Argas persicus and Carios vespertilionis Ticks Infesting Ducks, Domestic Fowls and Bats in Pakistan: First Report on Molecular Survey and Phylogenetic Position of Borrelia anserina
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
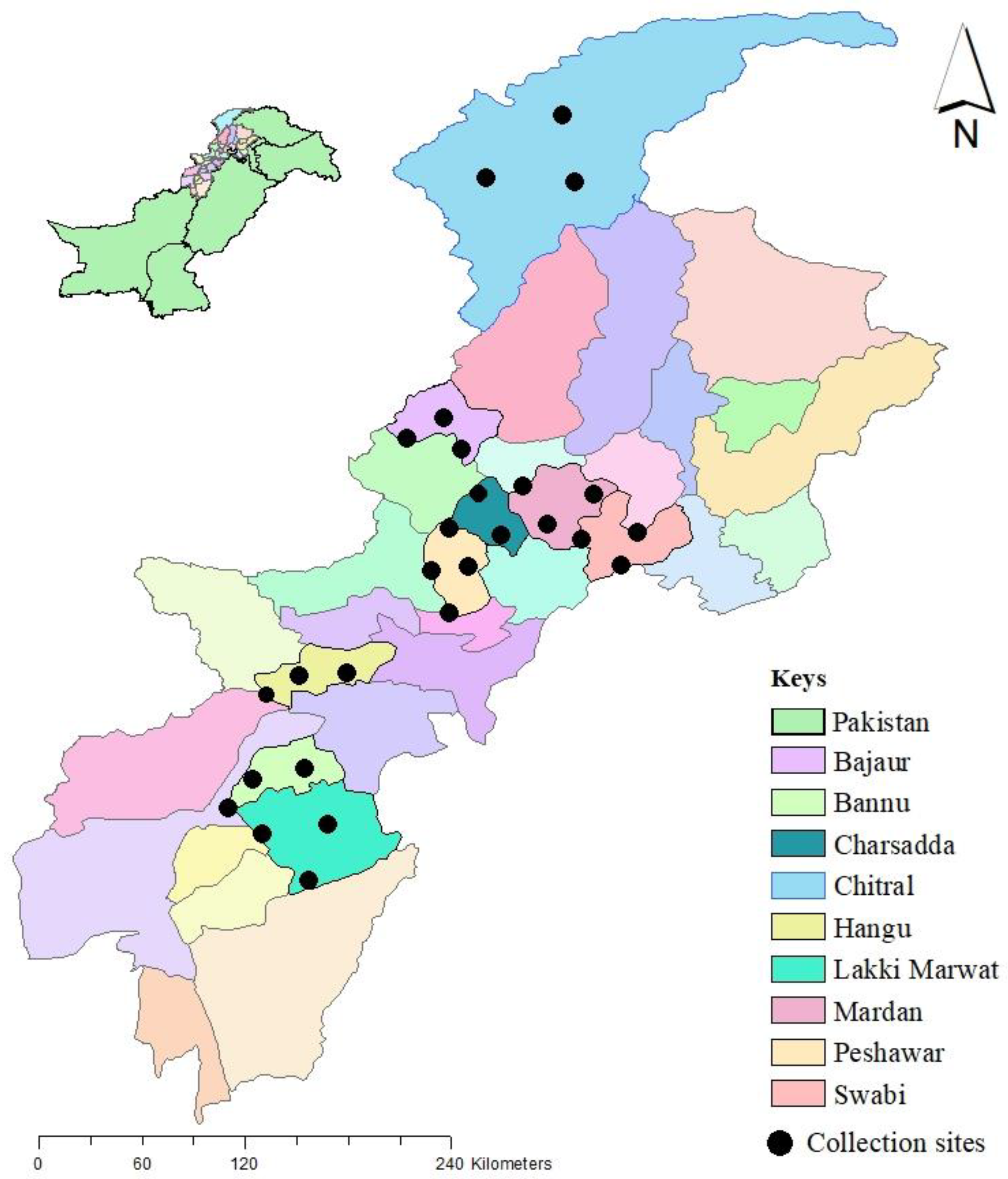
2.1. Collection Sites
2.2. Ethical Approval
2.3. Tick Collection, Preservations and Identification
2.4. Molecular Analyses
2.5. Sequence and Phylogenetic Analysis
2.6. Statistical Analyses
3. Results
3.1. Tick Infestation
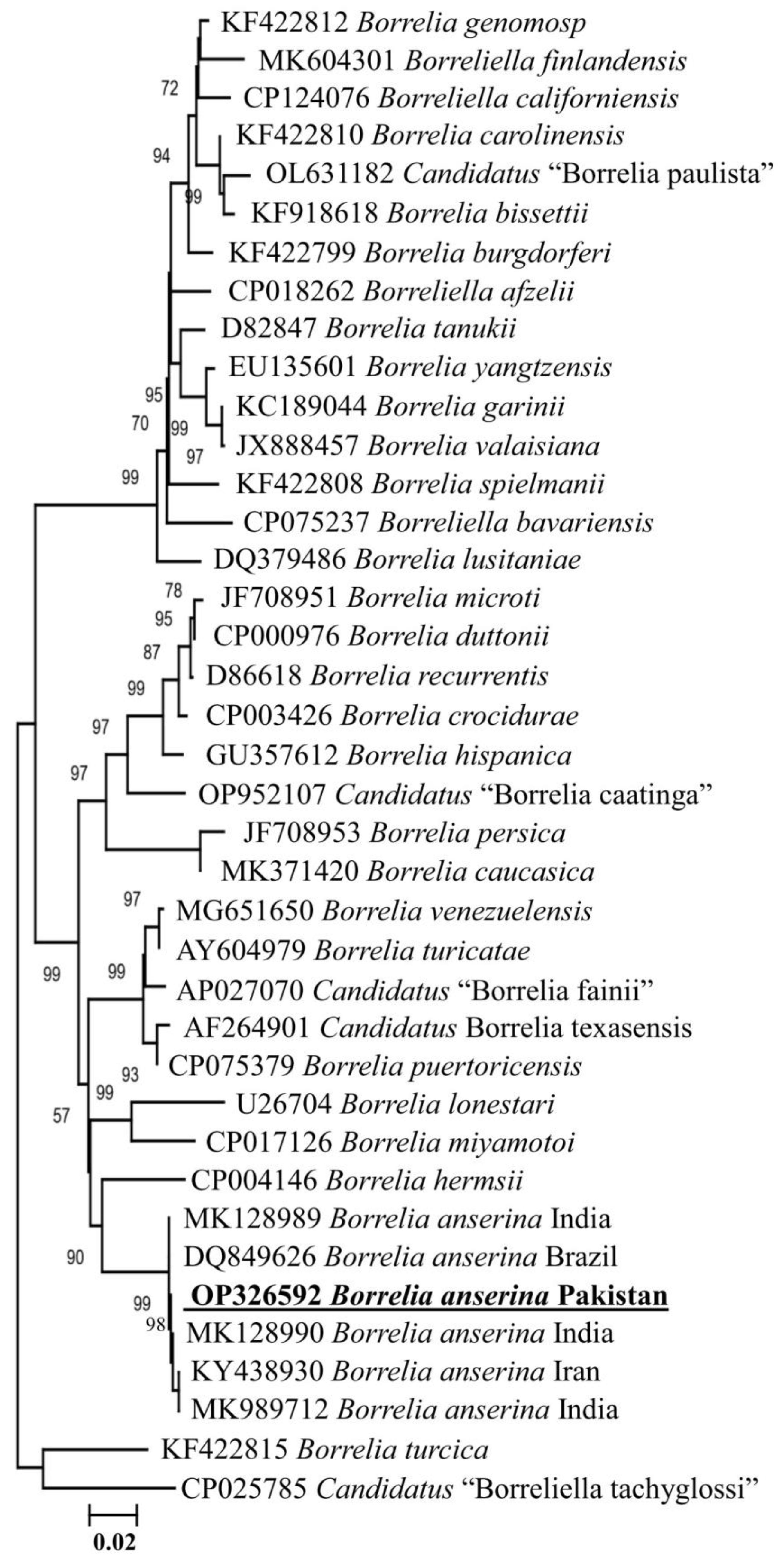
3.2. Detection of Borrelia anserina
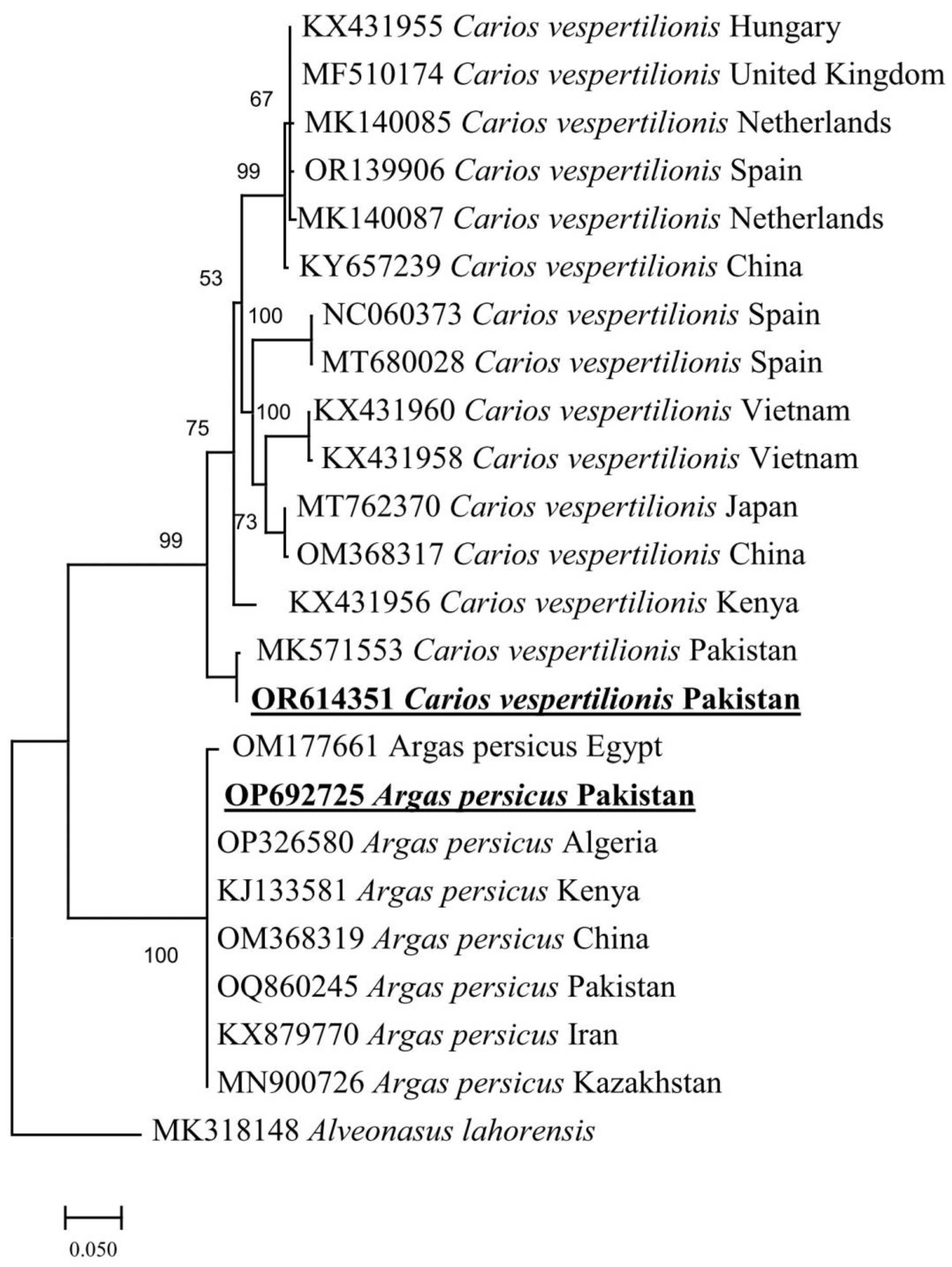
3.3. Molecular and Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turell, M.J.; Mores, C.N.; Lee, J.S.; Paragas, J.J.; Shermuhemedova, D.; Endy, T.P.; Khodjaev, S. Experimental transmission of Karshi and Langat (tick-borne encephalitis virus complex) viruses by Ornithodoros ticks (Acari: Argasidae). J. Med. Entomol. 2004, 41, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.; Muñoz-Leal, S.; Khan, M.Q.; Alouffi, A.S.; Labruna, M.B.; Ali, A. Life Cycle and Genetic Identification of Argas persicus Infesting Domestic Fowl in Khyber Pakhtunkhwa, Pakistan. Front. Vet. Sci. 2021, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Ouchene, N.; Nebbak, A.; Ouchene-Khelifi, N.A.; Dahmani, A.; Zeroual, F.; Khelef, D.; Bitam, I.; Benakhla, A.; Parola, P. Molecular detection of avian spirochete Borrelia anserina in Argas persicus ticks in Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2020, 68, 101408. [Google Scholar] [CrossRef] [PubMed]
- Brook, C.E.; Dobson, A.P. Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends Microbiol. 2015, 23, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Venzal, J.M.; González-Acuña, D.; Guglielmone, A.A. Argas (Persicargas) keiransi n. sp. (Acari: Argasidae), a parasite of the Chimango, Milvago c. chimango (Aves: Falconiformes) in Chile. J. Med. Entomol. 2003, 40, 766–769. [Google Scholar] [CrossRef]
- Khoury, C.; Maroli, M. The pigeon tick, Argas reflexus, and hazard for human health. Ann. Ist. Super. Sanita 2004, 40, 427–432. [Google Scholar]
- Elelu, N. Tick-borne relapsing fever as a potential veterinary medical problem. Vet. Med. Sci. 2018, 4, 271–279. [Google Scholar] [CrossRef]
- Lisbôa, R.S.; Teixeira, R.C.; Rangel, C.P.; Santos, H.A.; Massard, C.L.; Fonseca, A.H. Avian spirochetosis in chickens following experimental transmission of Borrelia anserina by Argas (Persicargas) miniatus. Avian Dis. 2009, 53, 166–168. [Google Scholar] [CrossRef]
- Cutler, S.; Abdissa, A.; Adamu, H.; Tolosa, T.; Gashaw, A. Borrelia in Ethiopian ticks. Ticks Tick Borne Dis. 2012, 3, 14–17. [Google Scholar] [CrossRef]
- Sakharoff, M.N. Spirochaeta anserina et la septicemie des oies. Ann. Inst. Pasteur 1891, 5, 564–566. [Google Scholar]
- Faccini-Martínez, Á.A.; Silva-Ramos, C.R.; Santodomingo, A.M.; Ramírez-Hernández, A.; Costa, F.B.; Labruna, M.B.; Muñoz-Leal, S. Historical overview and update on relapsing fever group Borrelia in Latin America. Parasit. Vectors 2022, 15, 196. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.; Wilhelmsson, P. First Record of a Suspected Human-Pathogenic Borrelia Species in Populations of the Bat Tick Carios vespertilionis in Sweden. Microorganisms 2021, 9, 1100. [Google Scholar] [CrossRef] [PubMed]
- Piksa, K.; Stańczak, J.; Biernat, B.; Górz, A.; Nowak-Chmura, M.; Siuda, K. Detection of Borrelia burgdorferi sensu lato and spotted fever group rickettsiae in hard ticks (Acari, Ixodidae) parasitizing bats in Poland. Parasitol. Res. 2016, 115, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.J.; Ruzic-Sabljic, E.; Potkonjak, A. Emerging borreliae–Expanding beyond Lyme borreliosis. Mol. Cell. Probes. 2016, 31, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Colunga-Salas, P.; Sánchez-Montes, S.; Volkow, P.; Ruíz-Remigio, A.; Becker, I. Lyme disease and relapsing fever in Mexico: An overview of human and wildlife infections. PLoS ONE 2020, 15, e0238496. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing fevers: Neglected tick-borne diseases. Front. Cell. Infect. Microbiol. 2018, 8, 98. [Google Scholar] [CrossRef]
- Barbour, A.G.; Schwan, T.G. Borrelia. In Bergey’s Manual of Systematics of Archea and Bacteria; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Margos, G.; Fingerle, V.; Cutler, S.; Gofton, A.; Stevenson, B.; Estrada-Peña, A. Controversies in bacterial taxonomy: The example of the genus Borrelia. Ticks Tick Borne Dis. 2020, 11, 101335. [Google Scholar] [CrossRef]
- Binetruy, F.; Garnier, S.; Boulanger, N.; Talagrand-Reboul, E.; Loire, E.; Faivre, B.; Noël, V.; Buysse, M.; Duron, O. A novel Borrelia species, intermediate between Lyme disease and relapsing fever groups, in neotropical passerine-associated ticks. Sci. Rep. 2020, 10, 10596. [Google Scholar] [CrossRef]
- DaMassa, A.J.; Adler, H.E. Avian spirochetosis: Natural transmission by Argas (Persicargas) sanchezi (Ixodoidea: Argasidae) and existence of different serologic and immunologic types of Borrelia anserina in the United States. Am. J. Vet. Res. 1979, 40, 154–157. [Google Scholar]
- Ataliba, A.C.; Resende, J.S.; Yoshinari, N.; Labruna, M.B. Isolation and molecular characterization of a Brazilian strain of Borrelia anserina, the agent of fowl spirochaetosis. Res. Vet. Sci. 2007, 83, 145–149. [Google Scholar] [CrossRef]
- Muñoz-Leal, S.; Marcili, A.; Fuentes-Castillo, D.; Ayala, M.; Labruna, M.B. A relapsing fever Borrelia and spotted fever Rickettsia in ticks from an Andean valley, central Chile. Exp. Appl. Acarol. 2019, 78, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Islam, N.; Khan, A.; Islam, Z.U.; Muñoz-Leal, S.; Labruna, M.B.; Ali, A. New records of Amblyomma gervaisi from Pakistan, with detection of a reptile-associated Borrelia sp. Ticks Tick Borne Dis. 2022, 13, 102047. [Google Scholar] [CrossRef] [PubMed]
- Elbir, H.; Sitlani, P.; Bergström, S.; Barbour, A.G. Chromosome and megaplasmid sequences of Borrelia anserina (Sakharoff 1891), the agent of avian spirochetosis and type species of the genus. Genome Announc. 2017, 5, e00018-17. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Budachetri, K.; Mukherjee, N.; Williams, J.; Kausar, A.; Hassan, M.J.; Adamson, S.; Dowd, S.E.; Apanskevich, D.; Arijo, A.; et al. A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 2017, 11, e0005681. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraal, H.; Varma, M.G.R. Haemaphysalis cornupunctata sp. n. and H. kashmirensis sp. n. from Kashmir, with Notes on H. sundrai Sharif and H. sewelli Sharif of India and Pakistan (Ixodoidea, Ixodidae). J. Parasitol. 1962, 48, 185–194. [Google Scholar] [CrossRef]
- Hoogstraal, H. Ticks and spirochetes. Acta Trop. 1979, 36, 133–136. [Google Scholar]
- Ullah, H.; Kontschán, J.; Takács, N.; Wijnveld, M.; Schötta, A.M.; Boldogh, S.A.; Sándor, A.D.; Szekeres, S.; Görföl, T.; Rasheed, S.B.; et al. A new Rickettsia honei related genotype, two novel soft tick haplotypes and first records of three mite species associated with bats in Pakistan. Syst. Appl. Acarol. 2019, 24, 2106–2118. [Google Scholar]
- Ali, A.; Zahid, H.; Zeb, I.; Tufail, M.; Khan, S.; Haroon, M.; Bilal, M.; Hussain, M.; Alouffi, A.S.; Muñoz-Leal, S. Risk factors associated with tick infestations on equids in Khyber Pakhtunkhwa, Pakistan, with notes on Rickettsia massiliae detection. Parasit. Vectors 2021, 14, 363. [Google Scholar] [CrossRef]
- Ali, A.; Shehla, S.; Zahid, H.; Ullah, F.; Zeb, I.; Ahmed, H.; Vaz Jr, I.; Tanaka, T. Molecular survey and spatial distribution of Rickettsia spp. in ticks infesting free-ranging wild animals in Pakistan (2017–2021). Pathogens 2022, 11, 162. [Google Scholar] [CrossRef]
- Ali, A.; Numan, M.; Khan, M.; Aiman, O.; Muñoz-Leal, S.; Chitimia-Dobler, L.; Labruna, M.B.; Nijhof, A.M. Ornithodoros (Pavlovskyella) ticks associated with a Rickettsia sp. in Pakistan. Parasit. Vectors 2022, 15, 138. [Google Scholar] [CrossRef]
- Alam, S.; Khan, M.; Alouffi, A.; Almutairi, M.M.; Ullah, S.; Numan, M.; Islam, N.; Khan, Z.; Aiman, O.; Zaman Safi, S.; et al. Spatio-Temporal Patterns of Ticks and Molecular Survey of Anaplasma marginale, with Notes on Their Phylogeny. Microorganisms 2022, 10, 1663. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Numan, M.; Alouffi, A.; Almutairi, M.M.; Zahid, H.; Khan, M.; Islam, Z.U.; Kamil, A.; Safi, S.Z.; Ahmed, H.; et al. Molecular Characterization and Assessment of Risk Factors Associated with Theileria annulata Infection. Microorganisms 2022, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Kohls, G.M.; Hoogstraal, H.; Clifford, C.M.; Kaiser, M.N. The subgenus Persicargas (Ixodoidea, Argasidae, Argas). 9. Redescription new world records of Argas (P.) persicus (Oken), resurrection, redescription, records of A. (P.) radiatus railliet A. (P.) sanchezi Dugès, A. (P.) miniatus Koch, New World ticks misidentified as A. (P.) persicus. Ann. Entomol. Soc. Am. 1970, 63, 590–606. [Google Scholar]
- Hoogstraal, H. African Ixodoidea; US Government Printing Office: Washington, DC, USA, 1956; Volume 1. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Stromdahl, E.Y.; Williamson, P.C.; Kollars, T.M.J.; Evans, S.R.; Barry, R.K.; Vince, M.A.; Dobbs, N.A. Evidence of Borrelia lonestari DNA in Amblyomma americanum (Acari: Ixodidae) removed from humans. J. Clin. Microbiol. 2003, 41, 5557–5562. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Labruna, M.B.; Whitworth, T.; Horta, M.C.; Bouyer, D.H.; McBride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004, 42, 90–98. [Google Scholar] [CrossRef]
- Inokuma, H.; Raoult, D.; Brouqui, P. Detection of Ehrlichia platys DNA in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island, Japan. J. Clin. Microbiol. 2000, 38, 4219–4221. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ali, A.; Khan, M.A.; Zahid, H.; Yaseen, P.M.; Qayash Khan, M.; Nawab, J.; Ur Rehman, Z.; Ateeq, M.; Khan, S.; Ibrahim, M. Seasonal dynamics, record of ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front. Physiol. 2019, 10, 793. [Google Scholar] [CrossRef]
- MacDonald, A.J. Abiotic and habitat drivers of tick vector abundance, diversity, phenology and human encounter risk in southern California. PLoS ONE 2018, 13, e0201665. [Google Scholar] [CrossRef]
- Ahmad, I.; Ullah, S.; Alouffi, A.; Almutairi, M.M.; Khan, M.; Numan, M.; Safi, S.Z.; Chitimia-Dobler, L.; Tanaka, T.; Ali, A. Description of Male, Redescription of Female, Host Record, and Phylogenetic Position of Haemaphysalis danieli. Pathogens 2022, 11, 1495. [Google Scholar] [CrossRef]
- Gilbert, L.; Aungier, J.; Tomkins, J.L. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: Implications for resilience to climate change? Ecol. Evol. 2014, 4, 1186–1198. [Google Scholar] [CrossRef]
- Numan, M.; Islam, N.; Adnan, M.; Zaman Safi, S.; Chitimia-Dobler, L.; Labruna, M.B.; Ali, A. First genetic report of Ixodes kashmiricus and associated Rickettsia sp. Parasit. Vectors 2022, 15, 378. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Climate change, biodiversity, ticks tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa: A Guide to Species Identification; Springer: Cham, Switzerland, 2018; Volume 404. [Google Scholar]
- Black, W.C.; Piesman, J. Phylogeny of hard-and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. USA 1994, 91, 10034. [Google Scholar] [CrossRef]
- Mans, B.J.; Kelava, S.; Pienaar, R.; Featherston, J.; de Castro, M.H.; Quetglas, J.; Reeves, W.K.; Durden, L.A.; Miller, M.M.; Laverty, T.M.; et al. Nuclear (18S-28S rRNA) and mitochondrial genome markers of Carios (Carios) vespertilionis (Argasidae) support Carios Latreille, 1796 as a lineage embedded in the Ornithodorinae: Re-classification of the Carios sensu Klompen and Oliver (1993) clade into its respective subgenera. Ticks Tick-Borne Dis. 2021, 12, 101688. [Google Scholar]
- Barbour, A.G. Chapter 16. Relapsing fever. In Tick-Borne Diseases of Humans; Goodman, J.L., Dennis, D.T., Sonenshine, D.E., Eds.; ASM Press: Washington, DC, USA, 2005; pp. 268–291. [Google Scholar]
- Parola, P.; Ryelandt, J.; Mangold, A.J.; Mediannikov, O.; Guglielmone, A.A.; Raoult, D. Relapsing fever Borrelia in Ornithodoros ticks from Bolivia. Ann. Trop. Med. Parasitol. 2011, 105, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, S.; Domínguez, L.; Troyo, A.; Venzal, J.M. Ticks infesting humans in Central America: A review of their relevance in public health. Curr. Res. Parasitol. Vector-Borne Dis. 2022, 2, 100065. [Google Scholar] [CrossRef]
- Rocha, S.C.; Velásquez, C.V.; Aquib, A.; Al-Nazal, A.; Parveen, N. Transmission Cycle of Tick-Borne Infections and Co-Infections, Animal Models and Diseases. Pathogens 2022, 11, 1309. [Google Scholar] [CrossRef] [PubMed]
- Socha, W.; Kwasnik, M.; Larska, M.; Rola, J.; Rozek, W. Vector-borne viral diseases as a current threat for human and animal health—One Health perspective. J. Clin. Med. 2022, 11, 3026. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, A.M.; Venzal, J.M.; Marcili, A.; Almeida, A.P.; González, E.M.; Labruna, M.B. Borrelia burgdorferi sensu lato infecting ticks of the Ixodes ricinus complex in Uruguay: First report for the Southern Hemisphere. Vector Borne Zoonotic Dis. 2013, 13, 147–153. [Google Scholar] [CrossRef]
- Nava, S.; Barbieri, A.M.; Maya, L.; Colina, R.; Mangold, A.J.; Labruna, M.B.; Venzal, J.M. Borrelia infection in Ixodes pararicinus ticks (Acari: Ixodidae) from northwestern Argentina. Acta Trop. 2014, 139, 1–4. [Google Scholar] [CrossRef]
- Sebastian, P.S.; Bottero, M.N.S.; Carvalho, L.; Mackenstedt, U.; Lareschi, M.; Venzal, J.M.; Nava, S. Borrelia burgdorferi sensu lato in Ixodes cfneuquenensis and Ixodes sigelos ticks from the Patagonian region of Argentina. Acta Trop. 2016, 162, 218–221. [Google Scholar]
- Muñoz-Leal, S.; Faccini-Martínez, Á.A.; Costa, F.B.; Marcili, A.; Mesquita, E.T.K.C.; Marques, E.P.; Labruna, M.B. Isolation and molecular characterization of a relapsing fever Borrelia recovered from Ornithodoros rudis in Brazil. Ticks Tick-Borne Dis. 2018, 9, 864–871. [Google Scholar] [CrossRef]
- Muñoz-Leal, S.; Faccini-Martínez, Á.A.; Teixeira, B.M.; Martins, M.M.; Serpa, M.C.A.; Oliveira, G.M.; Jorge, F.R.; Pacheco, R.C.; Costa, F.B.; Luz, H.R.; et al. Relapsing fever group borreliae in human-biting soft ticks, Brazil. Emerg. Infect. Dis. 2021, 27, 321. [Google Scholar] [CrossRef]
- Kaiser, A.; Seitz, A.; Strub, O. Prevalence of Borrelia burgdorferi sensu lato in the nightingale (Luscinia megarhynchos) and other passerine birds. Int. J. Med. Microbiol. 2002, 291, 75–79. [Google Scholar] [CrossRef]
| S# | Organism | Gene | Primers Sequence (5′-3′) | Amplicons Size | Reference |
|---|---|---|---|---|---|
| 1 | Tick | cox | HC02198: TAAACTTCAGGGTGACCAAAAAATCA LCO1490: GGTCAACAAATCATAAAGATATTG G | 710 bp | [38] |
| 2 | Tick | 16S | TTTGGGACAAGAAGACCCTATGAATTT ACATCGAGGTCGCAATCAATTTTATC | 250 bp | [2] |
| 3 | Borrelia | Fla LL Fla RL Fla SS Fla RS | ACATATTCAGATGCAGACAGAGGT GCAATCATAGCCATTGCAGATTGT AACAGCTGAAGAGCTTGGAATG CTTTGATCACTTATCATTCTAATAGC | 665 bp 354 bp | [37] |
| 4 | Rickettsia | gltA | CS-78: GCAAGTATCGGTGAGGATGTAAT CS-323: GCTTCCTTAAAATTCAATAAATCAGAT | 401 bp | [39] |
| 5 | Ehrlichia | 16S | EHR16SD: GGTACCYACAGAAGAAGTCC EHR16SR: TGCACTCATCGTTTACAG | 344 bp | [40] |
| Districts | Tick Species | Host | Observed/Infested (%) | No. of Collected Ticks F, M, N, L (n; %) | Detection of Borrelia anserina F, M, N, L | Total (%) |
|---|---|---|---|---|---|---|
| Peshawar | Argas persicus | Shelters | 25/12 (48) | 45, 40, 29, 17 (131; 7.2) | 2, 1, 1, 0 | 4 (10) |
| Domestic fowls | 167/99 (59) | 9, 7, 23, 31 (70; 3.9) | 1, 0, 0, 1 | 2 (5) | ||
| Carios vespertilionis | Bats | 5/1 (40) | 1, 2, 8, 80 (91; 5.0) | 0 | 0 | |
| Mardan | A. persicus | Shelters | 23/11 (47.82) | 47, 44, 41, 15 (147; 8) | 1, 0, 1, 0 | 2 (5) |
| Domestic fowls | 145/89 (61) | 7, 6, 17, 22 (52; 3) | 1, 0, 1, 1 | 3 (7.5) | ||
| C. vespertilionis | Bats | 6/1 (50) | 2, 3, 6, 87 (98; 5.4) | 0 | 0 | |
| Swabi | A. persicus | Shelters | 21/10 (47.61) | 40, 33, 39, 12 (124; 6.8) | 1, 0, 1, 0 | 2 (5) |
| Domestic fowls | 134/99 (74) | 6, 5, 5, 33 (49; 2.7) | 1, 0, 0, 0 | 1 (2.5) | ||
| C. vespertilionis | Bats | 5/1 (40) | 0, 0, 3, 58 (61; 3.3) | 0 | 0 | |
| Charsadda | A. persicus | Shelters | 20/12 (55) | 51, 45, 53, 13 (162; 9) | 1, 0, 1, 0 | 2 (5) |
| Domestic fowls | 116/91 (78) | 4, 3, 14, 11 (32; 1.8) | 0, 0, 0, 1 | 1 (2.5) | ||
| C. vespertilionis | Bats | 7/2 (43) | 4, 3, 12, 70 (89; 5) | 0 | 0 | |
| Chitral | A. persicus | Shelters | 21/5 (23.80) | 11, 9, 21, 3 (44; 2.4) | 1, 0, 0, 1 | 2 (5) |
| Domestic fowls | 142/45 (32) | 2, 3, 3, 6 (14; 1) | 1, 1, 1, 0 | 3 (7.5) | ||
| C. vespertilionis | Bats | 0 | 0 | 0 | 0 | |
| A. persicus | Ducks | 54/19 (35) | 12, 16, 13, 0 (41; 2.2) | 1, 0, 0, 0 | 1 (2.5) | |
| Lakki Marwat | A. persicus | Shelters | 21/7 (33.33) | 45, 33, 53, 9 (140; 7.7) | 1, 0, 0, 0 | 2 (5) |
| Domestic fowls | 112/69 (61) | 6, 3, 6, 14 (29; 1.6) | 1, 0, 1, 1 | 2 (5) | ||
| C. vespertilionis | Bats | 0 | 0 | 0 | 0 | |
| Bannu | A. persicus | Shelters | 20/9 (45) | 42, 32, 45, 11 (130; 7) | 0, 0, 1, 1 | 2 (5) |
| Domestic fowls | 121/66 (55) | 5, 4, 5, 15 (29; 1.6) | 1, 0, 1, 0 | 2 (5) | ||
| C. vespertilionis | Bats | 0 | 0 | 0 | 0 | |
| Bajaur | A. persicus | Shelters | 18/7 (38.88) | 35, 24, 31, 3 (93; 5) | 1, 1, 0, 0 | 2 (5) |
| Domestic fowls | 103/59 (57) | 5, 2, 5, 7 (19; 1) | 1, 0, 1, 1 | 3 (7.5) | ||
| C. vespertilionis | Bats | 0 | 0 | 0 | 0 | |
| Hangu | A. persicus | Shelters | 12/5 (41.66) | 30, 23, 27, 0 (80; 4.4) | 1, 0, 0, 1 | 2 (5) |
| Domestic fowls | 111/46 (41) | 4, 3, 7, 4 (18; 1) | 1, 0, 1, 0 | 2 (5) | ||
| C. vespertilionis | Bats | 4/2 (5) | 2, 2, 9, 62 (75; 4) | 0 | 0 | |
| Total | A. persicus | Shelters | 189/79 (42) | 358, 293, 347, 83 (1081; 59.4) | 10, 2, 5, 3 | 20 (13.6) |
| Domestic fowls | 1205/682 (57) | 48, 42, 90, 143 (323; 17.8) | 8, 1, 7, 4 | 20 (13.6) | ||
| C. vespertilionis | Bats | 27/7 (26) | 9, 10, 38, 357 (414; 22.8) | 0 | 0 | |
| Total | 415, 345, 475, 583 (1818) | 18, 3, 12, 7 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, H.; Alouffi, A.; Almutairi, M.M.; Ateeq, M.; Tanaka, T.; Chang, S.-C.; Chen, C.-C.; Ali, A. Argas persicus and Carios vespertilionis Ticks Infesting Ducks, Domestic Fowls and Bats in Pakistan: First Report on Molecular Survey and Phylogenetic Position of Borrelia anserina. Vet. Sci. 2023, 10, 628. https://doi.org/10.3390/vetsci10100628
Zahid H, Alouffi A, Almutairi MM, Ateeq M, Tanaka T, Chang S-C, Chen C-C, Ali A. Argas persicus and Carios vespertilionis Ticks Infesting Ducks, Domestic Fowls and Bats in Pakistan: First Report on Molecular Survey and Phylogenetic Position of Borrelia anserina. Veterinary Sciences. 2023; 10(10):628. https://doi.org/10.3390/vetsci10100628
Chicago/Turabian StyleZahid, Hafsa, Abdulaziz Alouffi, Mashal M. Almutairi, Muhammad Ateeq, Tetsuya Tanaka, Shun-Chung Chang, Chien-Chin Chen, and Abid Ali. 2023. "Argas persicus and Carios vespertilionis Ticks Infesting Ducks, Domestic Fowls and Bats in Pakistan: First Report on Molecular Survey and Phylogenetic Position of Borrelia anserina" Veterinary Sciences 10, no. 10: 628. https://doi.org/10.3390/vetsci10100628
APA StyleZahid, H., Alouffi, A., Almutairi, M. M., Ateeq, M., Tanaka, T., Chang, S.-C., Chen, C.-C., & Ali, A. (2023). Argas persicus and Carios vespertilionis Ticks Infesting Ducks, Domestic Fowls and Bats in Pakistan: First Report on Molecular Survey and Phylogenetic Position of Borrelia anserina. Veterinary Sciences, 10(10), 628. https://doi.org/10.3390/vetsci10100628









