Machine Learning Techniques for Canine Myxomatous Mitral Valve Disease Classification: Integrating Anamnesis, Quality of Life Survey, and Physical Examination
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchanan, J. Chapter 23: Prevalence of Cardiovascular Disorder. In Textbook of Canine and Feline Cardiology, 2nd ed.; Saunders W.B.: Philadelphia, PA, USA, 1999; pp. 457–470. ISBN 0-7216-4044-3. [Google Scholar]
- Bonnett, B.; Egenvall, A.; Hedhammar, Å.; Olson, P. Mortality in over 350,000 Insured Swedish Dogs from 1995–2000: I. Breed-, Gender-, Age- and Cause-Specific Rates. Acta Vet. Scand. 2005, 46, 105. [Google Scholar] [CrossRef]
- Egenvall, A.; Bonnett, B.; Hedhammar, Å.; Olson, P. Mortality in over 350,000 Insured Swedish Dogs from 1995–2000: II. Breed-Specific Age and Survival Patterns and Relative Risk for Causes of Death. Acta Vet. Scand. 2005, 46, 121. [Google Scholar] [CrossRef] [PubMed]
- Borgarelli, M.; Haggstrom, J. Canine Degenerative Myxomatous Mitral Valve Disease: Natural History, Clinical Presentation and Therapy. Vet. Clin. N. Am. Small Anim. Pract. 2010, 40, 651–663. [Google Scholar] [CrossRef]
- Häggström, J.; Hansson, K.; Kvart, C.; Swenson, L. Chronic Valvular Disease in the Cavalier King Charles Spaniel in Sweden. Vet. Rec. 1992, 131, 549–553. [Google Scholar] [PubMed]
- Boswood, A.; Häggström, J.; Gordon, S.G.; Wess, G.; Stepien, R.L.; Oyama, M.A.; Keene, B.W.; Bonagura, J.; MacDonald, K.A.; Patteson, M.; et al. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study—A Randomized Clinical Trial. J. Vet. Intern. Med. 2016, 30, 1765–1779. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Rush, J.E.; Farabaugh, A.E.; Must, A. Development and Evaluation of a Questionnaire for Assessing Health-Related Quality of Life in Dogs with Cardiac Disease. J. Am. Vet. Med. Assoc. 2005, 226, 1864–1868. [Google Scholar] [CrossRef]
- Häggström, J.; Höglund, K.; Borgarelli, M. An Update on Treatment and Prognostic Indicators in Canine Myxomatous Mitral Valve Disease. J. Small Anim. Pract. 2009, 50, 25–33. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM Consensus Guidelines for the Diagnosis and Treatment of Myxomatous Mitral Valve Disease in Dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Ljungvall, I.; Häggström, J. Chapter 251: Adult-Onset Valvular Heart Disease. In Textbook of Veterinary Internal Medicine, 8th ed.; Elsevier Saunders: Amsterdam, The Netherlands, 2017; Volume 2, pp. 1249–1269. ISBN 9780323462143. [Google Scholar]
- Borgarelli, M.; Savarino, P.; Crosara, S.; Santilli, R.A.; Chiavegato, D.; Poggi, M.; Bellino, C.; Rosa, G.L.; Zanatta, R.; Haggstrom, J.; et al. Survival Characteristics and Prognostic Variables of Dogs with Mitral Regurgitation Attributable to Myxomatous Valve Disease. J. Vet. Intern. Med. 2008, 22, 120–128. [Google Scholar] [CrossRef]
- Boswood, A.; Gordon, S.G.; Häggström, J.; Wess, G.; Stepien, R.L.; Oyama, M.A.; Keene, B.W.; Bonagura, J.; MacDonald, K.A.; Patteson, M.; et al. Longitudinal Analysis of Quality of Life, Clinical, Radiographic, Echocardiographic, and Laboratory Variables in Dogs with Preclinical Myxomatous Mitral Valve Disease Receiving Pimobendan or Placebo: The EPIC Study. J. Vet. Intern. Med. 2018, 32, 72–85. [Google Scholar] [CrossRef]
- Mattin, M.J.; Brodbelt, D.C.; Church, D.B.; Boswood, A. Factors Associated with Disease Progression in Dogs with Presumed Preclinical Degenerative Mitral Valve Disease Attending Primary Care Veterinary Practices in the United Kingdom. J. Vet. Intern. Med. 2019, 33, 445–454. [Google Scholar] [CrossRef]
- Sargent, J.; Muzzi, R.; Mukherjee, R.; Somarathne, S.; Schranz, K.; Stephenson, H.; Connolly, D.; Brodbelt, D.; Fuentes, V.L. Echocardiographic Predictors of Survival in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Cardiol. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Chetboul, V.; Athanassiadis, N.; Concordet, D.; Nicolle, A.; Tessier, D.; Castagnet, M.; Pouchelon, J.L.; Lefebvre, H.P. Observer-dependent Variability of Quantitative Clinical Endpoints: The Example of Canine Echocardiography. J. Vet. Pharmacol. Ther. 2004, 27, 49–56. [Google Scholar] [CrossRef]
- Oyama, M.A.; Rush, J.E.; O’Sullivan, M.L.; Williams, R.M.; Rozanski, E.A.; Petrie, J.-P.; Sleeper, M.M.; Brown, D.C. Perceptions and Priorities of Owners of Dogs with Heart Disease Regarding Quality versus Quantity of Life for Their Pets. J. Am. Vet. Med. Assoc. 2008, 233, 104–108. [Google Scholar] [CrossRef]
- Ngiam, K.Y.; Khor, I.W. Big Data and Machine Learning Algorithms for Health-Care Delivery. Lancet Oncol. 2019, 20, e262–e273. [Google Scholar] [CrossRef]
- Quer, G.; Arnaout, R.; Henne, M.; Arnaout, R. Machine Learning and the Future of Cardiovascular Care JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Boissady, E.; Comble, A.D.L.; Zhu, X.; Abbott, J.; Adrien-Maxence, H. Comparison of a Deep Learning Algorithm vs. Humans for Vertebral Heart Scale Measurements in Cats and Dogs Shows a High Degree of Agreement Among Readers. Front. Vet. Sci. 2021, 8, 764570. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Sung, J. An Automated Deep Learning Method and Novel Cardiac Index to Detect Canine Cardiomegaly from Simple Radiography. Sci. Rep. 2022, 12, 14494. [Google Scholar] [CrossRef] [PubMed]
- Valente, C.; Wodzinski, M.; Guglielmini, C.; Poser, H.; Chiavegato, D.; Zotti, A.; Venturini, R.; Banzato, T. Development of an Artificial Intelligence-Based Method for the Diagnosis of the Severity of Myxomatous Mitral Valve Disease from Canine Chest Radiographs. Front. Vet. Sci. 2023, 10, 1227009. [Google Scholar] [CrossRef] [PubMed]
- Strunz, C.M.C.; Marcondes-Santos, M.; Takada, J.Y.; Fragata, F.S.; Mansur, A.D.P. Quality of Life Score as a Predictor of Death in Dogs with Degenerative Mitral Valve Disease. Arq. Bras. Cardiol. 2017, 108, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.M.; Alessi, C.; Kittleson, M.D.; Linares-Villalba, S.; Engel-Manchado, J. Psychometric Properties of the Spanish Version of the Functional Evaluation of Cardiac Health Questionnaire “FETCH-QTM” for Assessing Health-Related Quality of Life in Dogs with Cardiac Disease. Top. Companion Anim. Med. 2020, 39, 100431. [Google Scholar] [CrossRef]
- Levine, S.A. Notes on the Gradation of the Intensity of Cardiac Murmurs. JAMA 1961, 177, 261. [Google Scholar] [CrossRef]
- Rishniw, M. Murmur Grading in Humans and Animals: Past and Present. J. Vet. Cardiol. 2018, 20, 223–233. [Google Scholar] [CrossRef] [PubMed]
- German, A.J.; Holden, S.L.; Moxham, G.L.; Holmes, K.L.; Hackett, R.M.; Rawlings, J. A Simple, Reliable Tool for Owners to Assess the Body Condition of Their Dog or Cat. Am. Soc. Nutr. 2006, 136, 2031–2033. [Google Scholar] [CrossRef] [PubMed]
- Acierno, M.J.; Brown, S.; Coleman, A.E.; Jepson, R.E.; Papich, M.; Stepien, R.L.; Syme, H.M. ACVIM Consensus Statement: Guidelines for the Identification, Evaluation, and Management of Systemic Hypertension in Dogs and Cats. J. Vet. Intern. Med. 2018, 32, 1803–1822. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.P.; Gaber, C.E.; Jacobs, G.J.; Kaplan, P.M.; Lombard, C.W.; Moise, N.S.; Moses, B.L. Recommendations for Standards in Transthoracic Two-Dimensional Echocardiography in the Dog and Cat. Vet. Radiol. Ultrasound 1994, 35, 173–178. [Google Scholar] [CrossRef]
- Cornell, C.C.; Kittleson, M.D.; Torre, P.D.; Häggström, J.; Lombard, C.W.; Pedersen, H.D.; Vollmar, A.; Wey, A. Allometric Scaling of M-Mode Cardiac Measurements in Normal Adult Dogs. J. Vet. Intern. Med. 2004, 18, 311–321. [Google Scholar] [CrossRef]
- Vezzosi, T.; Grosso, G.; Tognetti, R.; Meucci, V.; Patata, V.; Marchesotti, F.; Domenech, O. The Mitral INsufficiency Echocardiographic Score: A Severity Classification of Myxomatous Mitral Valve Disease in Dogs. J. Vet. Intern. Med. 2021, 35, 1238–1244. [Google Scholar] [CrossRef]
- Bryer, J.; Speerschneider, K. Package ‘Likert’. 2016. Available online: https://CRAN.R-project.org/package=likert (accessed on 28 February 2024).
- Ebbert, D. A Post Hoc Analysis for Pearson’s Chi-Squared Test for Count Data. 2022. Available online: https://CRAN.R-project.org/package=chisq.posthoc.test (accessed on 28 February 2024).
- Therneau, T.M.; Atkinson, E.J. An Introduction to Recursive Partitioning Using the RPART Routines. Rochester Mayo Found. 2022, 1, 1–60. [Google Scholar]
- Milborrow, S. Plotting Rpart Trees with the Rpart.Plot Package. 2021. Available online: https://CRAN.R-project.org/package=rpart.plot (accessed on 28 February 2024).
- Liaw, A.; Wiener, M. Breiman and Cutler’s Random Forests for Classification and Regression 2022. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; Team, R.C.; et al. Package Caret: Classification and Regression Training 2022. Kuhn, M. Building Predictive Models in R Using the caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Lisciandro, G.R.; Lisciandro, S.C. Lung Ultrasound Fundamentals, “Wet Versus Dry” Lung, Signs of Consolidation in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 1125–1140. [Google Scholar] [CrossRef]
- Wilshaw, J.; Rosenthal, S.L.; Wess, G.; Dickson, D.; Bevilacqua, L.; Dutton, E.; Deinert, M.; Abrantes, R.; Schneider, I.; Oyama, M.A.; et al. Accuracy of History, Physical Examination, Cardiac Biomarkers, and Biochemical Variables in Identifying Dogs with Stage B2 Degenerative Mitral Valve Disease. J. Vet. Intern. Med. 2021, 35, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Masic, I.; Begic, Z.; Naser, N.; Begic, E. Pediatric Cardiac Anamnesis: Prevention of Additional Diagnostic Tests. Int. J. Prev. Med. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Hatala, R.; Issenberg, S.B.; Kassen, B.; Cole, G.; Bacchus, C.M.; Scalese, R.J. Assessing Cardiac Physical Examination Skills Using Simulation Technology and Real Patients: A Comparison Study. Med. Educ. 2008, 42, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Flugelman, M.Y. History-Taking Revisited: Simple Techniques to Foster Patient Collaboration, Improve Data Attainment, and Establish Trust with the Patient. GMS J. Med. Educ. 2021, 38, Doc109. [Google Scholar] [CrossRef] [PubMed]
- López-Alvarez, J.; Elliott, J.; Pfeiffer, D.; Chang, Y.-M.; Mattin, M.; Moonarmart, W.; Hezzell, M.J.; Boswood, A. Clinical Severity Score System in Dogs with Degenerative Mitral Valve Disease. J. Vet. Intern. Med. 2015, 29, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Borgarelli, M.; Crosara, S.; Lamb, K.; Savarino, P.; Rosa, G.L.; Tarducci, A.; Haggstrom, J. Survival Characteristics and Prognostic Variables of Dogs with Preclinical Chronic Degenerative Mitral Valve Disease Attributable to Myxomatous Degeneration. J. Vet. Intern. Med. 2012, 26, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Häggström, J.; Boswood, A.; O’Grady, M.; Jöns, O.; Smith, S.; Swift, S.; Borgarelli, M.; Gavaghan, B.; Kresken, J.-G.; Patteson, M.; et al. Effect of Pimobendan or Benazepril Hydrochloride on Survival Times in Dogs with Congestive Heart Failure Caused by Naturally Occurring Myxomatous Mitral Valve Disease: The QUEST Study. J. Vet. Intern. Med. 2008, 22, 1124–1135. [Google Scholar] [CrossRef]
- Häggström, J.; Kvart, C.; Hansson, K. Heart Sounds and Murmurs: Changes Related to Severity of Chronic Valvular Disease in the Cavalier King Charles Spaniel. J. Vet. Intern. Med. 1995, 9, 75–85. [Google Scholar] [CrossRef]
- Pedersen, H.D.; Häggström, J.; Falk, T.; Mow, T.; Olsen, L.H.; Iversen, L.; Jensen, A.L. Auscultation in Mild Mitral Regurgitation in Dogs: Observer Variation, Effects of Physical Maneuvers, and Agreement with Color Doppler Echocardiography and Phonocardiography. J. Vet. Intern. Med. 1999, 13, 56–64. [Google Scholar] [CrossRef]
- Ljungvall, I.; Rishniw, M.; Porciello, F.; Ferasin, L.; Ohad, D.G. Murmur Intensity in Small-breed Dogs with Myxomatous Mitral Valve Disease Reflects Disease Severity. J. Small Anim. Pract. 2014, 55, 545–550. [Google Scholar] [CrossRef]
- Rasmussen, C.E.; Falk, T.; Zois, N.E.; Moesgaard, S.G.; Häggström, J.; Pedersen, H.D.; Åblad, B.; Nilsen, H.Y.; Olsen, L.H. Heart Rate, Heart Rate Variability, and Arrhythmias in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Intern. Med. 2012, 26, 76–84. [Google Scholar] [CrossRef]
- López-Alvarez, J.; Boswood, A.; Moonarmart, W.; Hezzell, M.J.; Lotter, N.; Elliott, J. Longitudinal Electrocardiographic Evaluation of Dogs with Degenerative Mitral Valve Disease. J. Vet. Intern. Med. 2014, 28, 393–400. [Google Scholar] [CrossRef]
- Boswood, A.; Gordon, S.G.; Häggström, J.; Vanselow, M.; Wess, G.; Stepien, R.L.; Oyama, M.A.; Keene, B.W.; Bonagura, J.; MacDonald, K.A.; et al. Temporal Changes in Clinical and Radiographic Variables in Dogs with Preclinical Myxomatous Mitral Valve Disease: The EPIC Study. J. Vet. Intern. Med. 2020, 34, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Valandro, M.A.; Pascon, J.P.E.; Pereira, D.T.P.; Mistieri, M.L.A. Exercise Training of Dogs with Myxomatous Valve Disease. Arq. Bras. Med. Veterinária E Zootec. 2016, 69, 325–332. [Google Scholar] [CrossRef][Green Version]
- Schober, K.E.; Hart, T.M.; Stern, J.A.; Li, X.; Samii, V.F.; Zekas, L.J.; Scansen, B.A.; Bonagura, J.D. Effects of Treatment on Respiratory Rate, Serum Natriuretic Peptide Concentration, and Doppler Echocardiographic Indices of Left Ventricular Filling Pressure in Dogs with Congestive Heart Failure Secondary to Degenerative Mitral Valve Disease and Dilated Cardiomyopathy. J. Am. Vet. Med. Assoc. 2011, 239, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Ohad, D.G.; Rishniw, M.; Ljungvall, I.; Porciello, F.; Häggström, J. Sleeping and Resting Respiratory Rates in Dogs with Subclinical Heart Disease. J. Am. Vet. Med. Assoc. 2013, 243, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Chalifoux, N.V.; Spielvogel, C.F.; Stefanovski, D.; Silverstein, D.C. Standardized Capillary Refill Time and Relation to Clinical Parameters in Hospitalized Dogs. J. Vet. Emerg. Crit. Car 2021, 31, 585–594. [Google Scholar] [CrossRef]
- Mrgan, M.; Rytter, D.; Brabrand, M. Capillary Refill Time Is a Predictor of Short-Term Mortality for Adult Patients Admitted to a Medical Department: An Observational Cohort Study. Emerg. Med. J. 2014, 31, 954. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.; Karlen, W.; Ansermino, J.M. Capillary Refill Time. Anesth. Analg. 2011, 113, 120–123. [Google Scholar] [CrossRef]
- Lobos, A.-T.; Menon, K. A Multidisciplinary Survey on Capillary Refill Time: Inconsistent Performance and Interpretation of a Common Clinical Test. Pediatr. Crit. Care Med. 2008, 9, 386–391. [Google Scholar] [CrossRef]
- Brabrand, M.; Hosbond, S.; Folkestad, L. Capillary Refill Time. Eur. J. Emerg. Med. 2011, 18, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.M.; Gouni, V.; Tissier, R.; Trehiou-Sechi, E.; Misbach, C.; Pouchelon, J.-L.; Lefebvre, H.P.; Chetboul, V. Systolic Arterial Blood Pressure in Small-Breed Dogs with Degenerative Mitral Valve Disease: A Prospective Study of 103 Cases (2007–2012). Vet. J. 2013, 197, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Visser, L.C.; Ciccozzi, M.M.; Sintov, D.J.; Sharpe, A.N. Echocardiographic Quantitation of Left Heart Size and Function in 122 Healthy Dogs: A Prospective Study Proposing Reference Intervals and Assessing Repeatability. J. Vet. Intern. Med. 2019, 33, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Wess, G.; Bauer, A.; Kopp, A. Echocardiographic Reference Intervals for Volumetric Measurements of the Left Ventricle Using the Simpson’s Method of Discs in 1331 Dogs. J. Vet. Intern. Med. 2021, 35, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Karsdorp, P.A.; Kindt, M.; Rietveld, S.; Everaerd, W.; Mulder, B.J.M. Interpretation Bias for Heart Sensations in Congenital Heart Disease and Its Relation to Quality of Life. Int. J. Behav. Med. 2008, 15, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Redelmeier, D.A.; Tu, J.V.; Schull, M.J.; Ferris, L.E.; Hux, J.E. Problems for Clinical Judgement: Obtaining a Reliable Past Medical History. CMAJ Can. Med. Assoc. J. 2001, 164, 809–813. [Google Scholar]
- Palmieri, J.J.; Stern, T.A. Lies in the Doctor-Patient Relationship: (Rounds in the General Hospital). Prim. Care Companion J. Clin. Psychiatry 2009, 11, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Noorbakhsh-Sabet, N.; Zand, R.; Zhang, Y.; Abedi, V. Artificial Intelligence Transforms the Future of Health Care. Am. J. Med. 2019, 132, 795–801. [Google Scholar] [CrossRef]
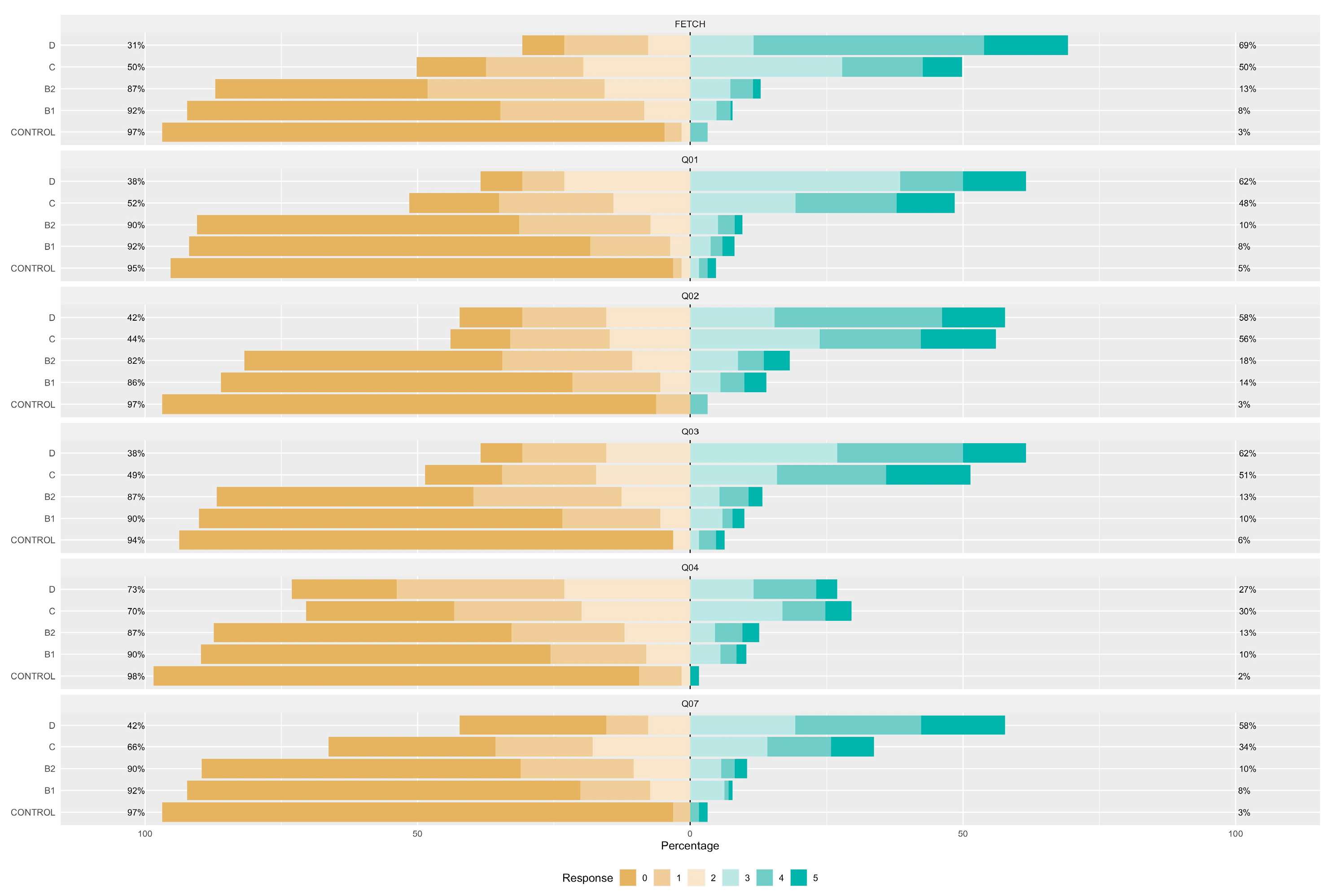
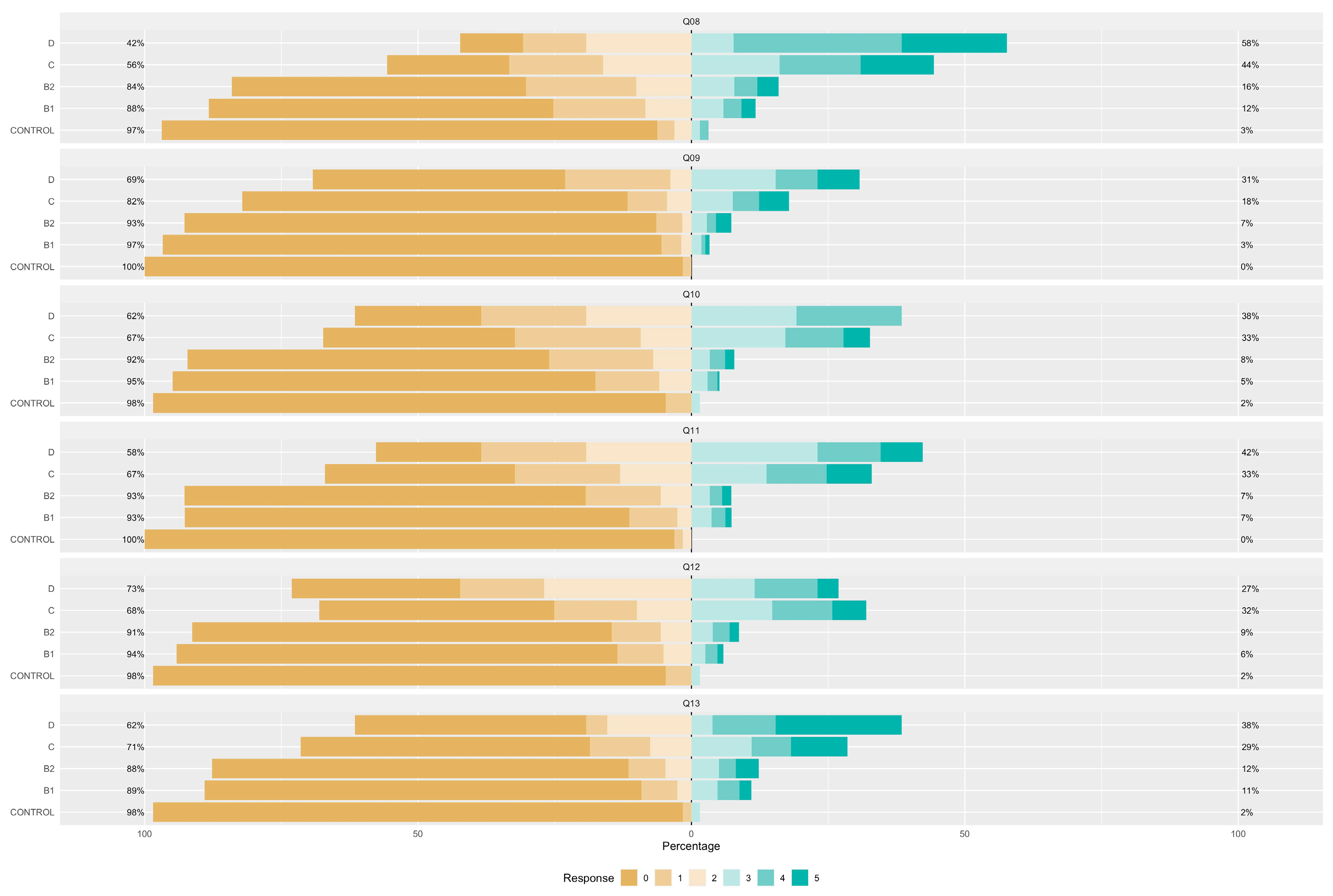
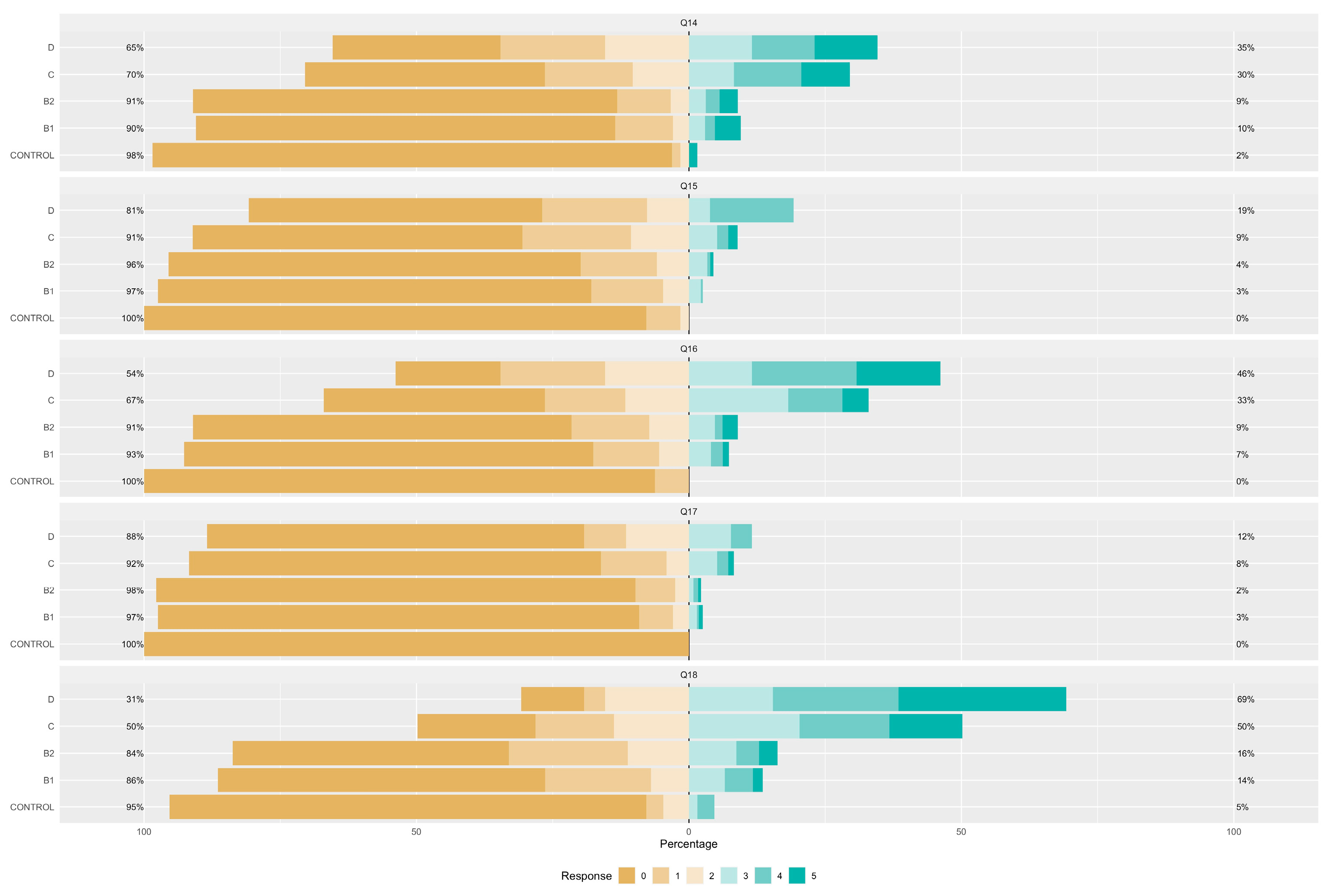
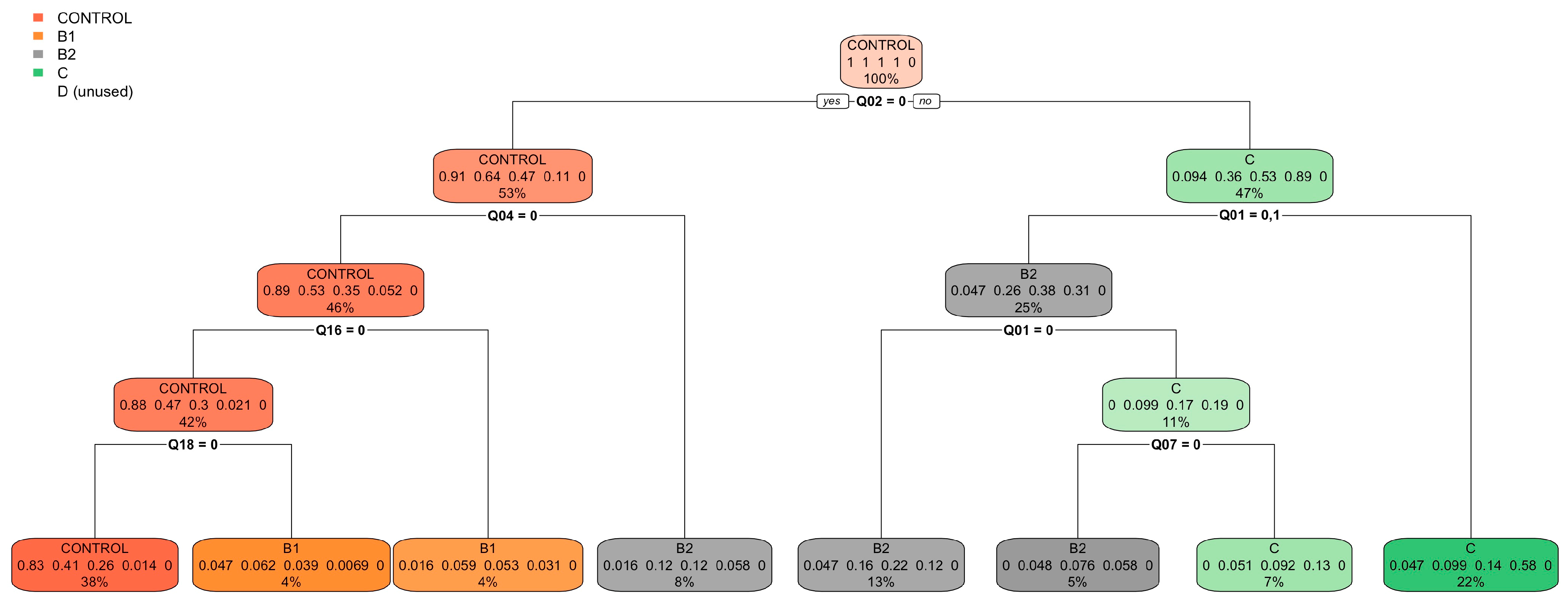
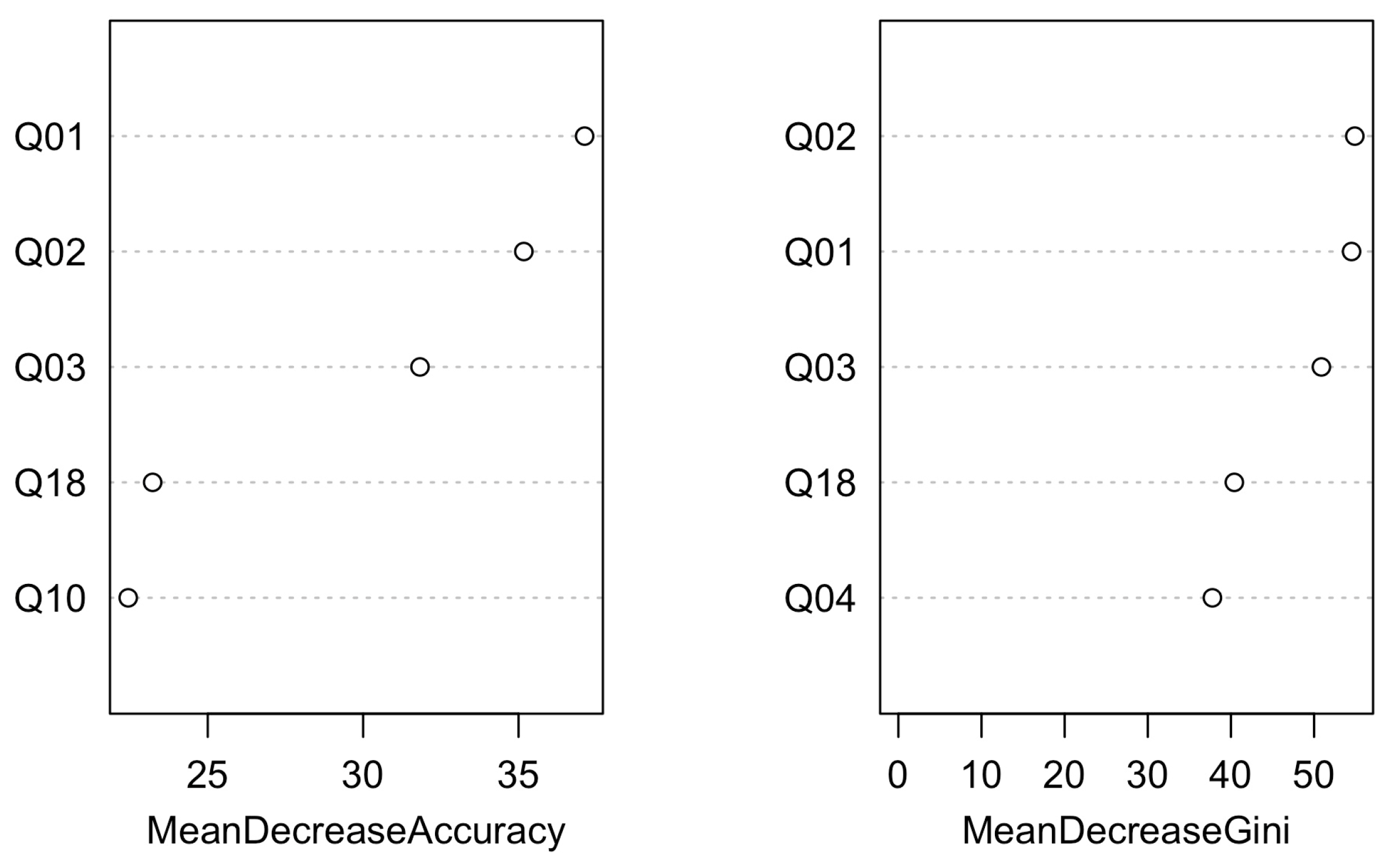

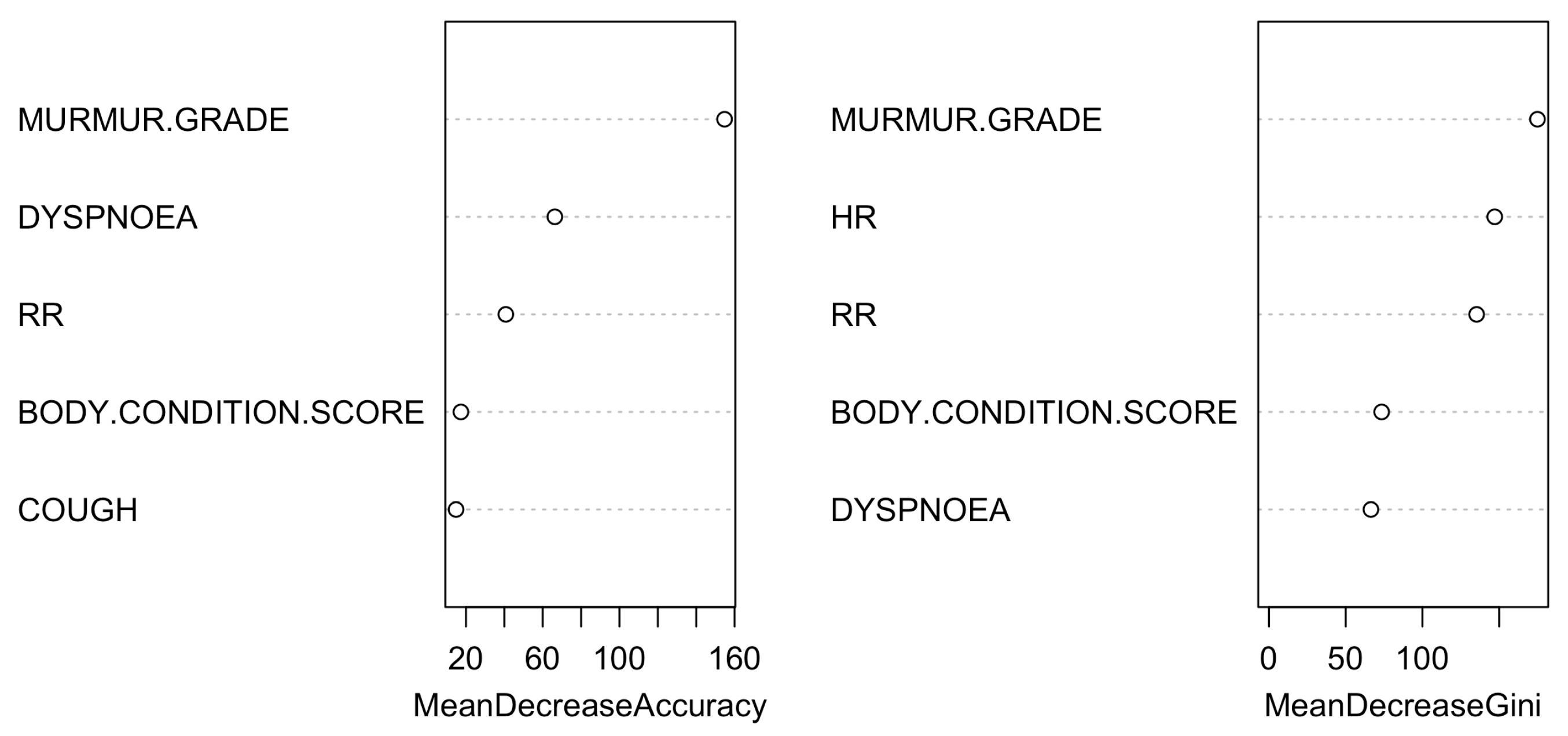
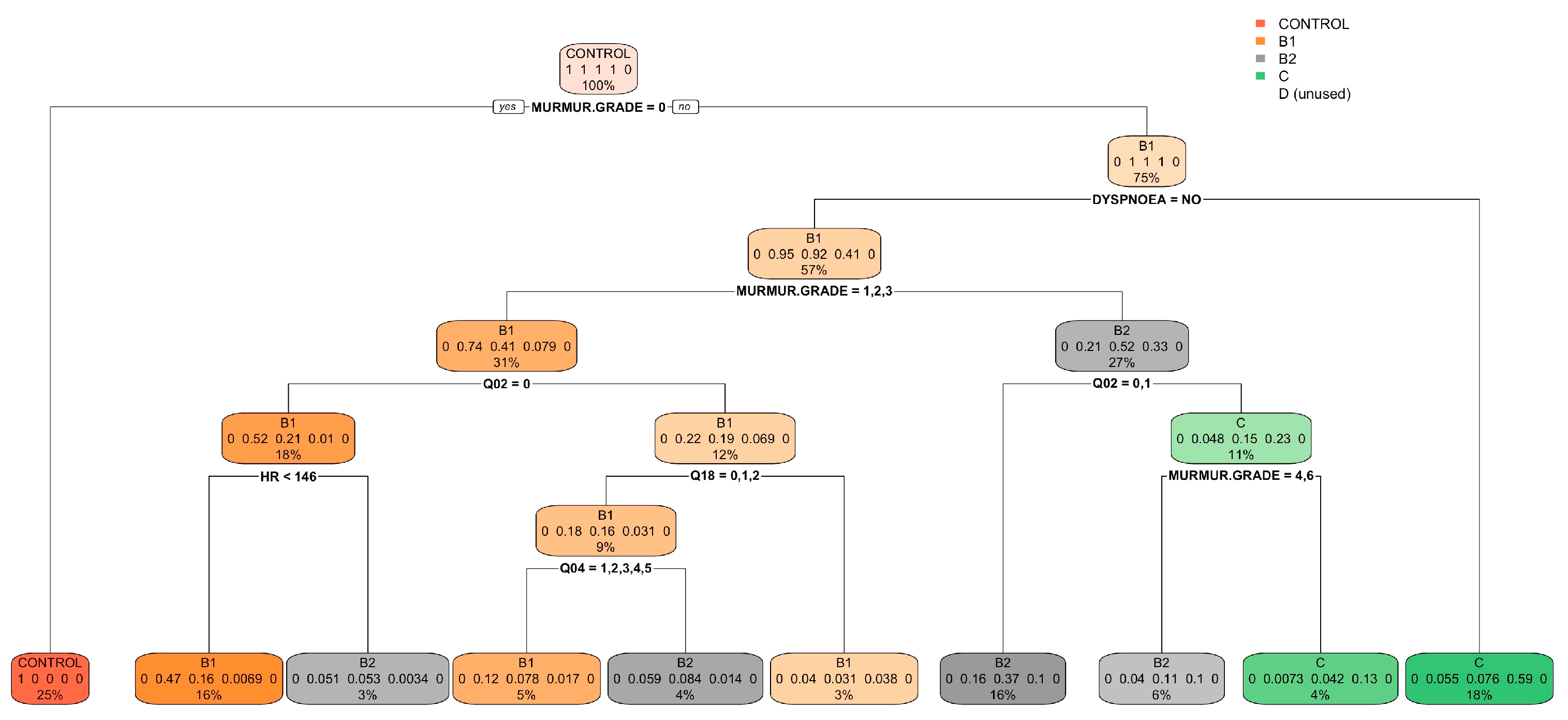
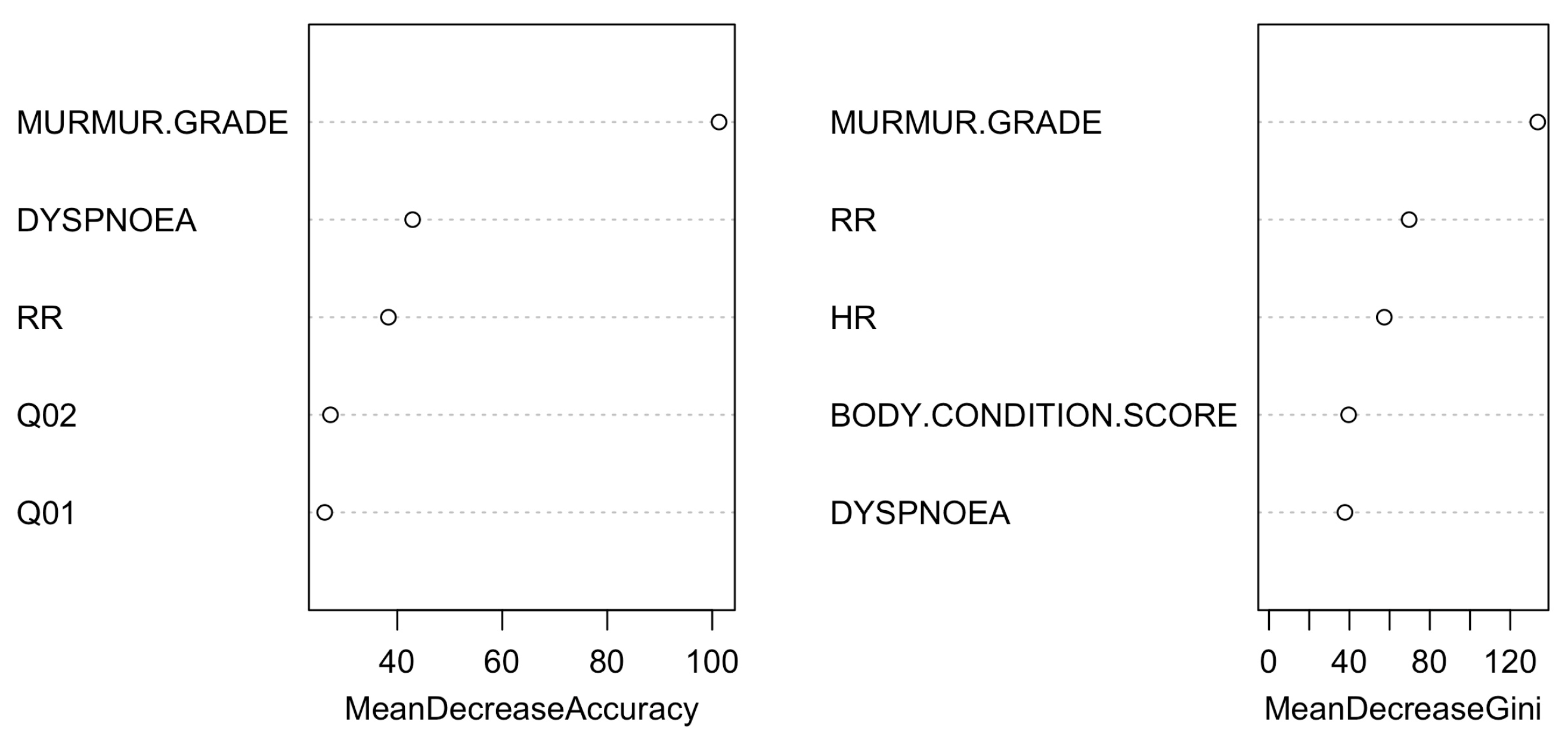
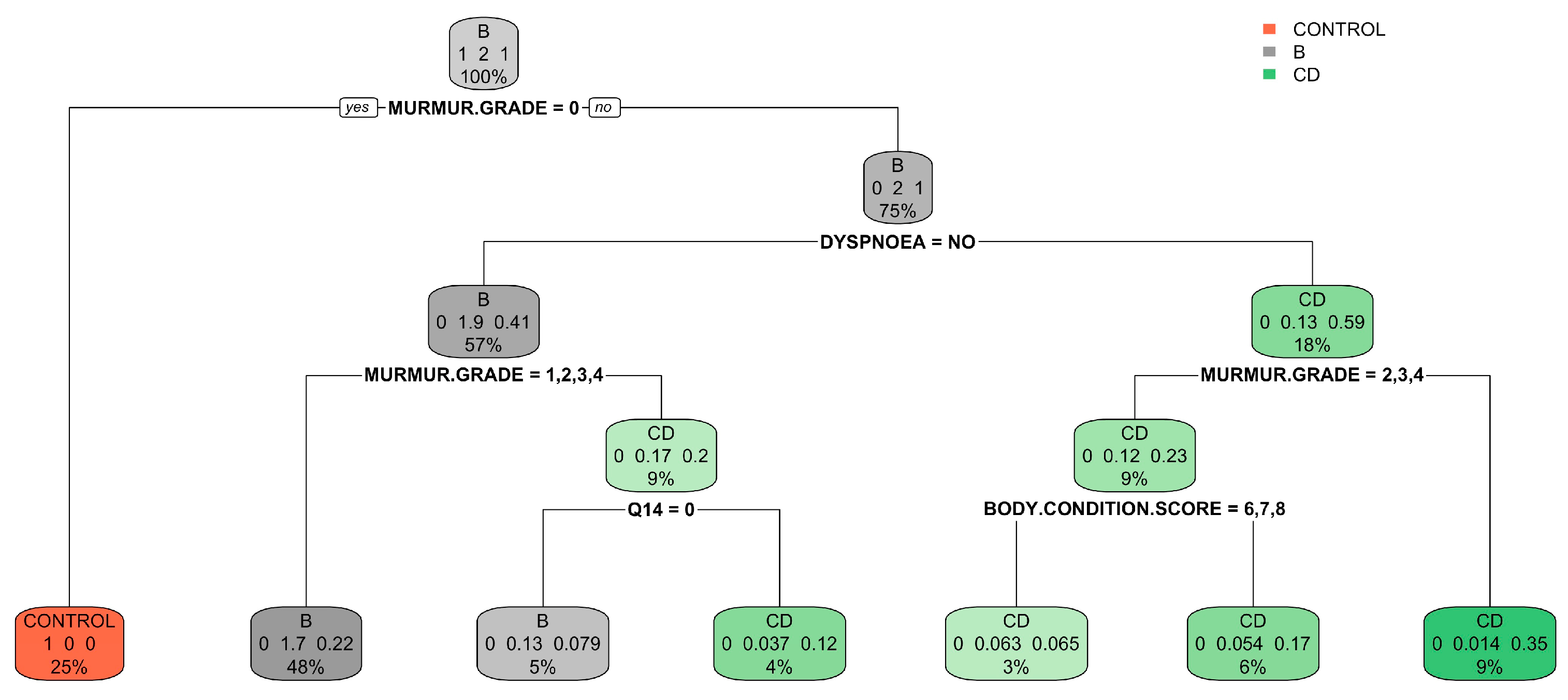

| Control Group (N = 64) | B1 (N = 273) | B2 (N = 357) | C (N = 291) | D (N = 26) | ||
|---|---|---|---|---|---|---|
| SEX | M | 36 (56.3%) | 139 (50.9%) | 175(49%) | 121 (41.6%) | 11 (42.3%) |
| F | 28 (43.8%) | 134 (49.2%) | 182 (51%) | 170 (58.4%) | 15 (57.7%) | |
| AGE (years) | ||||||
| Median (Min, max) | 6.0 (1.0, 16.0) | 11.0 (2.0, 19.0) | 12.0 (1.0, 18.0) | 12.0 (5.0, 18.0) | 12.8 (12.0, 17.0) | |
| BODY WEIGHT (Kg) | ||||||
| Median (Min, max) | 13.2 (2.5, 48.5) | 7 (1.0, 46.5) | 7.5 (1.5, 47.5) | 5.8 (1.5, 37.5) | 12.8 (10.0, 17.0) | |
| Control Group (N = 64) | B1 (N = 273) | B2 (N = 357) | C (N = 291) | D (N = 26) | |
|---|---|---|---|---|---|
| CROSSBREED | 33 (51.6%) | 101 (37.0%) | 132 (37.0%) | 93 (32.0%) | 12 (46.2%) |
| BEAGLE | 12 (18.8%) | 8 (2.9%) | 11 (3.1%) | 8 (2.7%) | 0 (0.0%) |
| YORKSHIRE TERRIER | 5 (7.8%) | 42 (15.4%) | 40 (11.2%) | 38 (13.1%) | 3 (11.5%) |
| CHIHUAHUA | 2 (3.1%) | 23 (8.4%) | 30 (8.4%) | 50 (17.2%) | 0 (0.0%) |
| MALTESE | 0 (0.0%) | 19 (7.0%) | 14 (3.9%) | 24 (8.2%) | 2 (7.7%) |
| POODLE | 0 (0.0%) | 9 (3.3%) | 20 (5.6%) | 24 (8.2%) | 2 (7.7%) |
| DACHSHUND | 0 (0.0%) | 11 (4.0%) | 19 (5.3%) | 10 (3.4%) | 0 (0.0%) |
| MINIATURE SCHNAUZER | 0 (0.0%) | 8 (2.9%) | 9 (2.5%) | 7 (2.4%) | 1 (3.8%) |
| SHIH TZU | 0 (0.0%) | 11 (4.0%) | 7 (2.0%) | 5 (1.7%) | 0 (0.0%) |
| COCKER SPANIEL | 0 (0.0%) | 3 (1.1%) | 12 (3.4%) | 5 (1.7%) | 0 (0.0%) |
| Control Group (N = 64) | B1 (N = 273) | B2 (N = 357) | C (N = 291) | D (N = 26) | ||
|---|---|---|---|---|---|---|
| Cough | Yes | 4 (6.3%) a | 55 (20.1%) b | 94 (26.3%) b | 169 (58.1%) c | 17 (65.4%) c |
| No | 60 (93,8%) | 218 (79.9%) | 263 (73.7%) | 122 (41.9%) | 9 (34.6%) | |
| Dyspnoea | Yes | 3 (4.7%) a | 15 (5.5%) a | 27 (7.6%) a | 171 (58.8%) b | 16 (61.5%) b |
| No | 61 (95.3%) | 258 (94.5%) | 330 (92.4%) | 120 (41.2%) | 10 (38.5%) | |
| Syncope | Yes | 1 (1.6%) ab | 7 (2.6%) a | 30 (8.4%) b | 48 (16.5%) c | 7 (26.9%) c |
| No | 63 (98.4%) | 266 (97.4%) | 327 (91.6%) | 243 (83.5%) | 19 (73.1%) | |
| Exercise | Yes | 1 (1.6%) a | 13 (4.8%) a | 36 (10.1%) b | 100 (34.4%) c | 14 (53.8%) c |
| Intolerance | No | 63 (98.4%) | 260 (95.2%) | 321 (89.9%) | 191 (65.6%) | 12 (46.2%) |
| Anorexia | Yes | 0 (0%) a | 10 (3.7%) a | 15 (4.2%) a | 42 (14.4%) b | 9 (34.6%) c |
| No | 64 (100%) | 263 (96.3%) | 342 (95.8%) | 249 (85.6%) | 17 (65.4%) | |
| Body weight loss | Yes | 0 (0%) a | 5 (1.8%) a | 6 (1.7%) a | 19 (6.5%) b | 8 (30.8%) c |
| No | 64 (100%) | 268 (98.2%) | 351 (98.3%) | 272 (93.5%) | 18 (69.2%) |
| Control Group (N = 64) | B1 (N = 273) | B2 (N = 357) | C (N = 291) | D (N = 26) | ||
|---|---|---|---|---|---|---|
| Murmur | Yes | 0 (0%) a | 273 (100%) b | 357 (100%) b | 291 (100%) b | 26 (100%) b |
| No | 64 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Murmur | No | 64(100%) a | 0 (0%) b | 0 (0%) c | 0 (0%) d | 0 (0%) e |
| grade | 1 | 0 (0%) | 18 (6.6%) | 3 (0.8%) | 0 (0%) | 0 (0%) |
| 2 | 0 (0%) | 71 (26%) | 28 (7.8%) | 4 (1.4%) | 1 (3.8%) | |
| 3 | 0 (0%) | 124 (45.4%) | 123 (34.5%) | 30 (10.3%) | 1 (3.8%) | |
| 4 | 0 (0%) | 56 (20.5%) | 143 (40.1%) | 97 (33.3%) | 5 (19.2%) | |
| 5 | 0 (0%) | 4 (1.5%) | 52 (14.6%) | 141 (48.5%) | 12 (46.2%) | |
| 6 | 0 (0%) | 0 (0%) | 8 (2.2%) | 19 (6.5%) | 7 (26.9%) | |
| CRT | >2 s | 0 (0%) a | 2 (0.7%) a | 1 (0.3%) a | 5 (1.7%) a | 3 (11.5%) b |
| <2 s | 64 (100%) | 271 (99.3%) | 356 (99.7%) | 286 (98.3%) | 23 (88.5%) | |
| HR | bpm | 107 [60, 176] a | 120 [60, 220] b | 124 [55, 230] b | 142 [60.0, 290] c | 150 [85.0, 260] c |
| RR | bpm | 24.0 [12, 60.0] a | 24.0 [15, 100] a | 24.0 [12, 90.0] a | 44.0 [16.0, 180] b | 40.0 [24.0, 210] b |
| SAP | mm Hg | 136 [101, 187] a | 134 [73, 206] a | 130 [79, 224] a | 140 [75, 210] a | 135 [95, 154] a |
| DAP | mm Hg | 84.0 [48, 158] a | 88.0 [48, 142] a | 87.0 [51, 150] a | 89.0 [36, 129] a | 92.0 [60, 113] a |
| MAP | mm Hg | 95.0 [65, 163] a | 98.0 [59, 147] a | 95.0 [70, 166] a | 98.0 [57, 148] a | 107 [71, 120] a |
| RT | °C | 38.0 [37.0, 40.0] a | 38.1 [35.4, 40.5] a | 38.2 [36.7, 39.7] a | 38.2 [35.0, 39.5] a | 38.1 [36.6, 39.3] a |
| FETCH-Q model | ||||||
| Control group | B1 | B2 | C | D | Class error | |
| Control group | 2 | 56 | 3 | 3 | 0 | 97% |
| B1 | 2 | 143 | 84 | 44 | 0 | 48% |
| B2 | 0 | 126 | 138 | 93 | 0 | 61% |
| C | 0 | 14 | 72 | 204 | 0 | 30% |
| D | 0 | 0 | 3 | 21 | 2 | 92% |
| Physical examination model | ||||||
| Control group | B1 | B2 | C | D | Class error | |
| Control group | 64 | 0 | 0 | 0 | 0 | 0% |
| B1 | 0 | 149 | 111 | 13 | 0 | 45% |
| B2 | 0 | 106 | 189 | 62 | 0 | 47% |
| C | 0 | 20 | 68 | 201 | 2 | 31% |
| D | 0 | 3 | 5 | 15 | 3 | 88% |
| FETCH-Q plus physical examination model | ||||||
| Control group | B1 | B2 | C | D | Class error | |
| Control group | 62 | 1 | 0 | 1 | 0 | 3% |
| B1 | 0 | 136 | 114 | 23 | 0 | 50% |
| B2 | 0 | 77 | 222 | 58 | 0 | 38% |
| C | 0 | 9 | 57 | 224 | 0 | 23% |
| D | 0 | 1 | 2 | 21 | 2 | 92% |
| Simplified Model | ||||
|---|---|---|---|---|
| Control Group | B | CD | Class Error | |
| Control group | 60 | 3 | 1 | 6% |
| B | 0 | 572 | 58 | 9% |
| CD | 0 | 84 | 232 | 27% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engel-Manchado, J.; Montoya-Alonso, J.A.; Doménech, L.; Monge-Utrilla, O.; Reina-Doreste, Y.; Matos, J.I.; Caro-Vadillo, A.; García-Guasch, L.; Redondo, J.I. Machine Learning Techniques for Canine Myxomatous Mitral Valve Disease Classification: Integrating Anamnesis, Quality of Life Survey, and Physical Examination. Vet. Sci. 2024, 11, 118. https://doi.org/10.3390/vetsci11030118
Engel-Manchado J, Montoya-Alonso JA, Doménech L, Monge-Utrilla O, Reina-Doreste Y, Matos JI, Caro-Vadillo A, García-Guasch L, Redondo JI. Machine Learning Techniques for Canine Myxomatous Mitral Valve Disease Classification: Integrating Anamnesis, Quality of Life Survey, and Physical Examination. Veterinary Sciences. 2024; 11(3):118. https://doi.org/10.3390/vetsci11030118
Chicago/Turabian StyleEngel-Manchado, Javier, José Alberto Montoya-Alonso, Luis Doménech, Oscar Monge-Utrilla, Yamir Reina-Doreste, Jorge Isidoro Matos, Alicia Caro-Vadillo, Laín García-Guasch, and José Ignacio Redondo. 2024. "Machine Learning Techniques for Canine Myxomatous Mitral Valve Disease Classification: Integrating Anamnesis, Quality of Life Survey, and Physical Examination" Veterinary Sciences 11, no. 3: 118. https://doi.org/10.3390/vetsci11030118
APA StyleEngel-Manchado, J., Montoya-Alonso, J. A., Doménech, L., Monge-Utrilla, O., Reina-Doreste, Y., Matos, J. I., Caro-Vadillo, A., García-Guasch, L., & Redondo, J. I. (2024). Machine Learning Techniques for Canine Myxomatous Mitral Valve Disease Classification: Integrating Anamnesis, Quality of Life Survey, and Physical Examination. Veterinary Sciences, 11(3), 118. https://doi.org/10.3390/vetsci11030118








