Development and Application of a TaqMan RT-qPCR for the Detection of Foot-and-Mouth Disease Virus in Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Materials
2.2. Nucleic Acid Extraction and Reverse Transcription
2.3. Primers and Probes
2.4. Construction and Characterization of Standard Plasmids
2.5. Preliminary Establishment of FDMV RT-qPCR
2.6. Optimization of RT-qPCR Reaction System
2.7. Establishment of the Standard Curve of RT-qPCR
2.8. Limit of Detection Determination
2.9. Specificity of FMDV RT-qPCR
2.10. Repeatability Test
2.11. Interference Assay
2.12. Detection of Clinical Samples
3. Results
3.1. Identification of Recombinant Plasmids
3.2. Primer Probe Selection
3.3. RT-qPCR Reaction System Optimization
3.4. Establishment of the Standard Curve
3.5. Sensitivity Test of FMDV RT-qPCR
3.6. Specificity of the Detection System
3.7. Repeatability Test of FMDV RT-qPCR
3.8. Interference Test of FMDV RT-qPCR
3.9. Clinical Sample Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, Y.J.; Lee, K.K.; Kim, J.W.; Chung, K.H.; Kim, S.J.; Yun, W.S.; Lee, C.S. Effective Diagnosis of Foot-And-Mouth Disease Virus (FMDV) Serotypes O and A Based on Optical and Electrochemical Dual-Modal Detection. Biomolecules 2021, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.E.; Burman, A.; Asfor, A.; Brocchi, E.; Grazioli, S.; Browning, C.; Ludi, A.; Tuthill, T.J.; King, D.P. Avidity of Polyclonal Antibodies to Foot-and-Mouth Disease Virus in Bovine Serum Measured Using Bio-Layer Interferometry. Viruses 2022, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Pega, J.; Di Giacomo, S.; Bucafusco, D.; Schammas, J.M.; Malacari, D.; Barrionuevo, F.; Capozzo, A.V.; Rodríguez, L.L.; Borca, M.V.; Pérez-Filgueira, M. Systemic Foot-and-Mouth Disease Vaccination in Cattle Promotes Specific Antibody-Secreting Cells at the Respiratory Tract and Triggers Local Anamnestic Responses upon Aerosol Infection. J. Virol. 2015, 89, 9581–9590. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Tummala, T.; Elliott, R.; Jain, V.; Brantley, W.; Hadorn, L.; Santra, S. Multimodal Magneto-Fluorescent Nanosensor for Rapid and Specific Detection of Blood-Borne Pathogens. ACS Appl. Nano Mater. 2019, 2, 5587–5593. [Google Scholar] [CrossRef] [PubMed]
- Arzt, J.; Belsham, G.J.; Lohse, L.; Bøtner, A.; Stenfeldt, C. Transmission of Foot-and-Mouth Disease from Persistently Infected Carrier Cattle to Naive Cattle via Transfer of Oropharyngeal Fluid. mSphere 2018, 3, e00365-18. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Yang, F.; Cao, W.; Tian, H.; Zhang, K.; Zheng, H.; Liu, X. Review of Seneca Valley Virus: A Call for Increased Surveillance and Research. Front. Microbiol. 2018, 9, 940. [Google Scholar] [CrossRef]
- Robinson, L.; Windsor, M.; McLaughlin, K.; Hope, J.; Jackson, T.; Charleston, B. Foot-and-mouth disease virus exhibits an altered tropism in the presence of specific immunoglobulins, enabling productive infection and killing of dendritic cells. J. Virol. 2011, 85, 2212–2223. [Google Scholar] [CrossRef]
- Xu, J.; Qian, P.; Wu, Q.; Liu, S.; Fan, W.; Zhang, K.; Wang, R.; Zhang, H.; Chen, H.; Li, X. Swine interferon-induced transmembrane protein, sIFITM3, inhibits foot-and-mouth disease virus infection in vitro and in vivo. Antivir. Res. 2014, 109, 22–29. [Google Scholar] [CrossRef]
- Banda, F.; Sinkala, Y.; Mataa, L.; Lebea, P.; Sikombe, T.; Kangwa, H.L.; Fana, E.M.; Mokopasetso, M.; Wadsworth, J.; Knowles, N.J.; et al. Characterization of Foot-and-Mouth Disease Viruses in Zambia-Implications for the Epidemiology of the Disease in Southern Africa. Viruses 2021, 13, 2195. [Google Scholar] [CrossRef]
- Bari, F.D.; Parida, S.; Asfor, A.S.; Haydon, D.T.; Reeve, R.; Paton, D.J.; Mahapatra, M. Prediction and characterization of novel epitopes of serotype A foot-and-mouth disease viruses circulating in East Africa using site-directed mutagenesis. J. Gen. Virol. 2015, 96, 1033–1041. [Google Scholar] [CrossRef]
- Kotecha, A.; Zhang, F.; Juleff, N.; Jackson, T.; Perez, E.; Stuart, D.; Fry, E.; Charleston, B.; Seago, J. Application of the thermofluor PaSTRy technique for improving foot-and-mouth disease virus vaccine formulation. J. Gen. Virol. 2016, 97, 1557–1565. [Google Scholar] [CrossRef]
- Bronsvoort, B.M.; Parida, S.; Handel, I.; McFarland, S.; Fleming, L.; Hamblin, P.; Kock, R. Serological survey for foot-and-mouth disease virus in wildlife in eastern Africa and estimation of test parameters of a nonstructural protein enzyme-linked immunosorbent assay for buffalo. Clin. Vaccine Immunol. CVI 2008, 15, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhmendak, D.; Mioulet, V.; King, D.P.; Burman, A.; Nfon, C.K. Combining a Universal Capture Ligand and Pan-Serotype Monoclonal Antibody to Develop a Pan-Serotype Lateral Flow Strip Test for Foot-and-Mouth Disease Virus Detection. Viruses 2022, 14, 785. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, P.; Li, Y.; Song, Y.; Liu, X.; Feng, N.; Jin, H.; Bai, Y.; Zhang, H.; Li, Y.; et al. A Visual Assay of a Loop-Mediated Isothermal Amplification Based Vertical Immunoassay for SARS-CoV-2 RNA Detection. Front. Microbiol. 2022, 13, 932698. [Google Scholar] [CrossRef]
- Muth, D.; Corman, V.M.; Meyer, B.; Assiri, A.; Al-Masri, M.; Farah, M.; Steinhagen, K.; Lattwein, E.; Al-Tawfiq, J.A.; Albarrak, A.; et al. Infectious Middle East Respiratory Syndrome Coronavirus Excretion and Serotype Variability Based on Live Virus Isolates from Patients in Saudi Arabia. J. Clin. Microbiol. 2015, 53, 2951–2955. [Google Scholar] [CrossRef]
- Tiwari, S.; Dhole, T.N. Assessment of enteroviruses from sewage water and clinical samples during eradication phase of polio in North India. Virol. J. 2018, 15, 157. [Google Scholar] [CrossRef]
- Ren, N.; Jinli, J.; Chen, Y.; Zhou, X.; Wang, J.; Ge, P.; Khan, F.A.; Zhang, L.; Hu, C.; Robertson, I.D. Identification of new diagnostic biomarkers for Mycobacterium tuberculosis and the potential application in the serodiagnosis of human tuberculosis. Microb. Biotechnol. 2018, 11, 893–904. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, Y.; Zeng, S.; Liu, X.; Li, Y.; Li, X.; Chen, W.; Li, Z.; Qin, Y.; Chen, J. Development and Application of RAA Nucleic Acid Test Strip Assay and Double RAA Gel Electrophoresis Detection Methods for ASFV and CSFV. Front. Mol. Biosci. 2021, 8, 811824. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Remarque, E.J.; Mortier, D.; Montomoli, E. Comparison of hemagglutination inhibition, single radial hemolysis, virus neutralization assays, and ELISA to detect antibody levels against seasonal influenza viruses. Influenza Other Respir. Viruses 2018, 12, 675–686. [Google Scholar] [CrossRef]
- Bivona, A.E.; Czentner, L.; Sanchez Alberti, A.; Cerny, N.; Cardoso Landaburu, A.C.; Nevot, C.; Estévez, O.; Marco, J.D.; Basombrio, M.A.; Malchiodi, E.L.; et al. Recombinant Cysteine Proteinase B from Leishmania braziliensis and Its Domains: Promising Antigens for Serodiagnosis of Cutaneous and Visceral Leishmaniasis in Dogs. J. Clin. Microbiol. 2019, 57, e00819-19. [Google Scholar] [CrossRef]
- Liu, L.; Liu, W.; Zheng, Y.; Jiang, X.; Kou, G.; Ding, J.; Wang, Q.; Huang, Q.; Ding, Y.; Ni, W.; et al. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes Infect. 2020, 22, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wan, Y.; Wu, X.; Zhang, Y.; Li, J.; Cui, T.; Sun, H.; Cui, H.; He, K.; Hui, G.; et al. Hapten designs based on aldicarb for the development of a colloidal gold immunochromatographic quantitative test strip. Front. Nutr. 2022, 9, 976284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gao, Q.; Wang, T.; Ke, Y.; Mo, F.; Jia, R.; Liu, W.; Liu, L.; Zheng, S.; Liu, Y.; et al. Development and evaluation of a serological test for diagnosis of COVID-19 with selected recombinant spike proteins. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Um, J.; Antigua, K.J.C.; Park, J.H.; Kim, Y.; Oh, S.; Kim, Y.I.; Choi, W.S.; Kim, S.G.; Jeong, J.H.; et al. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 998–1007. [Google Scholar] [CrossRef]
- Englezou, P.C.; Sapet, C.; Démoulins, T.; Milona, P.; Ebensen, T.; Schulze, K.; Guzman, C.A.; Poulhes, F.; Zelphati, O.; Ruggli, N.; et al. Self-Amplifying Replicon RNA Delivery to Dendritic Cells by Cationic Lipids. Mol. Ther. Nucleic Acids 2018, 12, 118–134. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, L.; Liu, C.; Ye, S.; Chen, W.; Li, D.; Huang, W. One-tube SARS-CoV-2 detection platform based on RT-RPA and CRISPR/Cas12a. J. Transl. Med. 2021, 19, 74. [Google Scholar] [CrossRef]
- Baloch, A.S.; Liu, C.; Liang, X.; Liu, Y.; Chen, J.; Cao, R.; Zhou, B. Avian Flavivirus Enters BHK-21 Cells by a Low pH-Dependent Endosomal Pathway. Viruses 2019, 11, 1112. [Google Scholar] [CrossRef]
- Hashem, M.A.; Maetani, F.; Kayesh, M.E.H.; Eiei, T.; Mochizuki, K.; Ito, A.; Sakurai, H.; Asai, T.; Tsukiyama-Kohara, K. Transmission of Koala Retrovirus from Parent Koalas to a Joey in a Japanese Zoo. J. Virol. 2020, 94, e00019-20. [Google Scholar] [CrossRef]
- Ruan, H.T.; Wang, R.L.; Li, H.T.; Liu, L.; Kuang, T.X.; Li, M.; Zou, K.S. Effects of sampling strategies and DNA extraction methods on eDNA metabarcoding: A case study of estuarine fish diversity monitoring. Zool. Res. 2022, 43, 192–204. [Google Scholar] [CrossRef]
- Guo, L.; Yang, S.L.; Wang, C.D.; Hou, R.; Chen, S.J.; Yang, X.N.; Liu, J.; Pan, H.B.; Hao, Z.X.; Zhang, M.L.; et al. Phylogenetic analysis of the haemagglutinin gene of canine distemper virus strains detected from giant panda and raccoon dogs in China. Virol. J. 2013, 10, 109. [Google Scholar] [CrossRef]
- Yi, J.; Wang, N.; Wu, J.; Tang, Y.; Zhang, J.; Zhu, L.; Rui, X.; Guo, Y.; Xu, Y. Development of a Droplet Digital Polymerase Chain Reaction for Sensitive Detection of Pneumocystis jirovecii in Respiratory Tract Specimens. Front. Med. 2021, 8, 761788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Das, A.; Zheng, W.; Porter, E.; Xu, L.; Noll, L.; Liu, X.; Dodd, K.; Jia, W.; Bai, J. Development and evaluation of multiplex real-time RT-PCR assays for the detection and differentiation of foot-and-mouth disease virus and Seneca Valley virus 1. Transbound. Emerg. Dis. 2020, 67, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Biswal, J.K.; Ranjan, R.; Mohapatra, J.K.; Rout, M.; Joshi, H.R.; Singh, R.P. Development of TaqMan Probe-Based One-Step RT-qPCR Assay Targeting 2B-NSP Coding Region for Diagnosis of Foot-and-Mouth Disease in India. Curr. Microbiol. 2023, 80, 245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.l.; Wang, Y.; Fang, J.C.q.P.F.Y.Y.Z.h. Establishment of a TaqMan probe-based qPCR assay for detecting microsporidia Enterospora epinepheli in grouper. J. Fish Dis. 2024, 47, e13893. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, G.; Liu, X.; Wu, W.; Yu, T.; Zhang, W.; Liu, X.; Cheng, G.; Wei, L.; Ni, L.; et al. Establishment and application of a quadruplex real-time RT-qPCR assay for differentiation of TGEV, PEDV, PDCoV, and PoRVA. Microb. Pathog. 2024, 191, 106646. [Google Scholar] [CrossRef]
- Alonso-Rebollo, A.; Ramos-Gómez, S.; Busto, M.D.; Ortega, N. Development and optimization of an efficient qPCR system for olive authentication in edible oils. Food Chem. 2017, 232, 827–835. [Google Scholar] [CrossRef]
- Pinheiro-de-Oliveira, T.F.; Fonseca, A.A., Jr.; Camargos, M.F.; Laguardia-Nascimento, M.; de Oliveira, A.M.; Cottorello, A.C.P.; Goes-Neto, A.; Barbosa-Stancioli, E.F. Development of a droplet digital RT-PCR for the quantification of foot-and-mouth virus RNA. J. Virol. Methods 2018, 259, 129–134. [Google Scholar] [CrossRef]
- El Bagoury, G.F.; Elhabashy, R.; Mahmoud, A.H.; Hagag, N.M.; El Zowalaty, M.E. Development and evaluation of one-step real-time RT-PCR assay for improved detection of foot-and-mouth disease virus serotypes circulating in Egypt. J. Virol. Methods 2022, 306, 114525. [Google Scholar] [CrossRef]
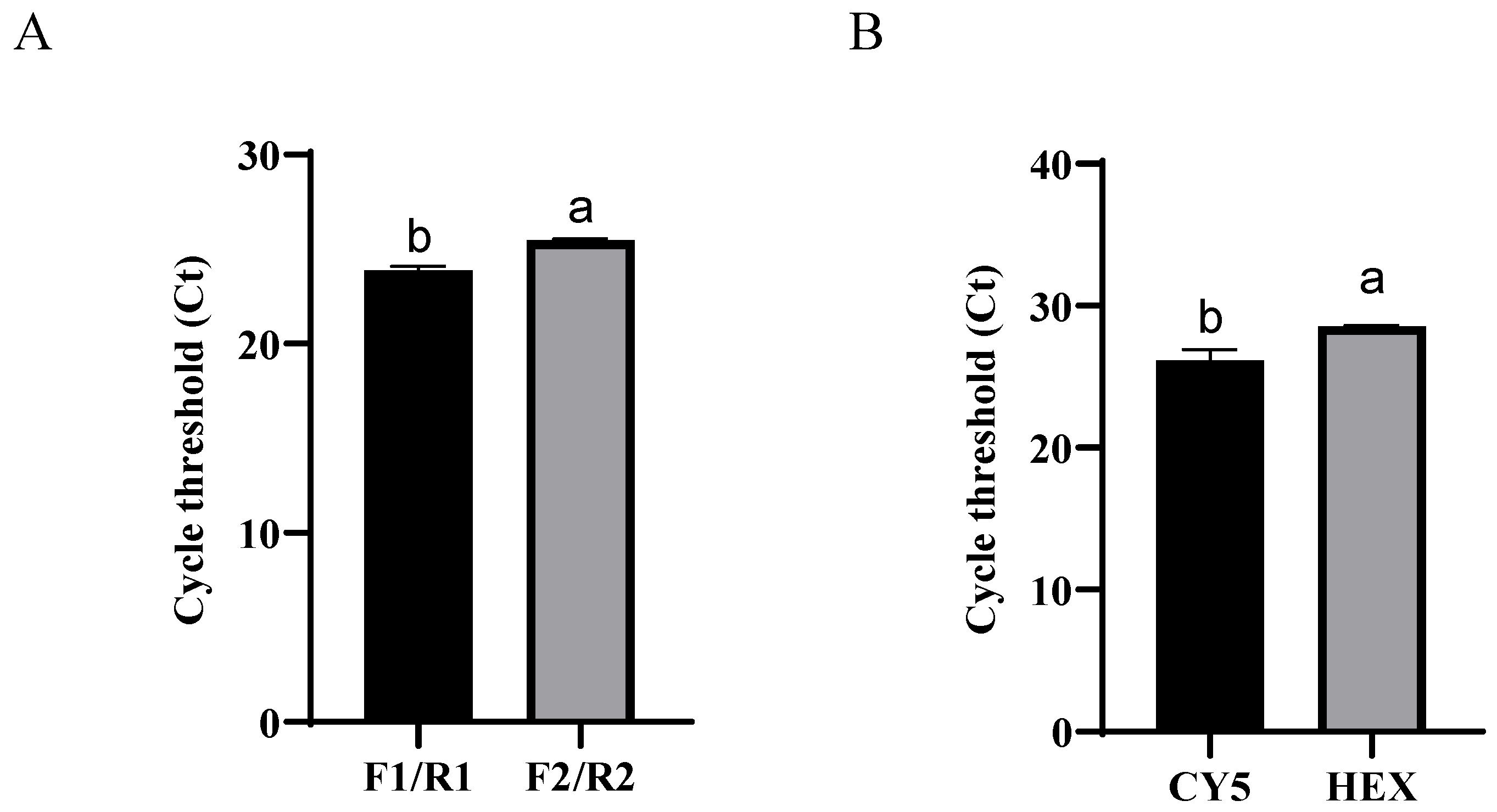



| Primer/Probe | Sequences (5′-3′) | Length (bp) | Product Length (bp) |
|---|---|---|---|
| FMVD-F1 | CCTCAAAAGACACTTCCACATGG | 23 | 99 |
| FMVD-R1 | CTCTCCTTTGCACGCCGTG | 19 | |
| FMVD-F2 | GAAGGACCTTACGAGGGACC | 20 | 124 |
| FMVD-R2 | CGACTTGCAAAAGATGGTCATGGG | 24 | |
| FMVD-P1 | HEX-CGAGGGTCTTCGAGGCCATCACAGGT-TAMRA | 26 | 99 |
| FMVD-P2 | CY5-CGAGGGTCTTCGAGGCCATCACAGGT-BHQ3 |
| Factor | Parameters | Plasmid Concentration (Copies/µL) | ||
|---|---|---|---|---|
| 6.43 × 104 | 6.43 × 105 | 6.43 × 106 | ||
| Primer concentration (µM) | 50 | 33.69 ± 0.55 | 29.76 ± 0.45 | 25.43 ± 0.32 |
| 70 | 33.34 ± 0.41 | 30.11 ± 0.05 | 25.15 ± 0.24 | |
| 90 | 34.74 ± 0.69 | 31.24 ± 0.16 | 25.82 ± 0.25 | |
| 100 | 33.61 ± 1.48 | 30.09 ± 0.20 | 25.59 ± 0.44 | |
| Probe concentration (µM) | 10 | 33.61 ± 0.62 | 28.63 ± 0.33 | 24.43 ± 0.23 |
| 20 | 34.20 ± 0.94 | 29.78 ± 0.29 | 25.81 ± 0.78 | |
| 30 | 34.20 ± 0.94 | 29.78 ± 0.29 | 25.81 ± 0.78 | |
| 50 | 33.54 ± 0.34 | 29.77 ± 0.66 | 24.69 ± 0.13 | |
| Annealing temperature (℃) | 58 | 24.98 ± 0.03 | 31.58 ± 0.99 | 35.12 ± 0.89 |
| 60 | 25.15 ± 0.07 | 30.88 ± 0.73 | 34.21 ± 0.35 | |
| 0.35 | 62 | 24.80 ± 0.01 | 30.36 ± 0.66 | 34.09 ± 0.39 |
| Concentrations of Standard Plasmid | Within-Group Variance | Between-Group Variance | ||
|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | |
| 6.43 × 107 copies/µL | 14.74 ± 0.26 | 1.78 | 14.85 ± 0.14 | 0.97 |
| 6.43 × 105 copies/µL | 24.82 ± 0.27 | 1.07 | 24.99 ± 0.19 | 0.75 |
| 6.43 × 103 copies/µL | 32.65 ± 0.24 | 0.74 | 32.32 ± 0.23 | 0.70 |
| Interference Reagents | Name | Ct (Mean ± SD) | CV (%) |
|---|---|---|---|
| Exogenous and endogenous interference with material | Blood of sheep | 25.42 ± 1.44 | 5.67 |
| Liver tissues | 23.44 ± 0.79 | 4.45 | |
| Intestinal contents | 21.42 ± 0.72 | 0.55 | |
| Lung tissues | 22.18 ± 0.62 | 1.71 | |
| Milk | 23.84 ± 0.94 | 3.35 | |
| Mucin | 21.94 ± 0.45 | 3.38 | |
| Oral swab | 22.48 ± 1.00 | 2.79 | |
| Manure | 24.31 ± 0.42 | 3.94 | |
| Animal feed | 21.01 ± 0.12 | 2.06 | |
| Reagent 1 a | 21.93 ± 0.73 | 3.40 | |
| Reagent 2 b | 22.49 ± 0.65 | 2.90 | |
| Reagent 3 c | 23.81 ± 0.67 | 2.81 | |
| Reagent 4 d | 22.81 ± 1.22 | 5.35 | |
| Positive control | Standard plasmid | 22.59 ± 1.39 | 6.13 |
| Negative control | ddH2O | N/A e | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, C.; Xiao, X.; Wang, M.; Sun, Y.; Jin, H.; Zhang, Y.; Zhao, H.; Cao, Q.; Yang, Y.; Yin, R. Development and Application of a TaqMan RT-qPCR for the Detection of Foot-and-Mouth Disease Virus in Pigs. Vet. Sci. 2024, 11, 541. https://doi.org/10.3390/vetsci11110541
Dong C, Xiao X, Wang M, Sun Y, Jin H, Zhang Y, Zhao H, Cao Q, Yang Y, Yin R. Development and Application of a TaqMan RT-qPCR for the Detection of Foot-and-Mouth Disease Virus in Pigs. Veterinary Sciences. 2024; 11(11):541. https://doi.org/10.3390/vetsci11110541
Chicago/Turabian StyleDong, Changying, Xingyu Xiao, Meiqi Wang, Yajuan Sun, Hui Jin, Yongzhe Zhang, Hongri Zhao, Qianyue Cao, Yanran Yang, and Rui Yin. 2024. "Development and Application of a TaqMan RT-qPCR for the Detection of Foot-and-Mouth Disease Virus in Pigs" Veterinary Sciences 11, no. 11: 541. https://doi.org/10.3390/vetsci11110541
APA StyleDong, C., Xiao, X., Wang, M., Sun, Y., Jin, H., Zhang, Y., Zhao, H., Cao, Q., Yang, Y., & Yin, R. (2024). Development and Application of a TaqMan RT-qPCR for the Detection of Foot-and-Mouth Disease Virus in Pigs. Veterinary Sciences, 11(11), 541. https://doi.org/10.3390/vetsci11110541




