Simple Summary
The poultry sector is an essential component of agriculture that has grown unusually over the past few decades. Salmonella is thought to live naturally in chickens and causes salmonellosis in humans. Many plant extracts, mainly essential oils, have had their active ingredients studied. Salmonella in chicken is resistant to the antimicrobial effects of the aromatic oil eugenol, which is present mainly in cinnamon and clove. Therefore, eugenol supplementation also improves gut health, thereby increasing overall well-being. Here, we reviewed the rising rates of salmonellosis worldwide and the factors contributing to its prevalence. Then, we suggested using eugenol as a natural feed supplement for reducing Salmonella in chicken.
Abstract
The poultry sector is an essential component of agriculture that has experienced unprecedented growth during the last few decades. It is especially true for the United States, where the average intake of chicken meat increased from 10 pounds (4.5 kg) per person in 1940 to 65.2 pounds (29.6 kg) per person in 2018, while the country produced 113 billion eggs in 2019 alone. Besides providing nutrition and contributing significantly to the economy, chicken is also a natural reservoir of Salmonella, which is responsible for salmonellosis in humans, one of the significant foodborne illnesses around the globe. The increasing use of chicken manure and antibiotics increases the spread of Salmonella and selects for multi-drug resistant strains. Various plant extracts, primarily essential oils, have been investigated for their antimicrobial activities. The multiple ways through which these plant-derived compounds exert their antimicrobial effects make the development of resistance against them unlikely. Eugenol, an aromatic oil primarily found in clove and cinnamon, has shown antimicrobial activities against various pathogenic bacteria. A few reports have also highlighted the anti-Salmonella effects of eugenol in chicken, especially in reducing the colonization by Salmonella Enteritidis and Salmonella Typhimurium, the primary Salmonella species responsible for human salmonellosis. Besides limiting Salmonella infection in chicken, the supplementation of eugenol also significantly improves intestinal health, improving overall well-being. In this review, we highlight the rising incidences of salmonellosis worldwide and the factors increasing its prevalence. We then propose the usage of eugenol as a natural feed supplement for containing Salmonella in chicken.
1. Introduction
Foodborne illnesses are one of the major causes of contributors to disease burden worldwide [1]. In the United States (US) alone, approximately 48 million people each year contract a foodborne disease, which costs around $90 billion to the US economy in healthcare expenses and reduces the economic activities of the affected individuals. Among many other foodborne pathogens, Salmonella from poultry is estimated to cause $2.8 billion in financial losses to the US [2,3]. Salmonella bacteria have emerged as the leading etiological agent of foodborne illnesses worldwide, posing a serious threat to public health. A significant number of foodborne infections worldwide are confirmed to be caused by Salmonella, which produces various disease symptoms collectively referred to as salmonellosis [4]. Because of the severe implications of Salmonella to public health, control of this pathogen in poultry has become a top priority of the US Department of Agriculture [USDA] [5]. Salmonella serovars have been shown to infect various domesticated animals, including cattle, poultry, pigs, and sheep. Infected animals may exhibit symptoms ranging from mild gastroenteritis to death in extreme cases of Salmonella infection [6]. Most cases of salmonellosis are attributed to eggs and chicken meat consumption. The human condition is commonly caused by a few serotypes, such as Salmonella Enteritidis (S. Enteritidis) and Salmonella Typhimurium (S. Typhimurium), with poultry serving as a primary reservoir [7,8]. However, there has been a recent increase in the prevalence of multidrug-resistant (MDR) Salmonella enterica [9]. Pietsch M. et al. have reported third-generation cephalosporin resistance phenotypes resistant to three or more antimicrobials [10].
The incidence of Salmonella infection in poultry varies by country. In some industrialized countries, roughly 1% of the poultry flock is infected with Salmonella. On the other hand, resource-poor countries can have as much as a 10% Salmonella infection rate in chickens due to challenging epidemiological settings [11]. The spread of pathogens originating in the chicken host, especially Salmonella, can spread faster and more efficiently due to the reuse of litter-containing bedding material and chicken droppings. Chicken producers increasingly use litter in farming due to the increased cost and difficulty procuring bedding material [12,13].
Moreover, chicken droppings are also extensively used as fertilizer due to the abundance of nitrogenous materials in the chicken feces. A study by Chinivasagam et al. [14] found that the farms that reuse litter had as much as 83% Salmonella prevalence compared to 68% in farms that disposed of litter after a single use by the flocks. Even if the manure is not reused and disposed of, the persistence problem of Salmonella still exists, as evidenced by the high prevalence of Salmonella in farms that regularly dispose of the chicken litter containing manure, as shown by Chinivasagam et al. [14]. Hence, research into effective ways of eradicating Salmonella from poultry is urgently needed to reduce the burden of salmonellosis and the economic destruction caused by it to the poultry industry and public healthcare [15].
Various management and treatment strategies have been proposed to reduce Salmonella infection and spread in chickens. Among these approaches, the pre-slaughter method is a primary method for controlling Salmonella in chickens [16]. The main goal behind this strategy is to reduce pathogen colonization in poultry through various intervention strategies, which can ultimately lead to a decrease in poultry meat and egg contamination. Various preslaughter approaches have been used over the years, such as vaccination, competitive exclusion of Salmonella in chickens by modulating gut bacteria, and the use of probiotics and prebiotics [17]. These techniques have shown varying levels of success in controlling Salmonella infection and colonization in chickens [18]. Various plants having therapeutic value have been used in animal and food production for centuries. These plants, especially their bioactive compounds and essential oils (EOs), are beneficial in containing Salmonella infection in chickens [19]. Secondary compounds produced by plants confer the main therapeutic action against pathogens. Such compounds are produced as byproducts of the interaction between plants and their environment [20].
The main advantage of plant-based compounds over antibiotics is multi-faceted antimicrobial action, as opposed to the limited antimicrobial mechanisms of antibiotics. The likelihood of developing bacterial resistance against plant-based antimicrobials is minimal [21]. EOs are one of the most potent plant-based resources, and among the essential oils, carvacrol, thymol, and eugenol have shown promising potential against Salmonella infections in chickens. Eugenol, a primary component of the EOs extracted from various plants but present in abundant amounts in clove, has been shown to kill many enteric pathogens, including Salmonella, selectively [22,23]. Moreover, one of the main advantages of plant-derived compounds is their non-destructive effects on the commensal microbiota in chickens. Therefore, plant-based extracts, especially EOs, can selectively kill various enteric pathogens without causing much harm to the gut commensal bacteria [22,24]. In the current review, we provide information on the destructive effects of Salmonella and the use of antimicrobials that increase this pathogen’s persistence. We then highlight the potential application of eugenol as an alternative to antibiotics for treating Salmonella in chicken.
2. Effect of Salmonellosis on Humans
Salmonella is a flagellated, facultative anaerobic, gram-negative bacillus. The genus belongs to Enterobacteriaceae, a large group of medically important enteric pathogens. Salmonella species are found in the microbiota that colonizes some humans and animals [25]. Salmonellosis is a zoonotic illness caused by Salmonella species, affecting humans and causing severe complications. It is a very common foodborne illness around the world. In the United States, the Center for Disease Control (CDC) estimates that salmonellosis affects about 1.2 million people and causes 450 deaths yearly [26].
Moreover, salmonellosis affects humans and farm animals, since Salmonella is endogenous in some animals, causing substantial livestock losses. Besides livestock, the most common animal reservoirs are birds, such as chickens, ducks, and turkeys [27]. Salmonella contamination of cattle and poultry may come through feed. The trends in animal feed and feed additives are tracked in the US by the FDA Center for Veterinary Medicine. Salmonella contamination of feed and making animals ill can also harm humans through direct contact with contaminated feed or through infected animals shedding the bacteria into human food and water supplies [28]. In humans, salmonellosis manifestations depend on the causative agents and the immune regulation inside the host. It can manifest as an asymptomatic carrier, gastroenteritis, enteric fevers, dehydration, arthritis, and septicemia. Nontyphoidal salmonellosis, caused by S. Enteritidis and S. Typhimurium, is associated with food poisoning cases [29].
It has been estimated that as high as 41% of retail chicken meat can contain Salmonella; this percentage is considered second after Campylobacter, the most prevalent bacterial pathogen in chicken, with an estimated prevalence of 70.6% [30]. Besides meat, chicken eggs are also a major source of the spread of salmonellosis [31]. It has been estimated that one in 20,000 eggs carry Salmonella. This might not sound like much, but the per capita consumption of chicken eggs is much higher than that of chicken meat, which makes it a significant source of Salmonella transmission to the public [32]. One of the major contributors of Salmonella in chicken products is thought to be fecal contamination. Because of this, the US Food Safety and Inspection Service (FSIS) has enacted mandatory inspection procedures for fecal contamination of poultry products for zero tolerance of fecal material in chicken meat and eggs. These procedures include the routine inspection of Salmonella, Campylobacter, and Escherichia species in chicken products [33].
3. Use of Antibiotics to Contain Salmonella in Chicken
The poultry sector has grown exponentially since the industrialization of poultry farming in the last century and is expected to grow further. The progress made in the chicken industry is attributed to multiple factors. The therapeutic usage of antibiotics, chicken vaccinations against communicable illnesses, organic nutrition, a healthy breeding environment, and gene selection are some of the contributing factors [34]. The success of this sector can in part be attributed to the use of antibiotics, which have been used at therapeutic and sub-therapeutic levels to protect the chicken from infectious diseases and enhance their economic output. Antibiotics help poultry birds mature and expand their body size by improving feed conversion capacity and minimizing disease occurrences [34,35].
Furthermore, antibiotics improve general animal well-being, and combining all these factors minimizes the cost of production associated with producing animals. As a result of the financial benefits of adding antibiotics to feed, food output improves, which is shared across value-added chains. Most cost savings associated with using antibiotics in animal agriculture are due to better feed conversion, improved litter and manure quality, and reduced mortality [36]. Despite these benefits, the ongoing use of antibiotics in chicken production generates public health and safety concerns about antimicrobial resistance and antibiotic residue accumulation in the environment and food supply [36,37]. The overuse of antibiotics in animal farming leads to the selection of antibiotic-resistant (ABR) genes in the bacteria residing in animals. These ABR genes can easily spread to bacterial populations in the environment, plants, and animals, including humans [38].
The spread of ABR is associated with increased medical care costs and mortality rates in humans due to increased antibiotic resistance. The European Union estimated that antibiotic-resistant infections cost around $1.5 billion, while around 25,000 people die yearly from these antibiotic-resistant bacterial infections [36,39]. However, suppose the increase in antibiotic resistance continues. In that case, it is feared that we will enter a post-antibiotic era that could cause the deaths of 10 million people by 2050 within a few decades. The high usage of antibiotics is also selected for the emergence of multidrug-resistant (MDR) Salmonella strains. The poultry sector is also the most significant contributor to the emergence of MDR Salmonella strains. It has been shown by many studies that most MDR Salmonella strains isolated from poultry belong to S. Enteritidis and S. Typhimurium, the causative agents of salmonellosis in humans [40]. There was a reported increase in the prevalence of multidrug-resistant (MDR) Salmonella enterica species, such as the third-generation cephalosporin resistance phenotypes and MDR-ACSSuT Salmonella enterica, which was reported to be resistant to ampicillin, chloramphenicol, streptomycin, sulphonamides, and tetracyclines. The resistance mechanisms were linked to int1-associated elements and extended-spectrum β-lactamase (ESBL) genes [9,10,40].
Moreover, the extensive use of antibiotics also destroys the chickens’ normal gut microbiota, leading to an imbalance in the microbial diversity of the chicken gastrointestinal (GI) tract. This disturbance of microbiota, called gut dysbiosis, causes a disturbance in the immune system and makes chickens vulnerable to various infectious and non-infectious diseases [36,37]. Thus, problems with the use of antibiotics in poultry farming are compelling scientists to discover reliable, cost-effective, and efficient means that could be used as an alternative to antibiotics in animal production. There are several alternatives proposed over the years. Various plants and their derivates are extensively studied due to their antioxidant, antiparasitic, anti-inflammatory, antiviral, and antibacterial activities [38,39].
4. Use of Chicken Manure in Poultry Farming and Spread of Salmonella
Animal manure is an excellent source of energy and nutrients; therefore, it is increasingly being used in agriculture and animal production, including poultry farming. However, besides containing these desirable features, animal manure is also rich in harmful pathogens and ABR genes. Chicken manure is untreated animal feces commonly used for agricultural and gardening [40,41]. It makes an excellent fertilizer that improves soil structure, moisture-holding capacity, and water infiltration. It is considered a cost-effective source of nitrogen, potassium, and phosphorus. Even though chicken excreta has several advantages, raw chicken manure fertilizer can transmit serious human infections [42]. Chickens are mostly reared on litter that contains bedding material and chicken feces or manure. Chicken manure contains various pathogens (many zoonotic), including Salmonella, which can spread easily between chickens in a flock as they all share common living conditions within a poultry farm. This increases the incidence of infectious diseases in chickens and humans who consume or encounter them [12,43]. Yang et al., 2016, investigated the effects of chicken manure on microbiome communities. Using genomic DNA analysis, the authors detected an increase in the soil’s pathogenic antibiotic-resistant bacteria population. Therefore, the authors demonstrated a potential threat to human health because of the improper usage of chicken excreta (Yang et al., 2016). Since Salmonella colonizes the gut of chickens, many outbreaks have been documented, related to improper chicken manure usage and handling of plant products [44]. Thus, increasing usage in various sectors can accelerate the spread of pathogens and ABR genes [45,46].
The factors that contribute to the spread of Salmonella in chicken manure include temperature, moisture, and litter usage [47]. Increased moisture and litter re-usage increases Salmonella prevalence in chicken manure, while temperatures unsupportive of the growth of Salmonella are associated with decreased Salmonella counts [14,47]. Even in the chickens infected with Salmonella strains, heterogeneity still exists in their ability to release Salmonella into the environment or transmit bacteria to other organisms [48]. The infected chickens that harbor and spread the Salmonella bacteria at higher levels than other chickens in the flock are called super-shedders for Salmonella. The trait can be found in genetically homogenous populations of host organisms, as shown by studies in inbred mice [49] and chickens [50]. Thus, factors other than host biology can also determine whether a host will be a super-shedder [48]. The transmission rate of these super-shedders makes them a focus for epidemiological studies and disease management. Empirical studies and modeling for numerous diseases have shown that 20% of individuals infected with pathogens contribute to 80% of the spread of any infection [51]. Therefore, strategies are required to decrease the transmission rate of Salmonella by chicken, especially super-shedders. There is insufficient information regarding what makes super chicken shedders for these bacteria. Hence, further research is required to determine the underlying reasons [48]. One factor contributing to the colonization and shedding of Salmonella in chickens includes gut microbial composition. Therefore, modulating the gut bacteria of chickens can help in decreasing the colonization of Salmonella in chickens and the eventual spread of this bacterium through feces [47].
5. Gut Microbiota in Chickens
Chickens acquire their microbiota shortly after birth, which is formed initially from the microorganisms inherited from mother hens and through the contamination of eggs by the environment [52]. This microbial community changes throughout the lifetime of chickens and is impacted by the nutritional intake, the environment surrounding chickens, and the sex and breeds of chickens [53]. The gut of chickens contains many microbial species dominated by bacteria [54]. It has been estimated that an individual chicken contains around 100 billion bacterial cells in the GI tract, far more than in any other organ of a chicken’s body. Most chicken GI tract bacteria are anaerobes, which can be obligatory and facultative. In a healthy chicken gut microbiota without dysbiosis, beneficial gram-positive bacteria predominate (as much as 85% of the total microbiome can contain gram-positive bacteria). Even in healthy chicken, Salmonella, other bacteria such as Escherichia coli (E. coli) and Campylobacters, can be present, but in much lower amounts than in the diseased chicken [55]. The gut microbiota can affect chicken physiology by contributing to plant polysaccharide digestion, toxin metabolism, and immunity against pathogens [56,57]. The gut microbiome also contributes to the chicken body mass [58]. This is mainly due to the involvement of microbiota in the generation of nutrients vital for chicken growth, such as amino acids, fatty acids, for example, short-chain fatty acids (SCFAs), vitamins, and ammonia [59].
The colonization of microbiota in the chicken gut also modulates the shape of the GI tract. It helps stimulate chickens’ innate and adaptive immune responses that help the host fight a foreign pathogen. Aside from GI tract shape modulation and immune stimulation, the gut microbiota, due to their advantage of inhabiting the host body earlier and adapting to it, resist the incoming bacteria from permanently colonizing the host. This mechanism is called competitive exclusion, one of the main ways the host body eliminates foreign pathogens [60]. An imbalance in the gut microbiota can cause diseases, low productivity and mass gain, and high mortality in chickens [61]. Several studies exist on the gut microbiota diversity and susceptibility to Salmonella infection in poultry [54,62,63]. Recently, a study by Litvak et al. [64] has shown that one of the commensal bacteria in the chicken gut (i.e., Enterobacteriaceae) protects neonate chickens from Salmonella infection by creating an oxygenated environment. The high oxygenation levels contain the colonization of Salmonella as they are toxic to the bacterium [64].
In most cases, infection with Salmonella does not follow any visible symptoms in chickens, and chickens remain asymptomatic carriers of Salmonella throughout their life. However, it has been experimentally shown that Salmonella infection causes suppression of the immune system, especially by increasing Treg cells in chicken, which increases their susceptibility to acquiring a microbial infection [65]. Therefore, it is important in the poultry industry to maintain a healthy microbiome in the chicken gut.
6. EOs and Their Usage in Chicken Farming
Using plant bioactive compounds in animal feeds has recently gained much attraction. These bioactive plant compounds are also called phytochemicals, phytogenics, or phytobiotics. When ingested into the body, these nutritional compounds are supposed to provide health benefits. A variety of such phytochemicals, when incorporated into animal feed, have been shown to increase body weight and decrease incidences of infectious diseases due to their antimicrobial activities and gut modulation in the animals ingesting such feed [66]. EOs are one of the most potent extracts of plants that can contain a variety of important phytochemicals from the plant. The most common methods for the extraction of EOs from plants include solvent extraction, steam distillation, and hydro distillation [67]. These plant extracts can be either terpenes or phenylpropenes based on the presence of these two major chemical compounds [68]. The two most accepted effects of EOs on the poultry gut include the stimulation of the secretion of digestive enzymes and the promotion of a favorable intestinal microbiota ecosystem. These effects increase feed utilization, reduce the risk of growth-depressing disorders, and decrease susceptibility to infections [69]. The antimicrobial effects of various EOs are generally well-studied. EOs are hydrophobic and work by partitioning the bacterial cell membrane’s lipophilic interior, causing the bacterial cells to burst and leak their cellular contents into the environment [22]. There are currently around 3000 EOs, with about 300 of them, including clove oil, being economically important [67].
6.1. Eugenol Oil from Clove, the Active Substance
Eugenol, known chemically as 4-allyl-2-methoxy phenol, is an essential aromatic oil obtained from various plant sources, such as basil, bay, cinnamon, cloves, ginger, nutmeg, pepper, thyme, turmeric, and tulsi. However, only clove and cinnamon were shown to contain considerably high amounts of eugenol [70]. The oil is present mostly in the buds of cloves, which can contain eugenol in the range of 49 to 90%. On the other hand, eugenol is found mostly in the barks of cinnamon, which can contain 20 to 50% eugenol [71]. This EO has many beneficial effects on different body systems [72]. It belongs to a class of phenylpropenes essential in providing flavor and scent to spices and herbs containing this compound. Eugenol has been demonstrated to function primarily as a pollinator attractant for plants and an antibacterial, antiparasitic, antiviral, and antifungal compound [68]. Pramod et al. [59] also documented that eugenol acts as a significant antioxidant, preventing cellular inflammation, and showed its analgesic effects on local tissues [73]. Eugenol is structurally smaller than many other compounds that have actively been used and identified as antimicrobials; therefore, it may provide new mechanistic insights. According to Mahboub [72], eugenol is soluble in alkaline aqueous solutions and was approved in the human diet. It can be assessed as a safer candidate for studying its antimicrobial properties [72]. According to the Research Institute for Fragrance Materials (RIFM), daily intake of eugenol less than 300 mg/kg/day is safe for consumption and poses no toxicity to the consumer [74]. However, for chickens, the recommended eugenol amount is 100 mg/kg/day according to the Additives and Products or Substances used in Animal Feed (FEEDAP) report of the European Food Safety Authority [EFSA] [75]. The beneficial effects of eugenol supplementation in chicken farming (studied and those that can be studied) are shown in Figure 1 and discussed in the further subsections.
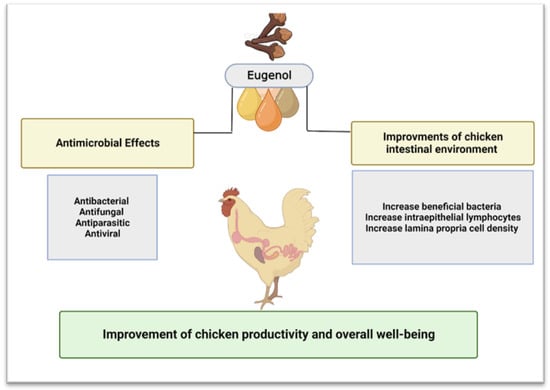
Figure 1.
Beneficial effects of eugenol supplementation in chickens. Created with BioRender.com, accessed on 5 December 2022.
6.1.1. Beneficial Effects of Eugenol on Chicken Gut
Supplementation of eugenol in chicken feed, alone or in addition to different plant extracts, has been previously shown to decrease pathogen counts, reduce disease severity by improving intestinal health, and increase the growth performance of chickens [76]. The beneficial effects of eugenol are related to a significant increase in the abundance of beneficial bacteria like Lactobacilli, immune cells, and intestinal epithelial cells, such as intraepithelial lymphocytes and lamina propria, respectively [77]. The Lactobacilli species are one of the most commonly known probiotic species employed in animal production. In the chickens, the strains belonging to Lactobacilli reduce Salmonella colonization, which ultimately reduces the latter’s fecal shedding [78]. Moreover, an increase in several lymphocytes is associated with improved pathogen clearance, while an increase in lamina propria cell density could help increase Lactobacilli numbers [77,79]. The beneficial effects of eugenol are also corroborated by research in mice. Eugenol administration through drinking water was reported to alter microbiota diversity in the colon and increase inner mucus thickness [80].
6.1.2. Antifungal, Antiparasitic, and Antiviral Effects of Eugenol
Currently, there are no published reports on the antifungal, antiviral, and antiparasitic effects of eugenol in chickens. However, the positive effects of eugenol against the fungi, viruses, and parasites that cause human diseases are well established. Carrasco et al. [81] showed the antifungal activities of eugenol against three human opportunistic pathogen yeasts, namely Candida albicans, C. neoformans, and Saccharomyces cerevisiae, with MIC values ranging from 125 μg/mL to more than 256 μg/mL [81]. During the 2014 Ebola virus outbreak in West Africa, Lane et al. [82] investigated the antiviral activity of Eugenol against Ebola and other viruses. The Ebola virus is the causative agent of fatal hemorrhagic fever in humans. The authors reported that eugenol had shown antiviral activity against Influenza A and Herpes Simplex virus types 1 and 2. Thus, the authors proposed using this natural product against the Ebola virus [82]. Eugenol has also been tested against parasites. Raja et al. [83] screened the inhibitory effect of eugenol derivatives on Leishmania Donovan, the causative agent of visceral leishmaniasis, a life-threatening human parasitic infection. Eugenol derivatives showed strong parasitic activity against this disease, inhibited leishmanial replication, and cleared the infection completely from liver cells [83]. The therapeutic effects of eugenol in humans against these pathogenic organisms can be replicated in chickens to investigate the therapeutic usage of eugenol against chicken fungal, parasitic, and viral diseases.
6.1.3. Antibacterial Effects of Eugenol
The antibacterial activities of eugenol have particularly captivated the attention of various scientists. The free hydroxyl groups in EOs confer antibacterial effects, and eugenol’s antibacterial properties are also due to its free hydroxyl group. This free hydroxyl group disrupts the bacterial cell membrane by altering the fatty acid composition, increasing the reactive oxygen species (ROS) concentration, or altering enzymatic activities. Moreover, the DNA damage effects of eugenol in bacterial cells have also been observed [84]. Recently, eugenol has been shown to decrease the motility and expression of molecules associated with pathogenicity and host cell attachment in chickens [85]. Eugenol has shown antibacterial activity against a variety of Gram-positive bacteria, such as Bacillus cereus, Bacillus subtilis, Enterococcus faecalis, Staphylococcus epidermidis, Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumonia, Listeria monocytogenes, and Gram-negative bacterial species, such as E. coli, Helicobacter pylori, Proteus Vulgaris, Salmonella choleraesuis, S. Typhi, S. Typhimurium, S. Enteritidis, and Yersinia enterocolitica [70,85,86,87]. Eugenol also has been shown to possess biofilm-eradicating capacity. Biofilms are one of the most resilient bacterial structures that allow bacteria to resist the harshest conditions, including the availability of antibiotics in the environment. At a concentration of 0.5 × the minimum inhibitory concentration (MIC), 50 percent inhibition was observed in biofilms generated by methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible Staphylococcus aureus (MSSA). However, combining carvacrol with eugenol lowered the minimum biofilm-eliminating concentration (MBEC) of already-formed biofilms to 99 percent, suggesting the synergistic effect of two EOs against bacterial biofilms [83]. Moreover, eugenol can also decrease the biofilm formed by S. Typhimurium by more than 50%, while the combinational use of cinnamaldehyde and eugenol increases antibiofilm activity against S. Typhimurium by 70% [88].
Anti-Salmonella Effects of Eugenol on Chickens
A handful of studies have reported the anti-Salmonella effects of eugenol. Based on a literature search, the scientific community’s interest in using eugenol for containing Salmonella in poultry is increasing. Incorporating eugenol in poultry feed has been shown to reduce S. Enteritidis colonization [89]. Moreover, Devi et al. [90] concluded that eugenol induced complete inhibition and reduced the viability of S. Typhi. The authors proposed that the main mechanism of action of eugenol against Salmonella was targeting the bacterial cell membrane [90]. Eugenol disrupts the bacterium’s cell membrane, which leads to the disruption of ATP synthesis, and the bacterial cells die due to the lack of energy [91]. In an in vitro chicken cecum model, eugenol treatment resulted in more than a five log10 CFU/mL decrease in S. Enteritidis bacterial cell counts [92].
The in vivo chicken models of eugenol supplementation have also shown promising results. It has been shown by Kollanoor-Johny et al. [86] that the addition of eugenol at 1% in chicken feed can significantly reduce (p < 0.05) the population of S. Enteritidis (>four log10 cfu/g) to 1.5 log10 cfu/g and two log10 cfu/g in the chicken cecum and cloaca, respectively. The supplementation of chicken feed with 0.75 and 1% eugenol significantly reduces (p < 0.05) S. Enteritidis counts in the chicken cecum (at least three log10 CFU/g or more S. Enteritidis reduction), which leads to reduced fecal shedding of the pathogen. The reduction in S. Enteritidis could be attributed to the eugenol-elicited reduced expression of genes involved in the invasion of chickens and the genes involved in metabolism, motility, the type III secretion system (T3SS), outer membrane proteins, and electron acceptor proteins [93,94]. Interestingly, supplementing feed with eugenol reduces chicken body weight gains (p < 0.05). This reduced weight gain could be due to reduced feed intake as the chickens with the eugenol-supplemented feed had significantly reduced (p < 0.05) feed intake in comparison with that of the control chicken groups [94]. One explanation for reduced feed intake due to eugenol or other EO supplementation is reduced palatability based on the strong smell and flavor. However, poultry shows tolerance for the moderate addition of EOs to feed [69]. Thus, other factors might have caused reduced feed intake, which might not have been considered by Kollanoor-Johny et al. [94].
Eugenol severely limits the motility of S. Typhimurium due to its destructive effects on fimbriae and decreases the expression of adhesion molecules and virulence factors. This not only decreases pathogenicity but also significantly reduces the S. Typhimurium counts in chickens, as shown by Zhao et al. [85], who reported a highly significant (p < 0.01) reduction of S. Typhimurium cells due to eugenol pretreatment. These bactericidal and bacteriostatic effects were due to the inhibition of the T3SS and mannose-sensitive type I fimbriae (TIF)-related adhesion virulence factors. The T3SS is a needle-like specialized apparatus capable of transporting effector proteins to host intestinal epithelial cells, critical for Salmonella pathogenicity. On the other hand, TIF is vital for the adherence of S. Typhimurium to host cells. Therefore, attenuating these genetic components lowers the pathogenicity and survivability of S. Typhimurium in chickens [67]. A summary of all studies performed on the anti-Salmonella effects in chickens is presented in Table 1. In contrast, an overview of the reduction of Salmonella colonization inside chicken guts is shown in Figure 2.

Table 1.
Effect of Eugenol supplementation on containing Salmonella colonization in chickens and spread.
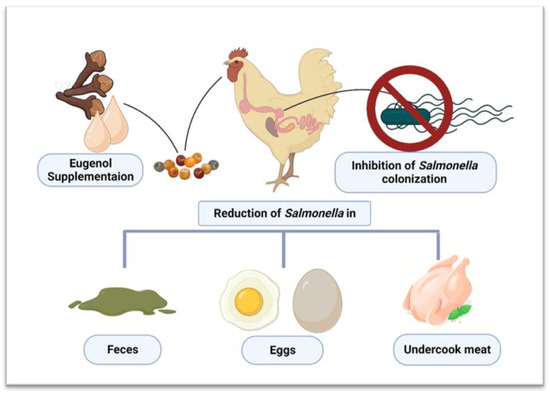
Figure 2.
Effect of Eugenol supplementation in controlling Salmonella colonization in chickens and its spread. Created with BioRender.com, 5 December 2022.
7. Conclusions
Salmonellosis is a zoonotic disease that is caused by the Salmonella bacterium. Poultry serves as one of the main reservoirs of Salmonella and is a significant source of its transmission to humans and the environment. In humans, Salmonella infections are responsible for salmonellosis, a disease that is among the most prominent foodborne zoonotic diseases. With the increasing use of chicken manure and antibiotics in the poultry industry, incidences of salmonellosis are expected to rise even further. Eugenol has the potential to serve as a safe alternative to antibiotics in poultry production for containing salmonellosis. Eugenol has been shown to improve the secretion of digestive enzymes and the overall diversity of the chicken gut microbiota.
Moreover, in vitro and in vivo models of Salmonella (especially of S. Enteritidis and S. Typhimurium, the causative agents of salmonellosis in humans) infection in chickens have shown the beneficial effects of eugenol. However, there is still a lack of mechanistic studies describing how eugenol supplementation reduces Salmonella in chickens. Moreover, only the effect of eugenol on Salmonella strains that cause salmonellosis in humans has been studied, highlighting the need to investigate eugenol’s effect on poultry disease-specific strains, such as S. Pullorum and S. Gallinarum as well. Both of these strains are one of the major causes of economic destruction to the poultry industry [83]. Therefore, studies exploring the molecular mechanisms of eugenol targeting Salmonella inside the chicken body and the therapeutic efficacy of eugenol against poultry-specific Salmonella strains are needed. Moreover, attention must also be paid to investigating the metabolic changes accompanying eugenol intake inside the bodies of chickens.
Author Contributions
Conceptualization, M.A., I.A.M. and Y.M.K.; methodology, M.A., I.A.M. and Y.M.K.; validation, M.A., I.A.M. and Y.M.K.; formal analysis Y.M.K., I.A.M. and M.A.; resources, Y.M.K.; data curation, I.A.M. and M.A.; writing—original draft preparation, I.A.M. and M.A.; writing—review and editing, M.A., I.A.M. and Y.M.K.; visualization, M.A.; supervision, Y.M.K.; project administration, Y.M.K.; funding acquisition, Y.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the University of Arkansas-Fayetteville, United States of America.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- USDA ERS—Food Availability and Consumption. Available online: https://www.ers.usda.gov/data-products/ag-and-food-statistics-charting-the-essentials/food-availability-and-consumption/ (accessed on 29 January 2023).
- Egg-STAT-Ic About Eggs. Available online: https://www.usda.gov/media/blog/2020/04/14/egg-stat-ic-about-eggs (accessed on 29 January 2023).
- Scharff, R.L. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. J. Food Prot. 2020, 83, 959–967. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in Foods by Using Essential Oils: A Review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- USDA Launches New Effort to Reduce Salmonella Illnesses Linked to Poultry. Available online: https://www.usda.gov/media/press-releases/2021/10/19/usda-launches-new-effort-reduce-salmonella-illnesses-linked-poultry (accessed on 29 January 2023).
- Karabasanavar, N.; Madhavaprasad, C.; Gopalakrishna, S.; Hiremath, J.; Patil, G.; Barbuddhe, S. Prevalence of Salmonella Serotypes S. Enteritidis and S. Typhimurium in Poultry and Poultry Products. J. Food Saf. 2020, 40, e12852. [Google Scholar] [CrossRef]
- Saleh, S.; Van Puyvelde, S.; Staes, A.; Timmerman, E.; Barbé, B.; Jacobs, J.; Gevaert, K.; Deborggraeve, S. Salmonella Typhi, Paratyphi A, Enteritidis and Typhimurium Core Proteomes Reveal Differentially Expressed Proteins Linked to the Cell Surface and Pathogenicity. PLoS Negl. Trop. Dis. 2019, 13, e0007416. [Google Scholar] [CrossRef]
- Alghoribi, M.F.; Doumith, M.; Alrodayyan, M.; Al Zayer, M.; Köster, W.L.; Muhanna, A.; Aljohani, S.M.; Balkhy, H.H.; Desin, T.S. S. Enteritidis and S. Typhimurium Harboring SPI-1 and SPI-2 Are the Predominant Serotypes Associated With Human Salmonellosis in Saudi Arabia. Front. Cell. Infect. Microbiol. 2019, 9, 187. [Google Scholar] [CrossRef]
- Campos, M.J.; Palomo, G.; Hormeño, L.; Ugarte, M.; Porrero, M.C.; Herrera-León, S.; Vadillo, S.; Píriz, S.; Quesada, A. Co-Occurrence of ACSSuT and Cephalosporin Resistance Phenotypes Is Mediated by Int1-Associated Elements in Nontyphoidal Salmonella Enterica from Human Infections in Spain. Microb. Drug Resist. 2013, 19, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, M.; Simon, S.; Meinen, A.; Trost, E.; Banerji, S.; Pfeifer, Y.; Flieger, A. Third Generation Cephalosporin Resistance in Clinical Non-Typhoidal Salmonella Enterica in Germany and Emergence of Bla CTX-M-Harbouring pESI Plasmids. Microb. Genom. 2021, 7, 000698. [Google Scholar] [CrossRef]
- Rychlik, I.; Elsheimer-Matulova, M.; Kyrova, K. Gene Expression in the Chicken Caecum in Response to Infections with Non-Typhoid Salmonella. Vet. Res. 2014, 45, 119. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.L.; Sharma, V.; Chapin, T.K.; Friedrich, L.M.; Larson, C.C.; Rodrigues, C.; Jay-Russell, M.; Schneider, K.R.; Danyluk, M.D. The Prevalence and Concentration of Salmonella Enterica in Poultry Litter in the Southern United States. PLoS ONE 2022, 17, e0268231. [Google Scholar] [CrossRef]
- Ngogang, M.P.; Ernest, T.; Kariuki, J.; Mouliom Mouiche, M.M.; Ngogang, J.; Wade, A.; van der Sande, M.A.B. Microbial Contamination of Chicken Litter Manure and Antimicrobial Resistance Threat in an Urban Area Setting in Cameroon. Antibiotics 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Chinivasagam, H.N.; Tran, T.; Blackall, P.J. Impact of the Australian Litter Reuse Practice on Salmonella in the Broiler Farming Environment. Food Res. Int. 2012, 45, 891–896. [Google Scholar] [CrossRef]
- Bierer, B.W. The Use of Nihydrazone Against Salmonella Typhimurium and Salmonella Gallinarum Infections in Turkeys. Poult. Sci. 1963, 42, 465–468. [Google Scholar] [CrossRef]
- Venkitanarayanan, K.; Kollanoor-Johny, A.; Darre, M.J.; Donoghue, A.M.; Donoghue, D.J. Use of Plant-Derived Antimicrobials for Improving the Safety of Poultry Products. Poult. Sci. 2013, 92, 493–501. [Google Scholar] [CrossRef]
- Acevedo-Villanueva, K.Y.; Renu, S.; Shanmugasundaram, R.; Akerele, G.O.; Gourapura, R.J.; Selvaraj, R.K. Salmonella Chitosan Nanoparticle Vaccine Administration Is Protective against Salmonella Enteritidis in Broiler Birds. PLoS ONE 2021, 16, e0259334. [Google Scholar] [CrossRef]
- Hofacre, C.L.; Rosales, A.G.; Costa, M.D.; Cookson, K.; Schaeffer, J.; Jones, M.K. Immunity and Protection Provided by Live Modified Vaccines Against Paratyphoid Salmonella in Poultry—An Applied Perspective. Avian Dis. 2021, 65, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B. Historical Review of Medicinal Plants′ Usage. Pharmacogn. Rev. 2012, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Mussarat, S.; Ali, R.; Ali, S.; Mothana, R.A.; Ullah, R.; Adnan, M. Medicinal Animals and Plants as Alternative and Complementary Medicine in Southern Regions of Khyber Pakhtunkhwa, Pakistan. Front. Pharmacol. 2021, 12, 801234. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.; Dias, C.; Saavedra, M.; Borges, F.; Simões, M. New Perspectives on the Use of Phytochemicals as an Emergent Strategy to Control Bacterial Infections Including Biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Song, F.; Liu, J.; Zhao, W.; Huang, H.; Hu, D.; Chen, H.; Zhang, H.; Chen, W.; Gu, Z. Synergistic Effect of Eugenol and Probiotic Lactobacillus Plantarum Zs2058 against Salmonella Infection in C57bl/6 Mice. Nutrients 2020, 12, 1611. [Google Scholar] [CrossRef]
- Ahmed, A.O.; Raji, M.A.; Mamman, P.H.; Kwanashie, C.N.; Raufu, I.A.; Aremu, A.; Akorede, G.J. Salmonellosis: Serotypes, Prevalence and Multi-Drug Resistant Profiles of Salmonella Enterica in Selected Poultry Farms, Kwara State, North Central Nigeria. Onderstepoort J. Vet. Res. 2019, 86, 1–8. [Google Scholar] [CrossRef]
- Baird-Parker, A.C. Foodborne Salmonellosis. Lancet 1990, 336, 1231–1235. [Google Scholar] [CrossRef]
- Andino, A.; Hanning, I. Salmonella Enterica: Survival, Colonization, and Virulence Differences among Serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef] [PubMed]
- Punchihewage-Don, A.J.; Hawkins, J.; Adnan, A.M.; Hashem, F.; Parveen, S. The Outbreaks and Prevalence of Antimicrobial Resistant Salmonella in Poultry in the United States: An Overview. Heliyon 2022, 8, e11571. [Google Scholar] [CrossRef]
- Williams, M.S.; Ebel, E.D. Temporal Changes in the Proportion of Salmonella Outbreaks Associated with 12 Food Commodity Groups in the United States. Epidemiol. Infect. 2022, 150, e126. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, J.M.; Totton, S.C.; Plishka, M.; Vriezen, E.R. Salmonella in Animal Feeds: A Scoping Review. Front. Vet. Sci. 2021, 8, 727495. [Google Scholar] [CrossRef]
- Wotzka, S.Y.; Nguyen, B.D.; Hardt, W.-D. Salmonella Typhimurium Diarrhea Reveals Basic Principles of Enteropathogen Infection and Disease-Promoted DNA Exchange. Cell Host Microbe 2017, 21, 443–454. [Google Scholar] [CrossRef]
- Thames, H.T.; Theradiyil Sukumaran, A. A Review of Salmonella and Campylobacter in Broiler Meat: Emerging Challenges and Food Safety Measures. Foods 2020, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, S.; Kim, H.; Kim, Y.; Kim, B.; Beuchat, L.R.; Ryu, J.-H. Fate of Mesophilic Aerobic Bacteria and Salmonella Enterica on the Surface of Eggs as Affected by Chicken Feces, Storage Temperature, and Relative Humidity. Food Microbiol. 2015, 48, 200–205. [Google Scholar] [CrossRef]
- Trampel, D.W.; Holder, T.G.; Gast, R.K. Integrated Farm Management to Prevent Salmonella Enteritidis Contamination of Eggs. J. Appl. Poult. Res. 2014, 23, 353–365. [Google Scholar] [CrossRef]
- Nam, I.S.; Kim, H.S.; Seo, K.M.; Ahn, J.H. Analysis of HACCP System Implementation on Productivity, Advantage and Disadvantage of Laying Hen Farm in Korea. Korean J. Poult. Sci. 2014, 41, 93–98. [Google Scholar] [CrossRef]
- Castanon, J.I.R. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.M.D.; Huque, K.S.; Kamaruddin, K.M.; Haque Beg, A. Global Restriction of Using Antibiotic Growth Promoters and Alternative Strategies in Poultry Production. Sci. Prog. 2018, 101, 52–75. [Google Scholar] [CrossRef]
- Muaz, K.; Riaz, M.; Akhtar, S.; Park, S.; Ismail, A. Antibiotic Residues in Chicken Meat: Global Prevalence, Threats, and Decontamination Strategies: A Review. J. Food Prot. 2018, 81, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Cogliani, C.; Goossens, H.; Greko, C. Restricting Antimicrobial Use in Food Animals: Lessons from Europe: Banning Nonessential Antibiotic Uses in Food Animals Is Intended to Reduce Pools of Resistance Genes | Scinapse. Available online: https://www.scinapse.io/papers/2330428806 (accessed on 29 January 2023).
- Castro-Vargas, R.E.; Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Antibiotic Resistance in Salmonella spp. Isolated from Poultry: A Global Overview. Vet. World 2020, 13, 2070–2084. [Google Scholar] [CrossRef]
- Sethiya, N.K. Review on Natural Growth Promoters Available for Improving Gut Health of Poultry: An Alternative to Antibiotic Growth Promoters. Asian J. Poult. Sci. 2015, 10, 1–29. [Google Scholar] [CrossRef]
- Sheffield, C.L.; Crippen, T.L.; Beier, R.C.; Byrd, J.A. Salmonella Typhimurium in Chicken Manure Reduced or Eliminated by Addition of LT1000. J. Appl. Poult. Res. 2014, 23, 116–120. [Google Scholar] [CrossRef]
- Abougabal, M. Possibility of broiler Production on reused litter. Egypt. Poult. Sci. J. 2019, 39, 405–421. [Google Scholar] [CrossRef]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Ateenyi Basamba, T. How Safe Is Chicken Litter for Land Application as an Organic Fertilizer?: A Review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ren, S.; Niu, T.; Guo, Y.; Qi, S.; Han, X.; Liu, D.; Pan, F. Distribution of Antibiotic-Resistant Bacteria in Chicken Manure and Manure-Fertilized Vegetables. Environ. Sci. Pollut. Res. 2013, 21, 1231–1241. [Google Scholar] [CrossRef]
- Mazza, L.; Xiao, X.; ur Rehman, K.; Cai, M.; Zhang, D.; Fasulo, S.; Tomberlin, J.K.; Zheng, L.; Soomro, A.A.; Yu, Z.; et al. Management of Chicken Manure Using Black Soldier Fly (Diptera: Stratiomyidae) Larvae Assisted by Companion Bacteria. Waste Manag. 2020, 102, 312–318. [Google Scholar] [CrossRef]
- Vaz, C.S.L.; Voss-Rech, D.; de Avila, V.S.; Coldebella, A.; Silva, V.S. Interventions to Reduce the Bacterial Load in Recycled Broiler Litter. Poult. Sci. 2017, 96, 2587–2594. [Google Scholar] [CrossRef]
- Kempf, F.; Menanteau, P.; Rychlik, I.; Kubasová, T.; Trotereau, J.; Virlogeux-Payant, I.; Schaeffer, S.; Schouler, C.; Drumo, R.; Guitton, E.; et al. Gut Microbiota Composition before Infection Determines the Salmonella Super- and Low-shedder Phenotypes in Chicken. Microb. Biotechnol. 2020, 13, 1611–1630. [Google Scholar] [CrossRef] [PubMed]
- Lawley, T.D.; Bouley, D.M.; Hoy, Y.E.; Gerke, C.; Relman, D.A.; Monack, D.M. Host Transmission of Salmonella Enterica Serovar Typhimurium Is Controlled by Virulence Factors and Indigenous Intestinal Microbiota. Infect. Immun. 2008, 76, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Menanteau, P.; Kempf, F.; Trotereau, J.; Virlogeux-Payant, I.; Gitton, E.; Dalifard, J.; Gabriel, I.; Rychlik, I.; Velge, P. Role of Systemic Infection, Cross Contaminations and Super-Shedders in Salmonella Carrier State in Chicken. Environ. Microbiol. 2018, 20, 3246–3260. [Google Scholar] [CrossRef] [PubMed]
- Black, Z.; Balta, I.; Black, L.; Naughton, P.J.; Dooley, J.S.G.; Corcionivoschi, N. The Fate of Foodborne Pathogens in Manure Treated Soil. Front. Microbiol. 2021, 12, 3873. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.J.; Dye, C.; Etard, J.-F.; Smith, T.; Charlwood, J.D.; Garnett, G.P.; Hagan, P.; Hii, J.L.K.; Ndhlovu, P.D.; Quinnell, R.J.; et al. Heterogeneities in the Transmission of Infectious Agents: Implications for the Design of Control Programs. Proc. Natl. Acad. Sci. USA 1997, 94, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chousalkar, K.K. Salmonella Typhimurium Infection Disrupts but Continuous Feeding of Bacillus Based Probiotic Restores Gut Microbiota in Infected Hens. J. Anim. Sci. Biotechnol. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Zhong, T.; Pandya, Y.; Joerger, R.D. 16S rRNA-Based Analysis of Microbiota from the Cecum of Broiler Chickens. Appl. Environ. Microbiol. 2002, 68, 124–137. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, L.; Doyle, M.P. Potential Competitive Exclusion Bacteria from Poultry Inhibitory to Campylobacter Jejuni and Salmonella. J. Food Prot. 2007, 70, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Choct, M. Managing Gut Health through Nutrition. Br. Poult. Sci. 2009, 50, 9–15. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. The Role of the Gut Microbiome in Shaping the Immune System of Chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef]
- Kohl, K.D. Diversity and Function of the Avian Gut Microbiota. J. Comp. Physiol. B 2012, 182, 591–602. [Google Scholar] [CrossRef]
- Santos, R.L. Pathobiology of Salmonella, Intestinal Microbiota, and the Host Innate Immune Response. Front. Immunol. 2014, 5, 252. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal Microbiome of Poultry and Its Interaction with Host and Diet. Gut Microbes 2013, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic Acids and Potential for Modifying the Avian Gastrointestinal Tract and Reducing Pathogens and Disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- Rabsch, W. Competitive Exclusion of Salmonella Enteritidis by Salmonella Gallinarum in Poultry. Emerg. Infect. Dis. 2000, 6, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Schneitz, C.; Koivunen, E.; Tuunainen, P.; Valaja, J. The Effects of a Competitive Exclusion Product and Two Probiotics on Salmonella Colonization and Nutrient Digestibility in Broiler Chickens. J. Appl. Poult. Res. 2016, 25, 396–406. [Google Scholar] [CrossRef]
- Litvak, Y.; Mon, K.K.Z.; Nguyen, H.; Chanthavixay, G.; Liou, M.; Velazquez, E.M.; Kutter, L.; Alcantara, M.A.; Byndloss, M.X.; Tiffany, C.R.; et al. Commensal Enterobacteriaceae Protect against Salmonella Colonization through Oxygen Competition. Cell Host Microbe 2019, 25, 128–139.e5. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Kogut, M.H.; Arsenault, R.J.; Swaggerty, C.L.; Cole, K.; Reddish, J.M.; Selvaraj, R.K. Effect of Salmonella Infection on Cecal Tonsil Regulatory T Cell Properties in Chickens. Poult. Sci. 2015, 94, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Moharreri, M.; Vakili, R.; Oskoueian, E.; Rajabzadeh, G. Effects of Microencapsulated Essential Oils on Growth Performance and Biomarkers of Inflammation in Broiler Chickens Challenged with Salmonella Enteritidis. J. Saudi Soc. Agric. Sci. 2022, 21, 349–357. [Google Scholar] [CrossRef]
- Micciche, A.; Rothrock, M.J.; Yang, Y.; Ricke, S.C. Essential Oils as an Intervention Strategy to Reduce Campylobacter in Poultry Production: A Review. Front. Microbiol. 2019, 10, 1058. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Saad, A.M.; Salem, H.M.; Ashry, N.M.; Abo Ghanima, M.M.; Shukry, M.; Swelum, A.A.; Taha, A.E.; El-Tahan, A.M.; et al. Essential Oils and Their Nanoemulsions as Green Alternatives to Antibiotics in Poultry Nutrition: A Comprehensive Review. Poult. Sci. 2022, 101, 101584. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.-M. Potential of Essential Oils for Poultry and Pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Khalil, A.A.; ur Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential Oil Eugenol: Sources, Extraction Techniques and Nutraceutical Perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- Moon, S.H.; Waite-Cusic, J.; Huang, E. Control of Salmonella in Chicken Meat Using a Combination of a Commercial Bacteriophage and Plant-Based Essential Oils. Food Control 2020, 110, 106984. [Google Scholar] [CrossRef]
- Mahboub, R. Structural Conformational Study of Eugenol Derivatives Using Semiempirical Methods. Adv. Chem. 2014, 2014, 1–5. [Google Scholar] [CrossRef]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A Natural Compound with Versatile Pharmacological Actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Kromidas, L.; La Cava, S.; et al. RIFM Fragrance Ingredient Safety Assessment, Ethylene Brassylate, CAS Registry Number 105-95-3. Food Chem. Toxicol. 2016, 97, S192–S200. [Google Scholar] [CrossRef]
- Scientific Opinion on the Safety and Efficacy of Liderfeed® (Eugenol) for Chickens for Fattening. EFSA J. 2015, 13, 4273. [CrossRef]
- Kumar, A.; Sharma, N.K.; Kheravii, S.K.; Keerqin, C.; Ionescu, C.; Blanchard, A.; Wu, S.-B. Potential of a Mixture of Eugenol and Garlic Tincture to Improve Performance and Intestinal Health in Broilers under Necrotic Enteritis Challenge. Anim. Nutr. 2022, 8, 26–37. [Google Scholar] [CrossRef]
- Agostini, P.S.; Solà-Oriol, D.; Nofrarías, M.; Barroeta, A.C.; Gasa, J.; Manzanilla, E.G. Role of In-Feed Clove Supplementation on Growth Performance, Intestinal Microbiology, and Morphology in Broiler Chicken. Livest. Sci. 2012, 147, 113–118. [Google Scholar] [CrossRef]
- VT Nair, D.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-Resistant Salmonella in the Food Supply and the Potential Role of Antibiotic Alternatives for Control. Foods 2018, 7, 167. [Google Scholar] [CrossRef]
- Matsumoto, S.; Nanno, M.; Watanabe, N.; Miyashita, M.; Amasaki, H.; Suzuki, K.; Umesaki, Y. Physiological Roles of Γδ T-cell Receptor Intraepithelial Lymphocytes in Cytoproliferation and Differentiation of Mouse Intestinal Epithelial Cells. Immunology 1999, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wlodarska, M.; Willing, B.P.; Bravo, D.M.; Finlay, B.B. Phytonutrient Diet Supplementation Promotes Beneficial Clostridia Species and Intestinal Mucus Secretion Resulting in Protection against Enteric Infection. Sci. Rep. 2015, 5, 9253. [Google Scholar] [CrossRef]
- Carrasco, H.; Raimondi, M.; Svetaz, L.; Liberto, M.D.; Rodriguez, M.V.; Espinoza, L.; Madrid, A.; Zacchino, S. Antifungal Activity of Eugenol Analogues. Influence of Different Substituents and Studies on Mechanism of Action. Molecules 2012, 17, 1002–1024. [Google Scholar] [CrossRef]
- Lane, T.; Anantpadma, M.; Freundlich, J.S.; Davey, R.A.; Madrid, P.B.; Ekins, S. The Natural Product Eugenol Is an Inhibitor of the Ebola Virus In Vitro. Pharm. Res. 2019, 36, 104. [Google Scholar] [CrossRef] [PubMed]
- Charan Raja, M.R.; Velappan, A.B.; Chellappan, D.; Debnath, J.; Kar Mahapatra, S. Eugenol Derived Immunomodulatory Molecules against Visceral Leishmaniasis. Eur. J. Med. Chem. 2017, 139, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial Activity of Eugenol and Essential Oils Containing Eugenol: A Mechanistic Viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, S.; Tian, Q.; Peng, W.; Tao, Y.; Bo, R.; Liu, M.; Li, J. Eugenol Exposure in Vitro Inhibits the Expressions of T3SS and TIF Virulence Genes in Salmonella Typhimurium and Reduces Its Pathogenicity to Chickens. Microb. Pathog. 2022, 162, 105314. [Google Scholar] [CrossRef]
- Kollanoor-Johny, A.; Upadhyay, A.; Baskaran, S.A.; Upadhyaya, I.; Mooyottu, S.; Mishra, N.; Darre, M.J.; Khan, M.I.; Donoghue, A.M.; Donoghue, D.J.; et al. Effect of Therapeutic Supplementation of the Plant Compounds Trans-Cinnamaldehyde and Eugenol on Salmonella Enterica Serovar Enteritidis Colonization in Market-Age Broiler Chickens. J. Appl. Poult. Res. 2012, 21, 816–822. [Google Scholar] [CrossRef]
- Charan Raja, M.R. Versatile and Synergistic Potential of Eugenol: A Review. Pharm. Anal. Acta 2015, 06, 1000367. [Google Scholar] [CrossRef]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R.. Evaluation of Antibiofilm Efficacy of Essential Oil Components Β-caryophyllene, Cinnamaldehyde and Eugenol Alone and in Combination against Biofilm Formation and Preformed Biofilms of Listeria Monocytogenes and Salmonella Typhimurium. Lett. Appl. Microbiol. 2020, 71, 195–202. [Google Scholar] [CrossRef]
- Pichika, M.; Mak, K.-K.; Kamal, M.; Ayuba, S.; Sakirolla, R.; Kang, Y.-B.; Mohandas, K.; Balijepalli, M.; Ahmad, S. A Comprehensive Review on Eugenol’s Antimicrobial Properties and Industry Applications: A Transformation from Ethnomedicine to Industry. Pharmacogn. Rev. 2019, 13, 1. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella Typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Wagle, B.R.; Arsi, K.; Shrestha, S.; Upadhyay, A.; Upadhyaya, I.; Bhargava, K.; Donoghue, A.; Donoghue, D.J. Eugenol as an Antimicrobial Wash Treatment reducesCampylobacter Jejuniin Postharvest Poultry. J. Food Saf. 2019, 39, e12704. [Google Scholar] [CrossRef]
- Kollanoor Johny, A.; Darre, M.J.; Donoghue, A.M.; Donoghue, D.J.; Venkitanarayanan, K. Antibacterial Effect of Trans-Cinnamaldehyde, Eugenol, Carvacrol, and Thymol on Salmonella Enteritidis and Campylobacter Jejuni in Chicken Cecal Contents in Vitro. J. Appl. Poult. Res. 2010, 19, 237–244. [Google Scholar] [CrossRef]
- Kollanoor Johny, A.; Frye, J.G.; Donoghue, A.; Donoghue, D.J.; Porwollik, S.; McClelland, M.; Venkitanarayanan, K. Gene Expression Response of Salmonella Enterica Serotype Enteritidis Phage Type 8 to Subinhibitory Concentrations of the Plant-Derived Compounds Trans-Cinnamaldehyde and Eugenol. Front. Microbiol. 2017, 8, 1828. [Google Scholar] [CrossRef]
- Kollanoor-Johny, A.; Mattson, T.; Baskaran, S.A.; Amalaradjou, M.A.; Babapoor, S.; March, B.; Valipe, S.; Darre, M.; Hoagland, T.; Schreiber, D.; et al. Reduction of Salmonella Enterica Serovar Enteritidis Colonization in 20-Day-Old Broiler Chickens by the Plant-Derived Compounds Trans -Cinnamaldehyde and Eugenol. Appl. Environ. Microbiol. 2012, 78, 2981–2987. [Google Scholar] [CrossRef]
- Ordóñez, G.; Llopis, N.; Peñalver, P. Efficacy of Eugenol Against a Salmonella Enterica Serovar Enteritidis Experimental Infection in Commercial Layers in Production. J. Appl. Poult. Res. 2008, 17, 376–382. [Google Scholar] [CrossRef]
- Upadhyaya, I.; Upadhyay, A.; Kollanoor-Johny, A.; Darre, M.; Venkitanarayanan, K. Effect of Plant Derived Antimicrobials on Salmonella Enteritidis Adhesion to and Invasion of Primary Chicken Oviduct Epithelial Cells in Vitro and Virulence Gene Expression. Int. J. Mol. Sci. 2013, 14, 10608–10625. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).