Multiple Endocrine Neoplasia with Multiple PGLs in Two Boxer Dogs: Morphological Features, Immunohistochemical Profile and SDHD Gene Mutation Screening
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Histological Processing
2.2. Immunohistochemistry
2.3. Genetic Analysis of SDHD Gene Polymorphism in Healthy and Neoplastic Tissue
3. Results
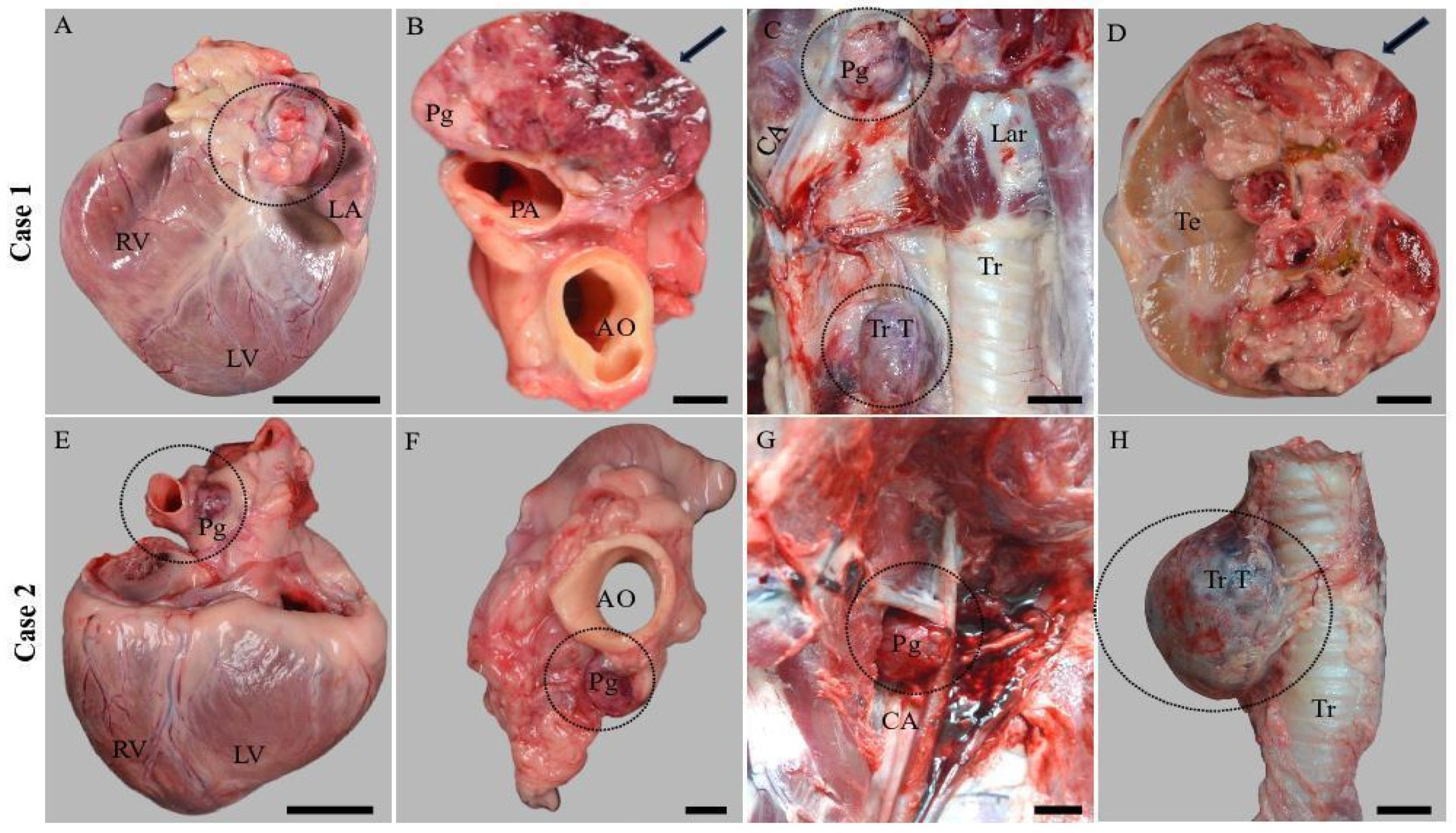
3.1. Gross Examination
3.2. Histological Examination
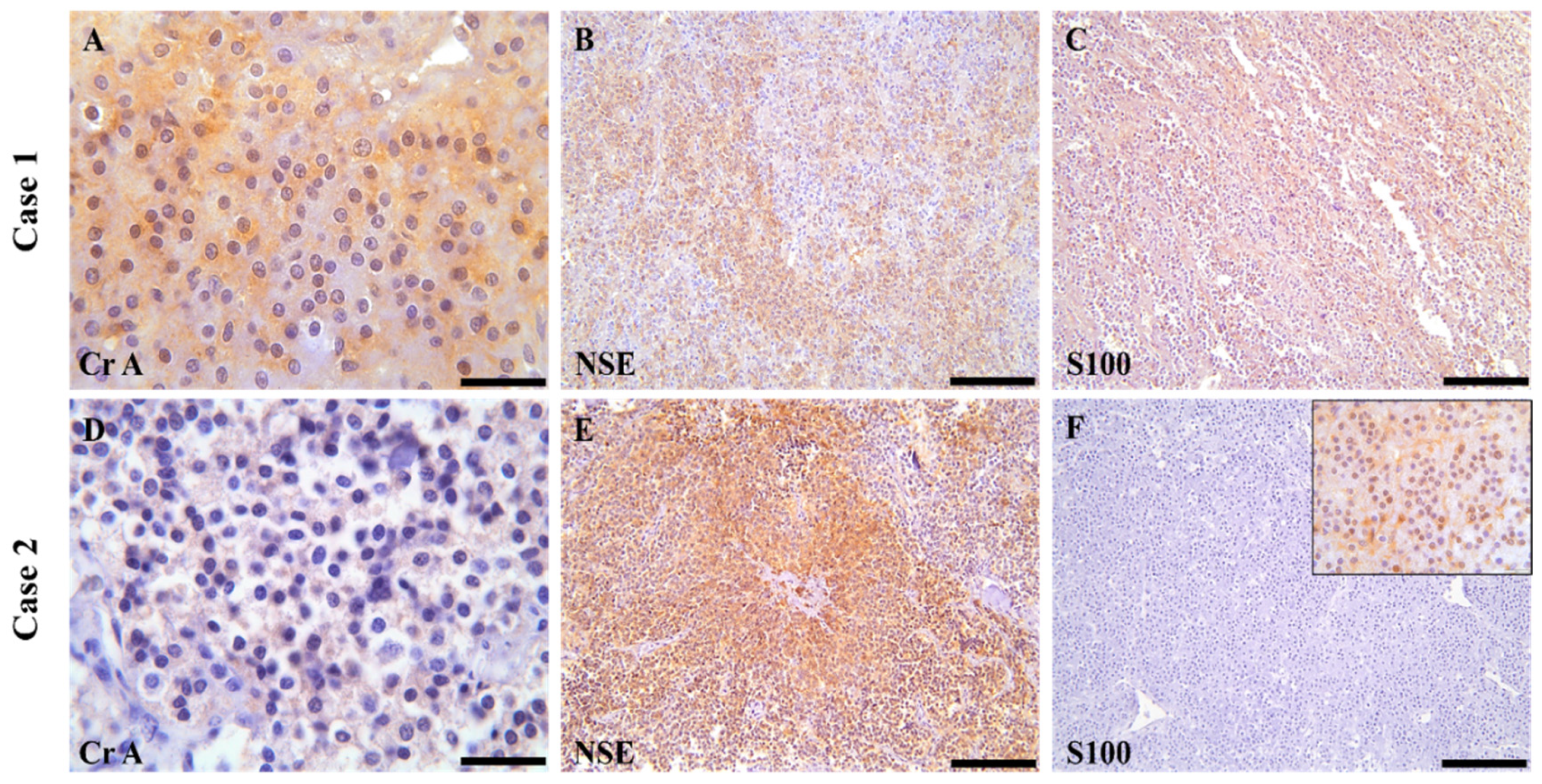
3.3. Immunohistochemical Examination
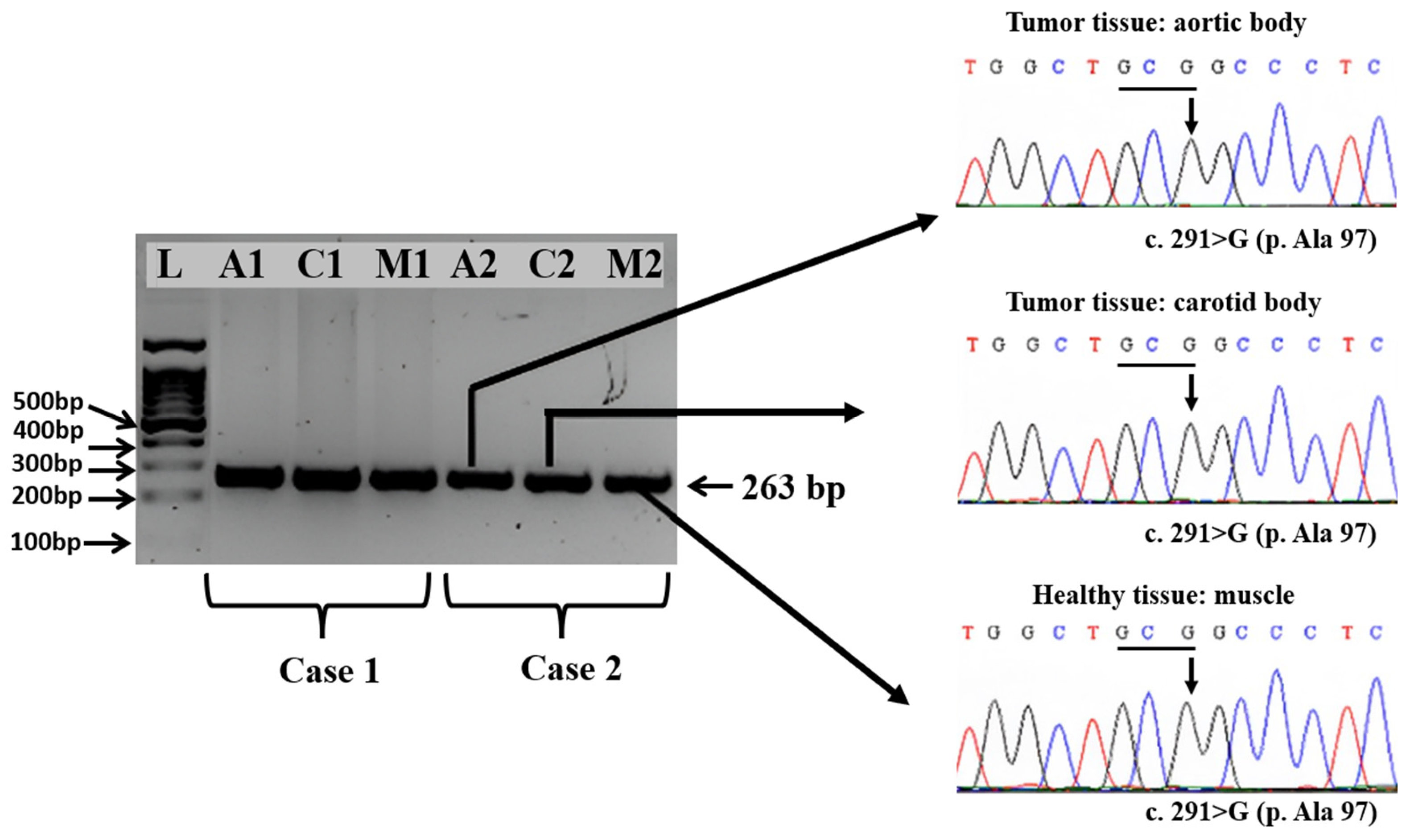
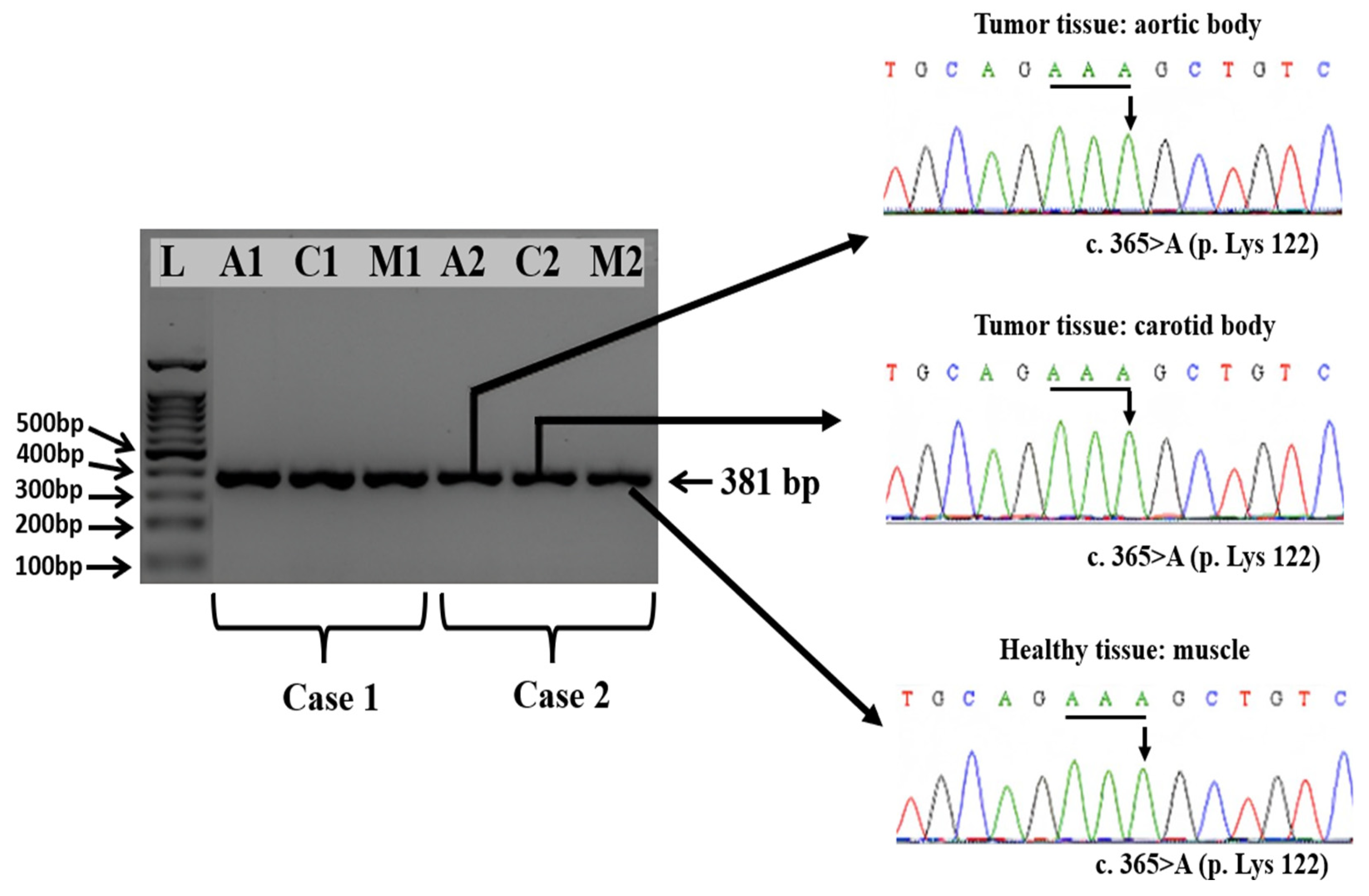
3.4. Amplification and Sequencing of Exons 2, 3, and 4 of the SDHD Gene from Healthy and Tumoral Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Aresu, L.; Tursi, M.; Iussich, S.; Guarda, F.; Valenza, F. Use of S-100 and Chromogranin A Antibodies as Immunohistochemical Markers on Detection of Malignancy in Aortic Body Tumors in Dog. J. Vet. Med. Sci. 2006, 68, 1229–1233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Salameh, A.; Cadiot, G.; Calender, A.; Goudet, P.; Chanson, P. Clinical Aspects of Multiple Endocrine Neoplasia Type 1. Nat. Rev. Endocrinol. 2021, 17, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Hensen, E.F. Head and Neck Paragangliomas: Genetics, Heredity and Clinical Characteristics; Leiden University: Leiden, The Netherlands, 2012; ISBN 9461912528. [Google Scholar]
- Lee, M.; Pellegata, N.S. Multiple Endocrine Neoplasia Type 4. Endocr. Tumor Syndr. Their Genet. 2013, 41, 63–78. [Google Scholar]
- Seabrook, A.J.; Harris, J.E.; Velosa, S.B.; Kim, E.; McInerney-Leo, A.M.; Dwight, T.; Hockings, J.I.; Hockings, N.G.; Kirk, J.; Leo, P.J. Multiple Endocrine Tumors Associated with Germline MAX Mutations: Multiple Endocrine Neoplasia Type 5? J. Clin. Endocrinol. Metab. 2021, 106, e1163–e1182. [Google Scholar] [CrossRef]
- Effraimidis, G.; Knigge, U.; Rossing, M.; Oturai, P.; Rasmussen, Å.K.; Feldt-Rasmussen, U. Multiple Endocrine Neoplasia Type 1 (MEN-1) and Neuroendocrine Neoplasms (NENs). Semin. Cancer Biol. 2022, 79, 141–162. [Google Scholar] [CrossRef]
- Mathiesen, J.S.; Effraimidis, G.; Rossing, M.; Rasmussen, Å.K.; Hoejberg, L.; Bastholt, L.; Godballe, C.; Oturai, P.; Feldt-Rasmussen, U. Multiple Endocrine Neoplasia Type 2: A Review. Semin. Cancer Biol. 2022, 79, 163–179. [Google Scholar] [CrossRef]
- Klöppel, G.; Willemer, S.; Stamm, B.; Häcki, W.H.; Heitz, P.U. Pancreatic Lesions and Hormonal Profile of Pancreatic Tumors in Multiple Endocrine Neoplasia Type I. An Immunocytochemical Study of Nine Patients. Cancer 1986, 57, 1824–1832. [Google Scholar] [CrossRef]
- Miller, A.D.; Masek-Hammerman, K.; Dalecki, K.; Mansfield, K.G.; Westmoreland, S.V. Histologic and Immunohistochemical Characterization of Pheochromocytoma in 6 Cotton-Top Tamarins (Saguinus Oedipus). Vet. Pathol. 2009, 46, 1221–1229. [Google Scholar] [CrossRef]
- De Cock, H.E.V.; MacLachlan, N.J. Simultaneous Occurrence of Multiple Neoplasms and Hyperplasias in the Adrenal and Thyroid Gland of the Horse Resembling Multiple Endocrine Neoplasia Syndrome: Case Report and Retrospective Identification of Additional Cases. Vet. Pathol. 1999, 36, 633–636. [Google Scholar] [CrossRef]
- Noszczyk-Nowak, A.; Nowak, M.; Paslawska, U.; Atamaniuk, W.; Nicpon, J. Cases with Manifestation of Chemodectoma Diagnosed in Dogs in Department of Internal Diseases with Horses, Dogs and Cats Clinic, Veterinary Medicine Faculty, University of Environmental and Life Sciences, Wroclaw, Poland. Acta Vet. Scand. 2010, 52, 35. [Google Scholar] [CrossRef]
- Blois, S.L.; Dickie, E.L.; Kruth, S.A.; Allen, D.G. Multiple Endocrine Diseases in Cats: 15 Cases (1997–2008). J. Feline Med. Surg. 2010, 12, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Dobromylskyj, M.J. Histopathological Evaluation of the Adrenal Glands in a Cat with Primary Hypoadrenocorticism and Multiple Endocrine Disease. J. Feline Med. Surg. Open Rep. 2022, 8, 20551169221125210. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Niitani, T.; Matsumoto, T.; Abe, A.; Ishida, K.; Aoki, T. Multiple Endocrine Neoplasia Type 1 with MEN1 Variant of Unknown Significance, in a Patient after the Diagnosed of Pancreatic Neuroendocrine Neoplasia. Int. Cancer Conf. J. 2024, 13, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Athey, J.M.; Vieson, M.D.; Bailey, K.; Rudmann, D.; Baumgartner, W.A.; Selting, K.A. Canine Thyroid Carcinomas: A Review with Emphasis on Comparing the Compact Subtype of Follicular Thyroid Carcinomas and Medullary Thyroid Carcinomas. Vet. Pathol. 2024, 61, 7–19. [Google Scholar] [CrossRef]
- Roccabianca, P.; Rondena, M.; Paltrinieri, S.; Pocacqua, V.; Scarpa, P.; Faverzani, S.; Scanziani, E.; Caniatti, M. Multiple Endocrine Neoplasia Type-I-like Syndrome in Two Cats. Vet. Pathol. 2006, 43, 345–352. [Google Scholar] [CrossRef]
- Lurye, J.C.; Behrend, E.N. Endocrine Tumors. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 1083–1110. [Google Scholar] [CrossRef]
- Whitlock, B.K.; Coffman, E.A.; Pugh, D.G. Diseases of the Endocrine System. In Sheep and Goat Medicine; Saunders: Maryland Heights, MO, USA, 2011; pp. 231–255. [Google Scholar]
- Rijnberk, A.; Kooistra, H.S. Clinical Endocrinology of Dogs and Cats: An Illustrated Text; Schlütersche: Hannover, Germany, 2010; ISBN 3899930916. [Google Scholar]
- Baba, A.I.; Câtoi, C. Endocrine Tumors. In Comparative Oncology; The Publishing House of the Romanian Academy: Bucharest, Romania, 2007. [Google Scholar]
- Cascón, A.; Calsina, B.; Monteagudo, M.; Mellid, S.; Díaz-Talavera, A.; Currás-Freixes, M.; Robledo, M. Genetic Bases of Pheochromocytoma and Paraganglioma. J. Mol. Endocrinol. 2023, 70, e220167. [Google Scholar] [CrossRef]
- Lefebvre, M.; Foulkes, W.D. Pheochromocytoma and Paraganglioma Syndromes: Genetics and Management Update. Curr. Oncol. 2014, 21, e8. [Google Scholar] [CrossRef]
- Hoff, A.O.; Cote, G.J.; Gagel, R.F. Multiple Endocrine Neoplasias. Annu. Rev. Physiol. 2000, 62, 377–411. [Google Scholar] [CrossRef]
- Holt, D.E.; Henthorn, P.; Howell, V.M.; Robinson, B.G.; Benn, D.E. Succinate Dehydrogenase Subunit D and Succinate Dehydrogenase Subunit B Mutation Analysis in Canine Phaeochromocytoma and Paraganglioma. J. Comp. Pathol. 2014, 151, 25–34. [Google Scholar] [CrossRef]
- Korpershoek, E.; Dieduksman, D.A.E.R.; Grinwis, G.C.M.; Day, M.J.; Reusch, C.E.; Hilbe, M.; Fracassi, F.; Krol, N.M.G.; Uitterlinden, A.G.; De Klein, A. Molecular Alterations in Dog Pheochromocytomas and Paragangliomas. Cancers 2019, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J. Succinate Dehydrogenase (SDH)-deficient Neoplasia. Histopathology 2018, 72, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Riemann, K.; Kupka, S.; Leistenschneider, P.; Sotlar, K.; Schmid, H.; Blin, N. Active Succinate Dehydrogenase (SDH) and Lack of SDHD Mutations in Sporadic Paragangliomas. Anticancer Res. 2005, 25, 2809–2814. [Google Scholar] [PubMed]
- Pacak, K. Preoperative Management of the Pheochromocytoma Patient. J. Clin. Endocrinol. Metab. 2007, 92, 4069–4079. [Google Scholar] [CrossRef]
- Aygun, N.; Uludag, M. Pheochromocytoma and Paraganglioma: From Epidemiology to Clinical Findings. Şişli Etfal Hastanesi Tip Bülteni 2020, 54, 159–168. [Google Scholar] [CrossRef]
- Buffet, A.; Burnichon, N.; Favier, J.; Gimenez-Roqueplo, A.-P. An Overview of 20 Years of Genetic Studies in Pheochromocytoma and Paraganglioma. Best. Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101416. [Google Scholar] [CrossRef]
- Tevosian, S.G.; Ghayee, H.K. Pheochromocytomas and Paragangliomas. Endocrinol. Metab. Clin. 2019, 48, 727–750. [Google Scholar] [CrossRef]
- Beatrice, L.; Boretti, F.S.; Sieber-Ruckstuhl, N.S.; Mueller, C.; Kümmerle-Fraune, C.; Hilbe, M.; Grest, P.; Reusch, C.E. Concurrent Endocrine Neoplasias in Dogs and Cats: A Retrospective Study (2004–2014). Vet. Rec. 2018, 182, 323. [Google Scholar] [CrossRef]
- Withrow, S.J.; Page, R.; Vail, D.M. Withrow and MacEwen’s Small Animal Clinical Oncology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012; ISBN 1437723624. [Google Scholar]
- Galac, S.; Korpershoek, E. Pheochromocytomas and Paragangliomas in Humans and Dogs. Vet. Comp. Oncol. 2017, 15, 1158–1170. [Google Scholar] [CrossRef]
- Bonagura, J.D.; Twedt, D.C. Kirk’s Current Veterinary Therapy XIV-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; ISBN 1437711529. [Google Scholar]
- Patnaik, A.K.; Erlandson, R.A.; Lieberman, P.H.; Welches, C.D.; Marretta, S.M. Extra-Adrenal Pheochromocytoma (Paraganglioma) in a Cat. J. Am. Vet. Med. Assoc. 1990, 197, 104–106. [Google Scholar] [CrossRef]
- Baysal, B.E.; Myers, E.N. Etiopathogenesis and Clinical Presentation of Carotid Body Tumors. Microsc. Res. Tech. 2002, 59, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Jubb, K.V.F.; Kennedy, P.C. CHAPTER 5—The Endocrine Glands. In Pathology of Domestic Animals; Jubb, K.V.F., Kennedy, P.C., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 320–353. ISBN 978-1-4832-3235-5. [Google Scholar]
- Ware, W.A.; Hopper, D.L. Cardiac Tumors in Dogs: 1982–1995. J. Vet. Intern. Med. 1999, 13, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Atasever, A.; Çam, Y. Aortic Body Tumor in a Dog. Turk. J. Vet. Anim. Sci. 2003, 27, 1241–1245. [Google Scholar]
- Kimura, N.; Watanabe, T.; Noshiro, T.; Shizawa, S.; Miura, Y. Histological Grading of Adrenal and Extra-Adrenal Pheochromocytomas and Relationship to Prognosis: A Clinicopathological Analysis of 116 Adrenal Pheochromocytomas and 30 Extra-Adrenal Sympathetic Paragangliomas Including 38 Malignant Tumors. Endocr. Pathol. 2005, 16, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kumaki, N.; Kajiwara, H.; Kameyama, K.; DeLellis, R.A.; Asa, S.L.; Osamura, R.Y.; Takami, H. Prediction of Malignant Behavior of Pheochromocytomas and Paragangliomas Using Immunohistochemical Techniques. Endocr. Pathol. 2002, 13, 149–156. [Google Scholar] [CrossRef]


| Antibody | Clone | Manufacturer | Dilution | Positive Control |
|---|---|---|---|---|
| Chromogranin A | 5H7 | Leica Biosystems Newcastle Ltd. Newcastle upon Tyne, UK | Ready to use | Endocrine pancreas |
| NSE | 22C9 | Leica Biosystems Newcastle Ltd. Newcastle upon Tyne, UK | Ready to use | Brain |
| S100 | Polyclonal | Leica Biosystems Newcastle Ltd. Newcastle upon Tyne, UK | Ready to use | Adipose tissue |
| Exon | Primers | Amplicon Size | Annealing Temperature |
|---|---|---|---|
| 2 | 2F: 5′-tgtcaggcctgttaaaagagaa-3′ 2R: 5′-cagatgtagagggccagagc-3’ | 227 bp | 60 °C |
| 3 | 3F: 5′-atgtgtgtttccccctttca-3′ 3R: 5′-atgagacaggctcacagcaa-3′ | 263 bp | 60 °C |
| 4 | 4F: 5′-tattactaggaaactcatagcccct-3′ 4R: 5′-gctgaagggcatgatagagc-3′ | 381 bp | 60 °C |
| Case | Histological Features of PGLs | Immunohistochemical Profile | SDHD Gene |
|---|---|---|---|
| Case 1 | Round to polygonal neoplastic endocrine cells arranged in the characteristic “Zellballen” pattern and separated by a fine fibrovascular stroma | PgA-Cr A(+); S 100(+), NSE (+) | |
| PgC-Cr A(+); S 100(+/−), NSE (+) | Exon 2: WT | ||
| Case 2 | PgA-Cr A(+); S 100(−), NSE (+) | Exon 3: WT | |
| PgA-Cr A(+); S 100(+), NSE (+) | Exon 4: WT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semzenisi, E.; Popa, R.; Toma, C.; Bâlteanu, V.-A.; Scurtu, I.C.; Pop, R.; Tăbăran, A.-F. Multiple Endocrine Neoplasia with Multiple PGLs in Two Boxer Dogs: Morphological Features, Immunohistochemical Profile and SDHD Gene Mutation Screening. Vet. Sci. 2024, 11, 586. https://doi.org/10.3390/vetsci11110586
Semzenisi E, Popa R, Toma C, Bâlteanu V-A, Scurtu IC, Pop R, Tăbăran A-F. Multiple Endocrine Neoplasia with Multiple PGLs in Two Boxer Dogs: Morphological Features, Immunohistochemical Profile and SDHD Gene Mutation Screening. Veterinary Sciences. 2024; 11(11):586. https://doi.org/10.3390/vetsci11110586
Chicago/Turabian StyleSemzenisi, Ecaterina, Roxana Popa, Corina Toma, Valentin-Adrian Bâlteanu, Iuliu Calin Scurtu, Romelia Pop, and Alexandru-Flaviu Tăbăran. 2024. "Multiple Endocrine Neoplasia with Multiple PGLs in Two Boxer Dogs: Morphological Features, Immunohistochemical Profile and SDHD Gene Mutation Screening" Veterinary Sciences 11, no. 11: 586. https://doi.org/10.3390/vetsci11110586
APA StyleSemzenisi, E., Popa, R., Toma, C., Bâlteanu, V.-A., Scurtu, I. C., Pop, R., & Tăbăran, A.-F. (2024). Multiple Endocrine Neoplasia with Multiple PGLs in Two Boxer Dogs: Morphological Features, Immunohistochemical Profile and SDHD Gene Mutation Screening. Veterinary Sciences, 11(11), 586. https://doi.org/10.3390/vetsci11110586






