1. Introduction
Environmental heat stress is a growing concern within the poultry industry. Broilers subjected to environmental heat stress were shown to experience hyperthermia along with a reduction in feed intake, nutrient absorption, and growth rate, as well as an increase in water intake, morbidity, and mortality [
1,
2,
3,
4]. The intestinal dysfunction associated with environmental heat stress involves oxidative stress, immune injury, and a weakening of the gut barriers. Particularly, the disruption of tight junction proteins within the gut increases permeability and allows for paracellular translocation of toxins and bacteria inside the body [
3,
4,
5,
6,
7]. Moreover, reduced secretory IgA content and number of epithelial lymphocytes in the duodenal, jejunal, and ileal mucosa further contribute to the reduction in immune protection at the gut barrier in broilers subjected to heat stress [
8,
9,
10]. The physiological stress placed upon the chickens by environmental heat stress may also account for the observed negative effects of heat stress on immune system development and function. Specifically, systemic effects of environmental heat stress on the chicken’s immune system include reductions in relative weights of primary and secondary lymphoid organs, antibody responses to SRBC, concentrations of IgA and cytokines in the blood, and innate immune activities, as indicated by differential protein expression profiles in spleens [
11,
12,
13,
14].
Although general suppression of the chicken’s immune system by environmental heat stress has been identified, there is a current lack of information concerning heat-stress effects on the inflammatory response to microbial infections. In general terms, the inflammatory response is defined as the recruitment of leukocytes, plasma proteins, and fluids from the blood to the affected tissue. Inflammatory responses that quickly recruit large numbers of appropriate leukocytes, activate antimicrobial activities, eliminate or contain the infection, and reestablish homeostasis, constitute a critically important mechanism of the innate immune system [
15,
16,
17].
Lipopolysaccharide (LPS), a cell wall component of Gram-negative bacteria and a potent inducer of an inflammatory response, is commonly used to evaluate innate immune response capabilities. In chickens, a one-time administration of LPS into a complex tissue, like the dermis of the skin, will typically result in vascular changes facilitating the rapid recruitment of heterophils from the blood. The influx of heterophils is accompanied and followed by monocyte/macrophage infiltration. The recruited leukocytes are stimulated to carry out various antimicrobial activities, including generating reactive oxygen species, expressing inflammatory cytokines and chemokines, activating enzymes, and producing various bactericidal substances. The local acute inflammatory response initiated by LPS is also associated with changes in peripheral blood cell and protein profiles [
18,
19,
20,
21,
22].
French et al. [
23] recently reported on a “two-window approach” to study the local and systemic acute inflammatory responses to intradermal (i.d.) injection of LPS in individual broilers [
23]. This approach involved minimally invasive techniques, allowing for repeated and concurrent sampling of an individual’s tissue and peripheral blood for ex vivo analyses. Specifically, in French et al. [
23], LPS was i.d. injected into the pulp of multiple growing feathers (GF) on each broiler [
24,
25]. GF and blood were collected before (0 h) and at 6 and 24 h post-GF-pulp injection. Laboratory analyses of collected samples revealed extensive recruitment of heterophils and monocytes/macrophages into the dermis of injected GF, reaching peak levels at 6 and 24 h post-injection, respectively. Local cellular activities in GF-pulps included the generation of reactive oxygen species (ROS), mRNA expression of inflammatory cytokines (e.g., interleukin-1 (IL-1), IL-6, IL-8), and antioxidant enzyme activity. In the blood, the concentrations of heterophils were elevated at 6 h and returned to baseline levels by 24 h, whereas lymphocyte concentrations dropped at 6 h and returned to pre-injection levels by 24 h [
23]. Overall, this model has proven effective in monitoring local and systemic inflammatory responses to microbes.
The objective of the current study was to examine the effects of cyclic, environmental heat stress on the local (GF-pulp) and systemic (blood) acute inflammatory responses to LPS injected into the dermis of GF-pulps in broilers reared at thermoneutral (TN) or cyclic heat stress (HS) temperatures. For TN-broilers, TN conditions followed step-down, standard industry temperature settings. From Day 4 onward, HS-broilers were subjected to cyclic environmental heat stress simulating hot summer days, with 35 °C for 14 h and TN-temperatures for the remaining hours of each day. The inflammatory response was induced by i.d. GF-pulp injections of LPS when the broilers were 37 days of age. GF and blood were collected before (0 h) and at 6 and 24 h post-injection for laboratory analyses. Aspects examined in GF-pulps included relative amounts and types of leukocyte populations present, ROS generation, and relative cytokine mRNA expression, and in blood, concentrations of various cell populations and plasma alpha-1 glycoprotein (AGP-1), an acute phase protein. The responses to i.d. GF-pulp injection of endotoxin-free PBS (vehicle injection control) were also examined in TN- and HS-broilers.
2. Materials and Methods
2.1. Experimental Animals and Rearing Conditions
Newly hatched Cobb 500 broiler chicks were tagged at hatch. Based on tag number, the chicks were then randomly assigned to eight chambers of the University of Arkansas System, Division of Agriculture’s (UADA) Poultry Environmental Research Laboratory (PERL) in Fayetteville, Arkansas. The eight environmental chambers were then randomly assigned to TN (4) or HS (4) temperature conditions, and each chamber was evenly split into two pens to produce eight pens per temperature treatment (16 pens total). Twenty-three birds were placed into each pen, on wood shavings with 10 birds/m2 stocking density. All protocols and procedures involving animals used in this trial were approved by the UA Institutional Animal Care and Use Committee (IACUC protocol #21018).
From Day 0 to 3, all chicks were reared at 32 °C. A standard industry step-down procedure was followed for TN temperature conditions, i.e., Day 4 to 6, 31 °C; Day 7 to 10, 29 °C; Day 11–14, 26 °C; and Day 15 onwards, 24 °C. From Day 4 onwards, HS chicks were subjected daily to 35 °C from 8 am to 10 pm (HS temperature; 14 h) and to TN temperature from 10 pm to 8 am (10 h), while TN chicks were kept at TN temperatures 24 h a day. For all treatment groups, diets and lighting schedules followed industry standards for broilers (i.e., Starter diet Day 0 to 10, Grower diet Day 11 to 28, and Finisher diet Day 28–42; 24 h of light Day 0 to 1; 23 h of light with 1 h of dark Day 2 to 7; 20 h of light with 4 h of dark Day 8 to 14; and 18 h of light with 6 h of dark Day 15 to 42).
2.2. Experimental Induction of the Inflammatory Response
At 18 days of age, four treatment groups were formed based on future GF-pulp injection of either
Salmonella typhimurium lipopolysaccharide (LPS; Sigma Chemical Company Saint Louis, MO, USA) prepared in endotoxin-free Dulbecco’s phosphate-buffered saline (PBS; Sigma, catalog number L6511) or PBS vehicle, and either TN or HS temperature conditions (i.e., TN-LPS, HS-LPS, TN-PBS and HS-PBS). Three broilers were randomly selected from each chamber, two for LPS (one per pen) and one for PBS (vehicle) injection, resulting in eight birds for each TN-LPS and HS-LPS, and four birds for each TN-PBS and HS-PBS treatment groups. A row of eight GFs were then plucked from each breast tract of the broilers to yield uniform, 19-day-old, regenerated GFs for injection when the broilers were 37 days of age. On Day 37, the pulp of 6 regenerated GFs from each breast tract (12 GF total/broiler) of TN-LPS and HS-LPS broilers were injected with 10 μL LPS (100 μg/mL; 1 μg/GF; 12 μg per broiler) or 10 μL of PBS for the LPS and PBS treatment groups, respectively, as described in [
26].
2.3. Blood and Growing Feather (GF) Sample Collections
One mL of heparinized blood and six GFs were collected from each bird before (0 h) and at 6 and 24 h post-intradermal (i.d.) GF-pulp injection. At each time-point, two of the collected GF were used to prepare pulp cell suspensions, while four GFs were snap-frozen in liquid nitrogen and stored at −80 °C. Blood was used to prepare blood smears and diluted blood cell suspensions, and to isolate plasma. Plasma was stored at −80 °C [
26].
2.4. Preparation of GF Pulp- and Blood-Cell Suspensions
Pulp cell suspensions were prepared as described previously [
23,
25]. Briefly, for each bird and time point, the entire pulp of two GFs was pulled out of the sheath and placed in 1 mL PBS containing 0.1% collagenase type IV (Life Technologies, Carlsbad, CA, USA) and 0.1% dispase II (Boehringer Mannheim, Mannheim, Germany). After a 10 min incubation at 37 °C, the pulps were gently pushed through a 60 μm nylon mesh while continuously adding ice-cold PBS. The pulp cell suspensions were washed twice in PBS at 250×
g for 4 min at 4 °C, and the pellet resuspended to a final volume of 0.5 mL with PBS. Cell suspensions were kept on ice until use for immunofluorescent staining or determination of ROS generation by kinetic fluorescence assay.
Heparinized blood was diluted 50-fold by adding 20 μL blood to 980 μL of PBS+ staining buffer (PBS, 1% bovine serum albumin, 0.1% sodium azide) [
27].
2.5. Immunofluorescent Staining and GF-Pulp- and Blood-Cell Population Analyses
Leukocyte populations in the GF-pulp and blood-cell suspensions were identified using direct, two- and three-color, immunofluorescent staining procedures, as described in [
23,
25]. A cocktail of mouse monoclonal antibodies (mAbs) to chicken CD41/61 conjugated to fluorescein isothiocyanate (FITC), KUL01 conjugated with phycoerythrin (PE), and CD45 conjugated to spectral red (SPRD) was used to identify thrombocytes, monocytes/macrophages, and total leukocytes, respectively. Another cocktail consisted of Bu-1-PE and CD3-SPRD mAbs to identify chicken B- and T-lymphocytes, respectively. Heterophils were identified based on size and internal complexity characteristics (forward and side scatter, respectively) of CD45
+ pulp and blood leukocytes, whereby for blood, total leukocytes were defined as CD45
+CD41/61
− cells as thrombocytes express low levels of CD45 [
23,
27]. Controls were included to detect non-specific binding of fluorescently labeled mAbs, determine cut-offs between positive and negative fluorescence, and to set compensations [
23]. Except for the CD41/61-FITC mAb purchased from Bio-Rad, Hercules, CA, USA, all mAbs were purchased from Southern Biotech, Birmingham, AL, USA. All cell samples were analyzed by flow cytometry using a BD C6-plus Accuri flow cytometer (Becton Dickinson, San Jose, CA, USA). Pulp data were expressed as the percentage of stained cells in the total pulp cell suspension, whereas for blood, cell concentrations (cells/μL) were reported. For both pulp and blood, lymphocyte data were calculated as the sum of T and B cells. For blood the heterophil to lymphocyte (H:L) ratios were calculated by dividing the concentrations of heterophils by the concentrations of lymphocytes. The proportions of blood basophils and eosinophils were determined by microscopic evaluation of at least 300 white blood cells (WBC) on Wright-stained blood smears prepared for each blood sample. The concentrations of basophils and eosinophils were then calculated by multiplying the concentration of WBC (CD45
+CD41/61
−) determined by flow cytometry by the percentage of basophils or eosinophils and dividing the products by 100.
2.6. Reactive Oxygen Species Generation Assay
Reactive oxygen species generation in pulp cell suspensions was determined for each pulp cell suspension by kinetic fluorescence assay using 2′,7′-dichlorofluorescin-diacetate (DCFDA, Sigma, St. Louis, MO, USA), as described in [
28]. Generation of fluorescence from the samples was determined via kinetic read for 1.0 h at 10 min intervals using a fluorescence microplate reader (Synergy HTX; BioTek, Winooski, VT, USA) set at 37 °C with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Data were acquired with Gen5 software 2.07 (BioTek). Appropriate controls for autofluorescence and background fluorescence were included in each assay. A line of best fit describing the relationship between fluorescence (a.u.) and time was calculated for each sample and the relative amount of ROS generation reported as the slope of the best fit equation [
23].
2.7. Isolation of RNA from GF-Pulps and Relative Gene Expression Analyses of Cytokines by Quantitative Reverse Transcription PCR
For each bird and time point, pulps were extracted from two frozen GFs and total RNA isolated, as described in [
23]. Total RNA quality and concentration were determined using NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA). RNA was transcribed to cDNA using the High-Capacity cDNA kit with MultiScribe Reverse Transcriptase following the manufacturer’s protocol (Thermo Fisher Scientific). All cDNA samples were diluted to a working concentration of 10 ng/μL with nuclease-free water and stored at −20 °C until used for relative gene expression analyses [
23].
Primers and probes for interleukin 1β (
IL1β),
IL6,
IL8 (
CXCL8), tumor-necrosis factor-α (
TNFα),
IL10, and transforming growth factor-β-1 (
TGFβ1) and the 28S reference gene, as well as the qPCR procedure were as described [
23]. Real-time qPCR was performed using TaqMan Universal Master Mix with UNG protocol in a 7500 Fast Real-Time PCR System (Thermo Fisher Scientific), as described in [
23]. Using 28S as the reference gene to calculate target gene delta Ct, the relative gene expression was determined as 40 minus delta Ct [
29].
2.8. Plasma Alpha-1 Acid Glycoprotein-1 Assay
Alpha-1 acid glycoprotein (AGP-1) concentrations (mg/mL) in plasma of blood samples collected before (0 h) and at 6 and 24 h following i.d. GF-pulp injection of lipopolysaccharide (LPS) or diluent (PBS) were determined by chicken AGP-1 ELISA following manufacturer’s protocol (Abcam, Waltham, MA, USA).
2.9. Statistical Analysis
Statistical analyses were conducted using Sigma Plot 13 Statistical Software (Systat Software, Inc., San Jose, CA). Three-way analysis of variance (ANOVA) was conducted to determine the effects of temperature (TN, HS), time (0, 6, 24 h), and injection (LPS, PBS) and their interactions. Significant interactions involving injection treatment were revealed. Hence, data for LPS and PBS injection were separately analyzed by 2-way ANOVA for GF-pulp data, or 2-way repeated measures (RM) ANOVA for blood data, to determine the effects of temperature and time, and temperature by time interactions. Shapiro–Wilk Normality test and Brown–Forsythe Equal variance tests were conducted for all ANOVAs; in the few cases when normality and equal variance tests failed (p ≤ 0.05), log transformation of data restored normality and equal variance (p > 0.05). In the presence of significant main effects, Holm–Sidak Pairwise Multiple Comparison Procedures were conducted as appropriate. In all cases, statistical significance was considered p ≤ 0.05.
4. Discussion
Inflammation is a highly conserved, innate immune system response that acts as a major first line of defense in the fight against microbial infection. As shown by French et al. [
23] using the “two window approach”, broilers exhibit all the basic hallmarks of LPS-stimulated inflammatory activities described [
17,
22]. Using a similar experimental approach as French et al. [
23], we examined the effects of cyclic, environmental heat-stress on the local (GF-pulp) and systemic (blood) inflammatory responses initiated by i.d. GF-pulp injection of LPS. In the current study, observations in TN-broilers fully corroborated those reported by [
23], further supporting the relevance of the insights gained from the two-window approach. Comparison of data from HS-broilers with those from TN-broilers revealed (1) suppressive effects of cyclic heat stress on various aspects of both the local and systemic inflammatory responses to LPS; (2) suppressive effects of cyclic heat stress on the response to sterile, endotoxin-free PBS injection (injection control); and (3), based on analysis of pre-injection samples, suppressive effects of cyclic heat stress on immune system development, including lower baseline ROS generation and mRNA expression of cytokines in GF-pulps (a skin derivative) and lower concentrations of circulating blood lymphocytes. To our knowledge, this is the first comprehensive assessment of the effects of environmental, cyclic heat stress on the local and systemic innate immune system activities in response to LPS and vehicle injection, as well as on aspects of baseline immune system status in broilers.
4.1. Suppressive Effects of Cyclic, Environmental Heat Stress on the Local and Systemic Responses to LPS Injected into the Dermis of GF-Pulps
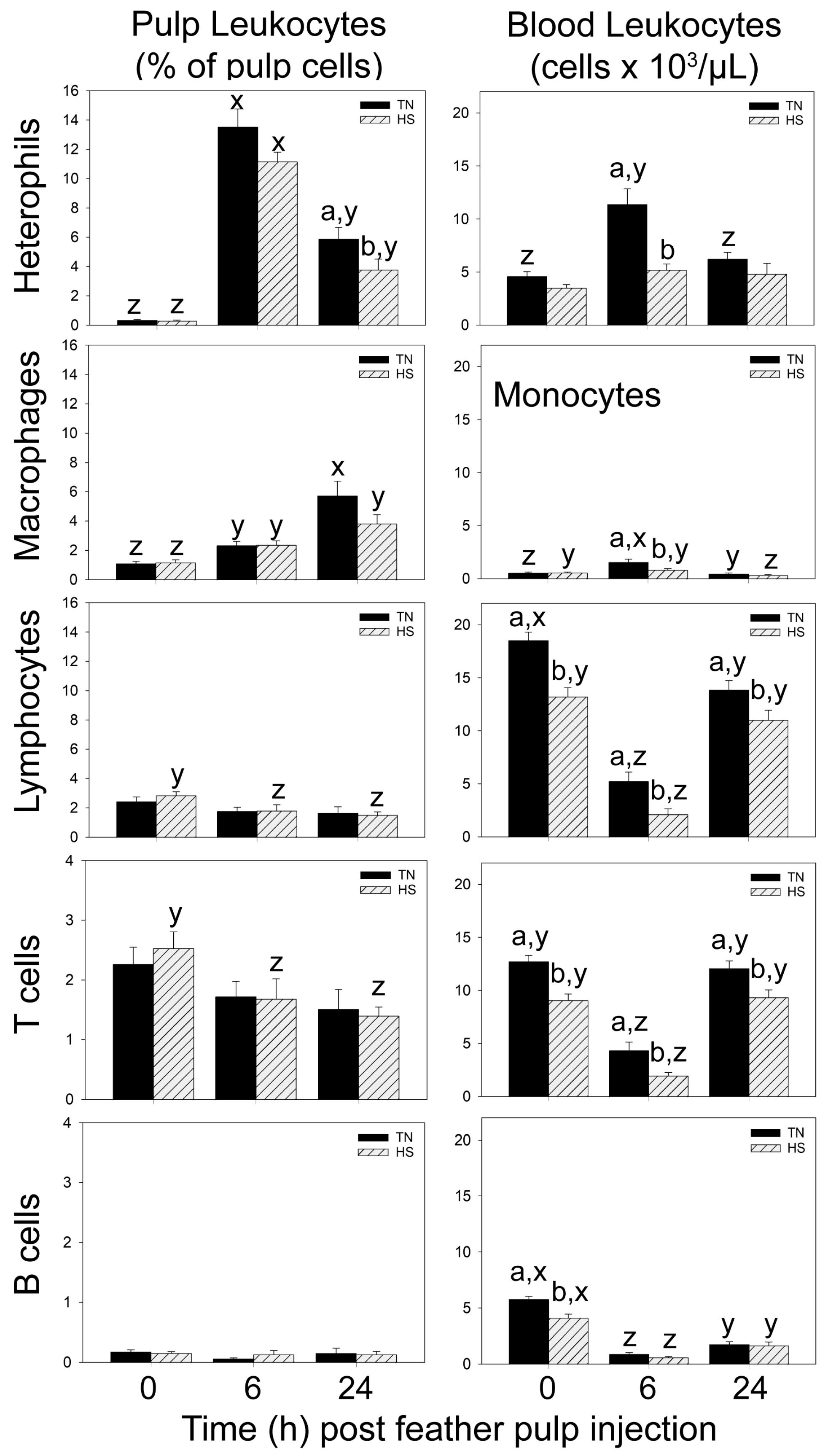
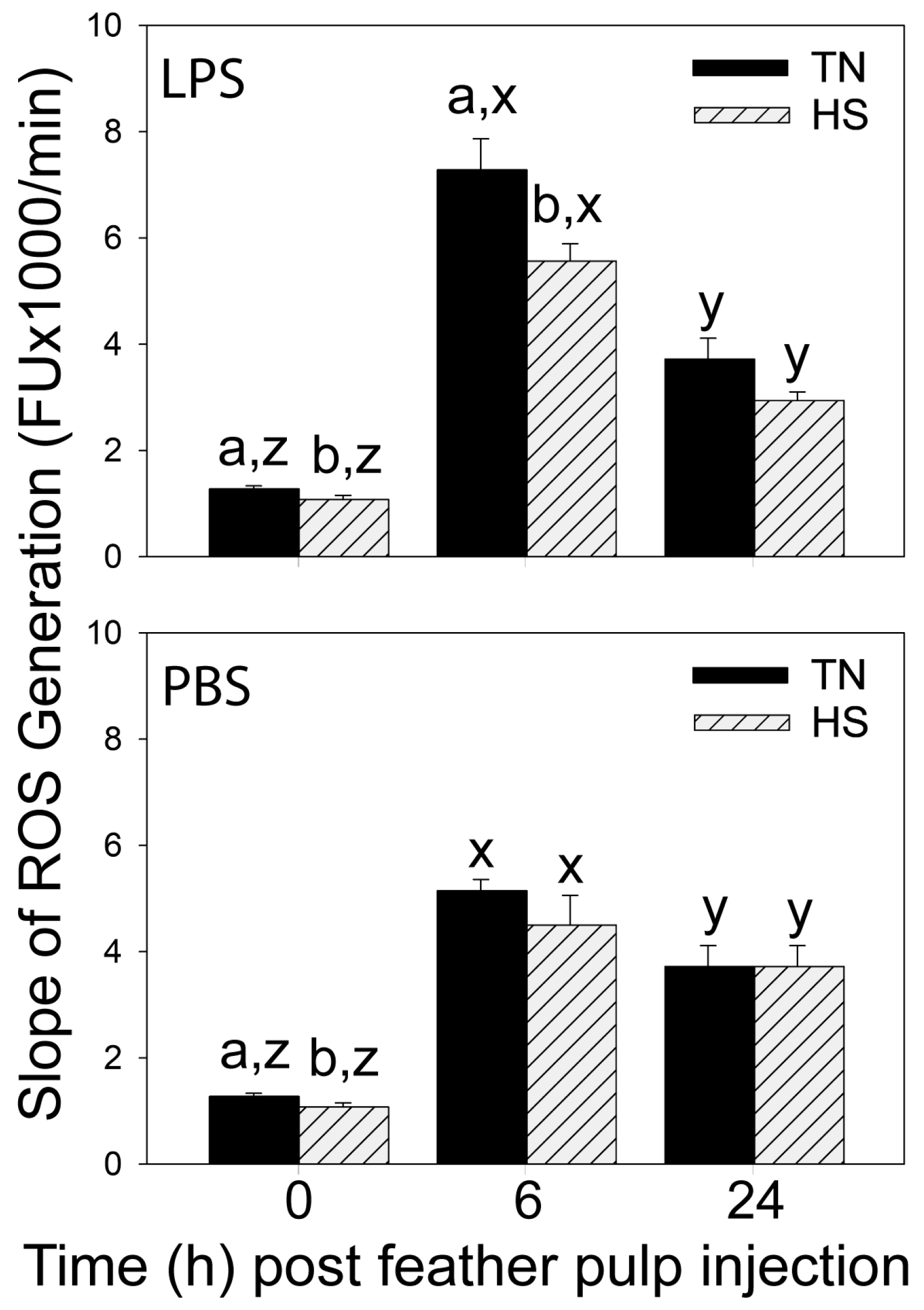
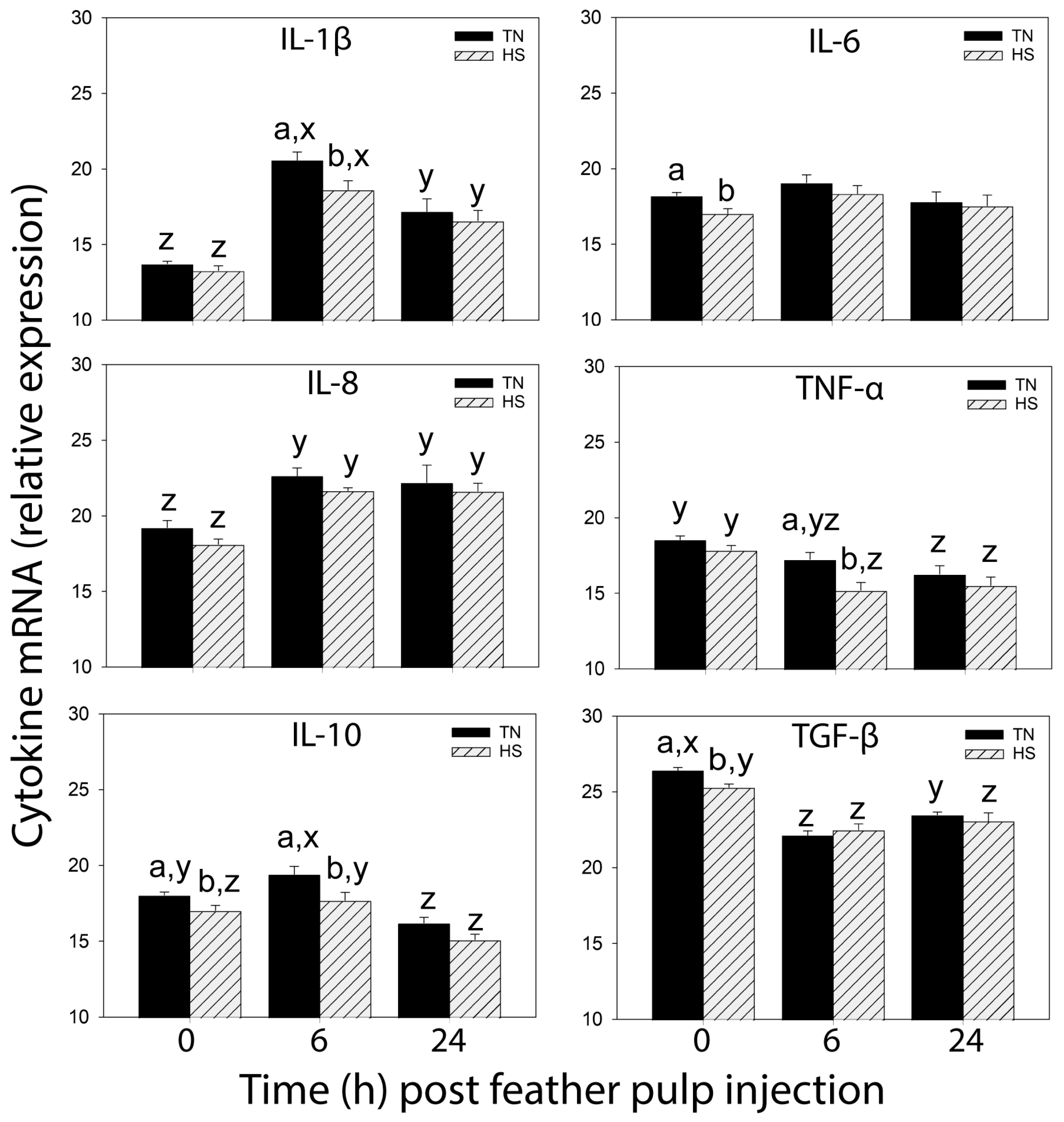
In both HS- and TN-broilers, LPS administration stimulated the recruitment of heterophils and monocytes/macrophages, but not lymphocytes, from the blood into the injected GF-pulps. While heterophil and monocyte/macrophage infiltration was similar at 6 h p.i. in both HS- and TN-broilers, suppressive effects of heat stress on heterophil- and monocyte/macrophage-infiltration at the site of LPS injection were evident at 24 h. Specifically, at 24 h p.i., heterophil levels (% pulp cells) were lower in GF-pulps of HS- than TN-broilers, and in HS-broilers, there was no increase in macrophages from 6 to 24 h p.i. In both HS- and TN-broilers, ROS generation and relative mRNA expressions of IL-1β, IL-8, and IL-10 peaked at 6 h p.i. However, the 6 h peak in ROS generation and in relative mRNA expression of IL-1β and IL-10 were lower in GF-pulps of HS- than TN-broilers. Moreover, the drop in mRNA expression of TNF-α occurred earlier (6 h p.i.) in HS- than TN-broilers, and, although TGF-β1 mRNA expression dropped at 6 h p.i. in both HS- and TN-broilers, it only increased again by 24 h p.i. in TN-broilers. Taken together, the temporal and quantitative differences between the LPS-induced local inflammatory responses of HS- and TN-broilers attest to the suppressive effects of cyclic heat stress not only on leukocyte recruitment and infiltration, but also on the functional activities of resident/infiltrating leukocytes and/or other GF-pulp cells. Similar suppressive effects of heat stress on the inflammatory response were observed when broilers were subjected to heat stress and infected with Gram-negative bacteria (i.e.,
Salmonella Enteritidis [
12],
Escherichia coli [
10]).
A reduced ability in HS-broilers to fully respond to the inflammatory activity initiated by LPS in the GF-pulp can also be observed in the peripheral blood. In the blood, the anticipated [
23] increases in the heterophil and monocyte concentrations at 6 h post-GF-pulp injection of LPS only occurred in TN-broilers. In HS-broilers, the heterophil concentrations did not change post-LPS injection and the monocyte concentration gradually dropped below pre-injection levels at 24 h. These observations suggest a lower ability of HS-broilers to communicate the need for more heterophils and macrophages at the site of inflammation and/or to produce/release additional heterophils and monocytes into the circulation [
17,
30,
31].
One effect of i.d. GF-pulp injection of LPS that was similar in magnitude in HS- and TN-broilers is the extensive drop in the concentrations of circulating lymphocytes at 6 h p.i. and their subsequent return to near baseline levels by 24 h p.i. In both HS- and TN-broilers, the 6 h drop in lymphocyte concentrations was due to reduced concentrations of T and B cells, with T cells returning to pre-injection concentrations at 24 h p.i., while B cells remained below pre-injection concentrations. The drop in blood lymphocyte concentrations in response to LPS administration has been consistently reported, but the mechanisms are not well understood and have primarily been attributed to the lymphotoxic effects of LPS [
21,
22,
23,
31]. However, as discussed below, a drop in blood lymphocyte concentrations was also observed in TN- and HS-broilers when sterile, endotoxin-free PBS (injection control) was injected into the dermis of GF-pulps, suggesting that the drop in circulating concentrations of lymphocytes post-LPS administration may in part be due to physiological effects associated with GF-pulp injection and handling of the broilers [
23].
Other blood cell populations were also affected by i.d. GF-pulp injection of LPS. Included are a drop in eosinophil and an increase in thrombocyte concentrations at 6 h post-LPS injection in both TN- and HS-broilers. For TN-broilers, this observation differs somewhat from [
23], which reported no change in eosinophils, and increased concentrations of thrombocytes at 24 h p.i. These discrepancies in eosinophil and thrombocytes concentrations changes in TN-broilers compared to French et al.’s [
23] broilers may be due to the different methods employed to assess blood cell concentrations. Specifically, in the current study, a combination of immunofluorescence-based flow cytometry and Wright-stained blood smear evaluations were used to identify thrombocytes and eosinophils, respectively. In a previous study [
23], proportions and concentrations of leukocytes were determined by automated hematology (i.e., Cell Dyn, Abbott Diagnostics, Abbott Park, IL, USA) calibrated for chicken blood cells.
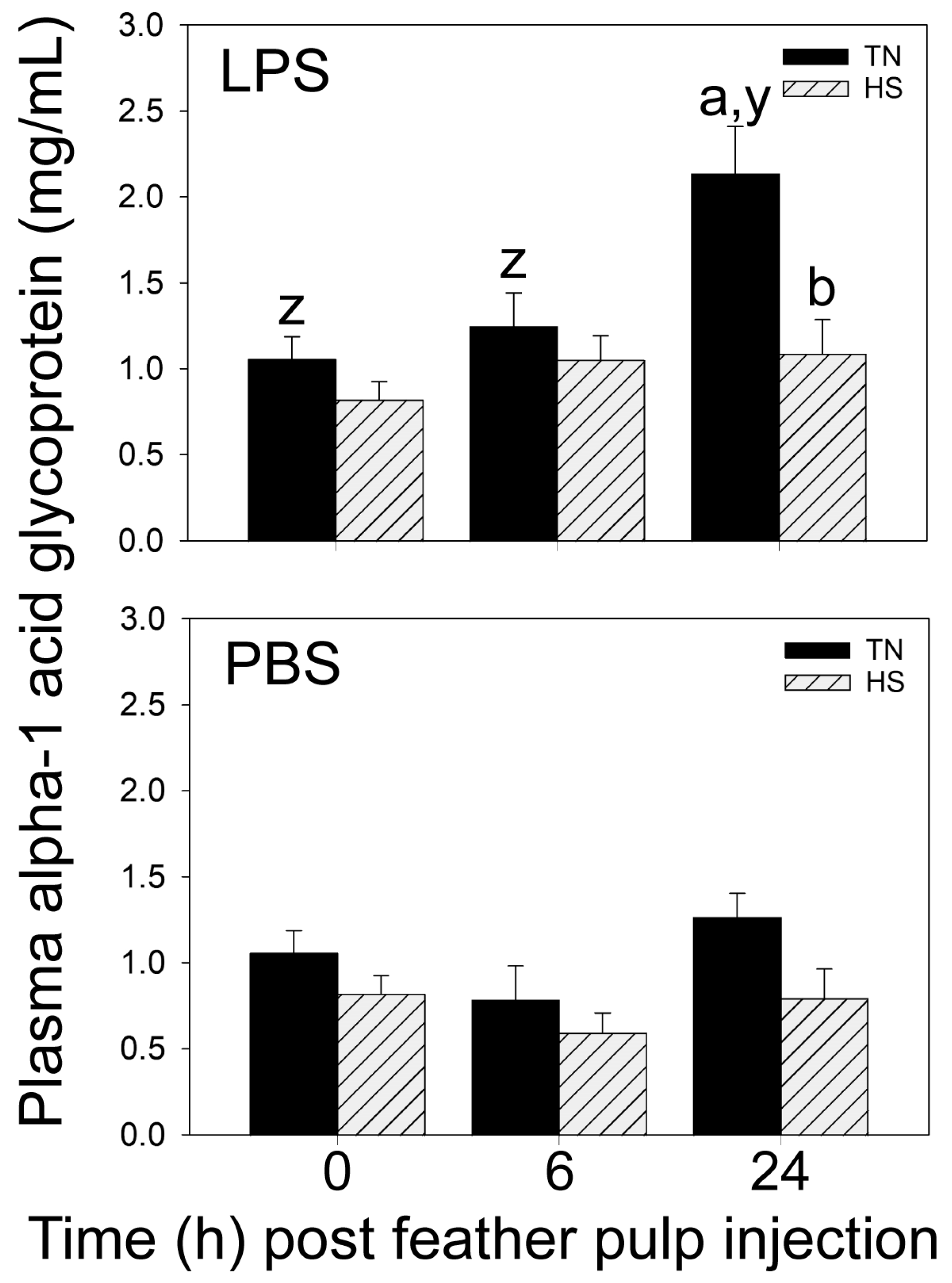
Lastly, plasma AGP-1 concentrations increased to the highest levels in TN-broilers at 24 h after i.d. GF-pulp injection of LPS, whereas in HS-broilers, plasma AGP-1 concentrations did not change over time. Considering the protective and regulatory role of acute phase proteins such as AGP-1, this too is a negative effect of environmental HS on the innate immune system’s efforts to control inflammatory damage and restore homeostasis [
17,
32,
33].
Collectively, our observations support suppressive effects of cyclic, environmental heat stress on the broilers’ ability to effectively respond to LPS administration and, hence, to challenges by Gram-negative bacteria.
4.2. Suppressive Effects of Cyclic Heat Stress on the Local and Systemic Inflammatory Responses to Injection of Sterile, Endotoxin-Free PBS into the Dermis of GF-Pulps
In both TN- and HS-broilers, few effects of the vehicle injection, namely sterile, endotoxin-free PBS (injection control), were observed both at the injection site and in the peripheral blood circulation. In PBS-injected GF, heterophil levels (% pulp cells) increased reaching peak levels at 6 h in both TN- and HS-broilers. However, while monocyte/macrophage infiltration was highest at 24 h p.i. in TN-broilers, it did not change from 6 to 24 h in HS-broilers. These responses in TN- and HS-broilers were similar to those observed with LPS, but the relative quantity of infiltrating cells was many folds lower with PBS than LPS. Furthermore, the small changes in heterophil and monocyte/macrophage presence in PBS-injected GF-pulps of TN-broilers are consistent with observations by French et al. [
23]. The increases in local inflammatory activities such as ROS generation and the relative mRNA expression of proinflammatory cytokines IL-1β and IL-8, albeit lower than with LPS, further reflect local inflammatory activity initiated by injection of PBS due to tissue damage and activation of repair processes. Other than lower expression of IL-1β mRNA in HS- than TN-broilers at 6 h post i.d. PBS injection, there were no significant differences in local inflammatory activity due to cyclic heat stress. Like with LPS, lymphocytes were not recruited to GF-pulps in response to PBS injection. However, lower lymphocyte levels were observed at 24 h p.i. in GF-pulps of both HS- and TN-broilers, whereby this decrease was due to lower T cell presence. This small drop in GF-pulp lymphocytes observed here with the PBS-injections was not observed in [
23], although they reported a drop in GF-pulp lymphocytes when LPS was injected. We do not have an explanation for these inconsistencies in GF-pulp lymphocyte levels, other than that the significance of these small proportional changes varies from experiment to experiment.
The increases in heterophils and monocytes/macrophages in GF-pulps post-PBS injection were not accompanied by changes in heterophil and monocyte concentrations in the blood circulation, in both HS- and TN-broilers. However, as mentioned above, a drop in blood lymphocyte concentrations was also observed in both HS- and TN-broilers in response to PBS injection. This observation suggests a contributing role of the stress associated with handling and injection to the drop in circulating lymphocyte concentrations seen with LPS [
33]. It should be noted that the drop in circulating lymphocytes in TN-broilers was less extensive with PBS than with LPS, whereas in HS-broilers, the drop was almost identical in response to PBS or LPS. Hence, cyclic heat stress exacerbated this effect in HS-injection controls, suggesting a bigger role of physiological stress in the drop in lymphocyte concentrations in the HS-group. Moreover, in HS-broilers, the blood T cell concentrations dropped from 9.07 ± 0.60 × 10
3 cells/µL before PBS injection to 3.07 ± 0.81 × 10
3 cells/µL at 6 h p.i., whereas in TN-broilers, the drop was less extensive (i.e., from 12.7 ± 0.58 × 10
3 cells/µL to 8.59 ± 0.98 × 10
3 cells/µL). B cell concentrations dropped to a similar extent in HS- and TN-broilers; and, unlike T cells, which recovered to pre-injection concentrations at 24 h p.i., B cells remained at low concentrations at 24 h p.i. in both groups. Hence, the physiological stress associated with injections and handling of the broilers may affect the B cell compartment to a larger extent than T cells. The basis for this observation needs further investigation.
The exacerbating effect of cyclic heat stress on the reduction of circulating concentrations of lymphocytes in HS-broilers is further supported by the increase in H:L ratios at 6 h post-PBS-injection in HS- but not in TN-broilers. It is particularly notable that this increase in H:L ratio in HS-broilers was solely due to the drop in blood lymphocytes, as there was no increase in heterophil concentrations at this time-point.
The injection of sterile, endotoxin-free PBS into GF-pulps also did not affect the blood concentrations of basophils, eosinophils, thrombocytes, and erythrocytes. Moreover, i.d. injection of PBS without a microbial component did not stimulate an increase in plasma AGP-1 concentrations. This observation agrees with AGP-1′s role as an acute phase protein in cell protection and anti-inflammatory activities in response to microbial infection [
17,
32,
33].
Taken together, few effects of the control injection and few differences between HS- and TN-broilers in the local and systemic inflammatory responses to PBS were observed. The extensive drop in blood lymphocytes in HS-broilers and significant increase in the H:L ratio at 6 h post-injection of sterile, endotoxin-free PBS suggests additional physiological stress in HS-broilers and an impaired ability of the HS-broilers to cope with the stress of being handled and with the tissue damage associated with the GF-pulp injection.
4.3. Suppressive Effects of Cyclic Heat Stress on Baseline Levels of Immune Cells and Activities in GF-Pulps and Blood
In GF-pulps, there were no differences in baseline levels (0 h) of heterophils, macrophages and lymphocytes, and in relative mRNA expression of pro-inflammatory cytokines IL-1β, IL-8 and TNF-α, between HS- and TN-broilers. Considering that the presence and production of inflammatory cells and cytokines are dependent on inflammatory stimuli, it is not surprising that baseline tissue levels are similar between HS- and TN-broilers. However, baseline ROS generation and relative expression of IL-6, IL-10, and TGF-β1 mRNA was lower in HS- compared to TN-broilers. These differences between HS- and TN-broilers may be due to suppressive effect of heat stress on metabolic activities of resident leukocytes and epidermal and dermal pulp cells [
11,
12,
13].
It should be noted that, except for lymphocytes, the baseline levels of all other blood cells (i.e., RBC, thrombocytes, heterophils, monocytes, eosinophils, and basophils) were not different for broilers reared under HS- versus TN-conditions. Hence, it appears that cyclic heat stress alone did not affect hematopoiesis of myeloid cells. Rather, the lower concentrations of circulating T- and B-lymphocytes point towards an effect of cyclic heat stress on lymphocyte development in the thymus and bursa of Fabricius, respectively. In a similar study by Quinteiro-Filho et al. [
10], 35-day-old broilers were subjected to 10 h of HS (36 °C), with control birds kept at TN (21 °C) temperatures for 24 h per day, for one week. At 42 days of age, HS broilers were reported to have elevated serum corticosterone concentrations, decreased relative weights (% BW) of the thymus and bursa of Fabricius, and reduced
Staphylococcus aureus-induced reactive oxygen species generation (ROS) by macrophages in vitro. These alterations in the thymic and bursal weights, as well as ex vivo macrophage ROS generation, were attributed to the elevated levels of the stress hormone, corticosterone [
10]. Corticosterone, as well as sex steroids, are known to drive regression of primary lymphoid organs by reducing the levels of immature lymphocytes (e.g., CD4
+CD8
+ thymocytes) and hence, the weight of these organs [
17]. Moreover, corticosterone and other glucocorticoids are known to have anti-inflammatory properties, explaining the reduced ROS generation in response to
S. aureus stimulation of macrophages in the Quinteiro-Filho et al. [
10] study.
While corticosterone concentrations were not measured in the current study, our observations of reduced local and systemic acute inflammatory responses and reduced circulating levels of T- and B-lymphocytes in HS broilers are likely due to elevated levels of heat-stress-associated stress hormones. In avian species, the H:L ratio is often used as an indicator of physiological stress [
31,
34,
35]. With this assumption, the similarly low H:L ratios before PBS/LPS injection suggests that environmental, cyclic heat stress did not impose an additional stress on the broilers. This may be due to habituation of HS-broilers to the heat over the 33 days of high temperatures from 8:00 am to 10 pm. On the other hand, the 6 h increase in H:L ratio following PBS-injection in HS- but not in TN-broilers may be interpreted as an indicator of increased physiological stress in HS-broilers, as there was no microbial stimulus or non-sterile injury influencing the H:L ratio. The great increase in H:L ratio at 6 h post-LPS injection in both HS- and TN-broilers, however, is primarily due to the inflammatory response to LPS and, hence, “immunological stress”. This observation serves as a reminder that in order to use the H:L ratio as an indicator of physiological stress, microbial infection status of the chicken has to be considered. Based on the current study, it appears that increased H:L ratios during an inflammatory response in TN-broilers involve both an increase in blood heterophil concentrations and a drop in the concentrations of blood lymphocytes, while changes in H:L ratios due to physiological stress may be due to a drop in lymphocyte concentrations only.