin. Nutr. 2011, 94, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Abdullah; Zhang, C.; Li, Y.; Zhang, H.; Wang, J.; Feng, F. Effects of Dietary Glycerol Monolaurate on the Growth Performance, Digestive Enzymes, Body Composition and Non-Specific Immune Response of White Shrimp (Litopenaeus vannamei). Aquac. Rep. 2020, 18, 100535. [Google Scholar] [CrossRef]
- Jang, I.S.; Ko, Y.H.; Kang, S.Y.; Lee, C.Y. Effect of a Commercial Essential Oil on Growth Performance, Digestive Enzyme Activity and Intestinal Microflora Population in Broiler Chickens. Anim. Feed Sci. Technol. 2007, 134, 304–315. [Google Scholar] [CrossRef]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Peterson, M.L. Glycerol Monolaurate Antibacterial Activity in Broth and Biofilm Cultures. PLoS ONE 2012, 7, e40350. [Google Scholar] [CrossRef]
- Lin, Y.; Li, D.; Ma, Z.; Che, L.; Feng, B.; Fang, Z.; Xu, S.; Zhuo, Y.; Li, J.; Hua, L.; et al. Maternal Tributyrin Supplementation in Late Pregnancy and Lactation Improves Offspring Immunity, Gut Microbiota, and Diarrhea Rate in a Sow Model. Front Microbiol. 2023, 14, 1142174. [Google Scholar] [CrossRef]

| Items | CON | ATLL | 0.1% GML | 0.2% GML | SEM | p-Value |
|---|---|---|---|---|---|---|
| Sow Back Fat Thickness, mm | ||||||
| Day 90 of Gestation | 14.81 | 15.06 | 15.07 | 15.38 | 0.43 | 0.98 |
| Farrowing, within 24h | 16.00 | 15.30 | 15.14 | 13.80 | 0.36 | 0.56 |
| Day 21 of Lactation | 14.64 | 15.33 | 14.43 | 13.03 | 0.35 | 0.69 |
| Total Back Fat Loss | −1.47 | −1.08 | −1.14 | −1.68 | 0.22 | 0.89 |
| Fecal Scores | 1.66 | 1.42 | 1.41 | 1.58 | 0.08 | 0.64 |
| Items | CON | ATLL | 0.1% GML | 0.2% GML | SEM | p-Value |
|---|---|---|---|---|---|---|
| Farrowing Duration, min | 195.4 b | 154 ab | 120.53 a | 185.81 b | 9.26 | 0.01 |
| Delivery Interval, min | 23.6 b | 19.59 ab | 13.1 a | 12.75 a | 1.48 | 0.02 |
| Litter Size, n | ||||||
| Total Born | 11.69 | 13.13 | 12.80 | 13.73 | 0.46 | 0.29 |
| Born Alive | 11.00 | 11.75 | 11.73 | 12.57 | 0.43 | 0.45 |
| Weaning | 9.28 | 10.81 | 10.09 | 11.25 | 0.32 | 0.60 |
| Litter Weight, kg | ||||||
| Day 0 of Birth | 15.18 | 14.82 | 14.86 | 17.11 | 0.56 | 0.24 |
| Day of Weaning | 62.82 | 59.42 | 60.11 | 66.73 | 1.65 | 0.33 |
| Average Pig Body Weight, kg | ||||||
| Day 0 of Birth | 1.39 | 1.30 | 1.36 | 1.31 | 0.04 | 0.37 |
| Day of Weaning | 6.29 | 6.09 | 6.39 | 6.50 | 0.13 | 0.46 |
| Fecal Score of Piglets | 0.57 | 0.56 | 0.52 | 0.36 | 0.01 | 0.22 |
| Diarrhea Rate of Piglets, % | 1.46 | 1.14 | 0.92 | 0.84 | 0.32 | 0.08 |
| Items | CON | ATLL | 0.1% GML | 0.2% GML | SEM | p-Value |
|---|---|---|---|---|---|---|
| G90-D0, kg | 2.88 | 2.87 | 2.84 | 2.86 | 0.01 | 0.21 |
| L1-L7, kg | 4.1 ab | 3.9 a | 4.07 ab | 4.19 b | 0.03 | 0.03 |
| L8-L14, kg | 6.27 | 5.79 | 5.9 | 6.19 | 0.09 | 0.2 |
| L15-L21, kg | 6.34 | 5.8 | 5.88 | 6.19 | 0.08 | 0.06 |
| L1-L21, kg | 5.57 | 5.16 | 5.27 | 5.51 | 0.06 | 0.07 |
| Items | CON | ATLL | 0.1% GML | 0.2% GML | SEM | p-Value |
|---|---|---|---|---|---|---|
| Colostrum | ||||||
| Fat, % | 6.65 | 7.75 | 7.33 | 6.92 | 0.31 | 0.17 |
| Protein, % | 14.55 | 16.29 | 16.99 | 17.59 | 0.57 | 0.08 |
| Lactose, % | 2.75 | 2.65 | 2.40 | 2.48 | 0.08 | 0.40 |
| Total Solids, % | 28.56 | 31.75 | 30.99 | 30.94 | 0.76 | 0.33 |
| IgA, mg/ml | 4.80 | 3.26 | 4.49 | 5.83 | 0.56 | 0.46 |
| Milk on Day 20 of Lactation | ||||||
| Fat, % | 6.65 | 6.86 | 6.99 | 7.25 | 0.29 | 0.22 |
| Protein, % | 5.02 | 5.08 | 5.05 | 5.09 | 0.12 | 0.35 |
| Lactose, % | 5.52 | 5.25 | 5.25 | 5.22 | 0.07 | 0.65 |
| Total Solids, % | 19.32 | 20.60 | 19.93 | 20.10 | 0.33 | 0.54 |
| Items | CON | ATLL | 0.1% GML | 0.2% GML | SEM | p-Value |
|---|---|---|---|---|---|---|
| Day 1 of lactation | ||||||
| T-AOC, U/mL | 2.89 | 2.73 | 2.73 | 2.67 | 0.10 | 0.95 |
| T-SOD, U/mL | 78.02 | 75.18 | 74.37 | 76.23 | 0.76 | 0.38 |
| MDA, nmol/mL | 4.94 | 4.15 | 5.09 | 5.20 | 0.38 | 0.48 |
| GSH-Px, U/mL | 785.53 | 739.42 | 779.27 | 676.22 | 19.70 | 0.18 |
| Day 21 of lactation | ||||||
| T-AOC, U/mL | 3.01 | 3.38 | 3.29 | 3.02 | 0.14 | 0.73 |
| T-SOD, U/mL | 54.27 | 50.38 | 54.28 | 53.78 | 1.41 | 0.75 |
| MDA, nmol/mL | 3.94 a | 5.13 b | 4.59 ab | 4.55 ab | 0.19 | 0.04 |
| GSH-Px, U/mL | 667.60 | 558.86 | 660.51 | 567.36 | 30.10 | 0.58 |
| Weaning piglets | ||||||
| T-AOC, U/mL | 3.38 | 3.40 | 4.07 | 5.154 | 0.14 | 0.43 |
| T-SOD, U/mL | 23.73 a | 38.25 ab | 39.80 ab | 49.53 b | 3.08 | 0.02 |
| MDA, nmol/mL | 7.31 | 8.315 | 8.07 | 7.09 | 0.41 | 0.27 |
| GSH-Px, U/mL | 140.73 a | 210.70 b | 145.86 a | 187.91 ab | 10.29 | 0.03 |
| Items | CON | ATLL | 0.1% GML | 0.2% GML | SEM | p-Value |
|---|---|---|---|---|---|---|
| Day 1 of lactation | ||||||
| IL-1β, ng/L | 202.18 | 188.38 | 186.77 | 199.66 | 6.88 | 0.76 |
| TNF-α, ng/L | 264.53 b | 203.27 a | 198.09 a | 220.67 a | 16.73 | 0.05 |
| IL-6, ng/L | 61.94 | 63.10 | 56.69 | 48.49 | 3.45 | 0.45 |
| IL-10, ng/L | 124.73 | 122.59 | 123.50 | 123.69 | 5.24 | 1.00 |
| Day 21 of lactation | ||||||
| IL-1β, ng/L | 181.64 | 185.91 | 172.90 | 179.62 | 10.07 | 0.98 |
| TNF-α, ng/L | 367.28 c | 322.02 b | 338.63 b | 303.53 a | 34.14 | 0.05 |
| IL-6, ng/L | 60.69 | 58.77 | 68.40 | 59.23 | 4.03 | 0.84 |
| IL-10, ng/L | 124.19 a | 129.44 a | 154.39 b | 140.41 ab | 6.54 | 0.05 |
| Weaning piglets | ||||||
| IL-1β, ng/L | 68.58 | 85.12 | 77.80 | 64.51 | 4.16 | 0.31 |
| TNF-α, ng/L | 115.54 b | 99.191 b | 110.35 b | 76.76 a | 12.45 | 0.01 |
| IL-6, ng/L | 40.85 a | 57.70 ab | 29.35 a | 85.64 b | 6.65 | 0.01 |
| IL-10, ng/L | 66.76 | 58.98 | 77.42 | 51.39 | 4.23 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yang, M.; Ma, Z.; Che, L.; Feng, B.; Fang, Z.; Xu, S.; Zhuo, Y.; Li, J.; Wang, J.; et al. Glycerol Monolaurate Complex Improved Antioxidant, Anti-Inflammation, and Gut Microbiota Composition of Offspring in a Sow–Piglet Model. Vet. Sci. 2025, 12, 24. https://doi.org/10.3390/vetsci12010024
Li D, Yang M, Ma Z, Che L, Feng B, Fang Z, Xu S, Zhuo Y, Li J, Wang J, et al. Glycerol Monolaurate Complex Improved Antioxidant, Anti-Inflammation, and Gut Microbiota Composition of Offspring in a Sow–Piglet Model. Veterinary Sciences. 2025; 12(1):24. https://doi.org/10.3390/vetsci12010024
Chicago/Turabian StyleLi, Dan, Min Yang, Zhao Ma, Lianqiang Che, Bin Feng, Zhengfeng Fang, Shengyu Xu, Yong Zhuo, Jian Li, JiHhua Wang, and et al. 2025. "Glycerol Monolaurate Complex Improved Antioxidant, Anti-Inflammation, and Gut Microbiota Composition of Offspring in a Sow–Piglet Model" Veterinary Sciences 12, no. 1: 24. https://doi.org/10.3390/vetsci12010024
APA StyleLi, D., Yang, M., Ma, Z., Che, L., Feng, B., Fang, Z., Xu, S., Zhuo, Y., Li, J., Wang, J., Zhang, Z., Wu, Z., Lin, T., Wu, D., & Lin, Y. (2025). Glycerol Monolaurate Complex Improved Antioxidant, Anti-Inflammation, and Gut Microbiota Composition of Offspring in a Sow–Piglet Model. Veterinary Sciences, 12(1), 24. https://doi.org/10.3390/vetsci12010024









