Accelerated Aging of the Traditional Greek Distillate Tsipouro Using Wooden Chips. Part I: Effect of Static Maceration vs. Ultrasonication on the Polyphenol Extraction and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Wooden Chips
2.3. Distillate
2.4. Aging Treatments
2.5. Sampling and Determinations
2.6. Statistical Analysis
3. Results and Discussion
3.1. Polyphenol Extraction Kinetics
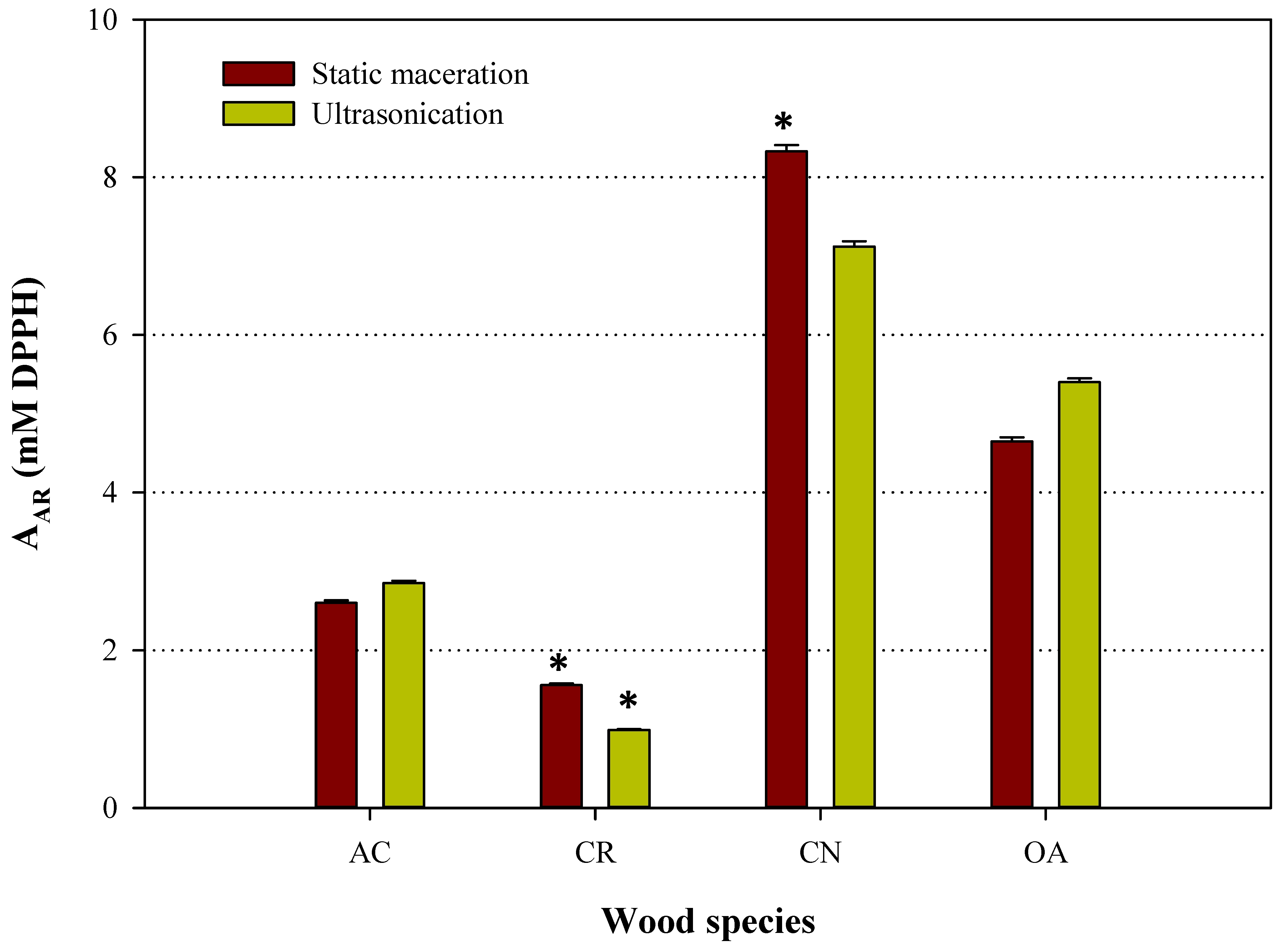
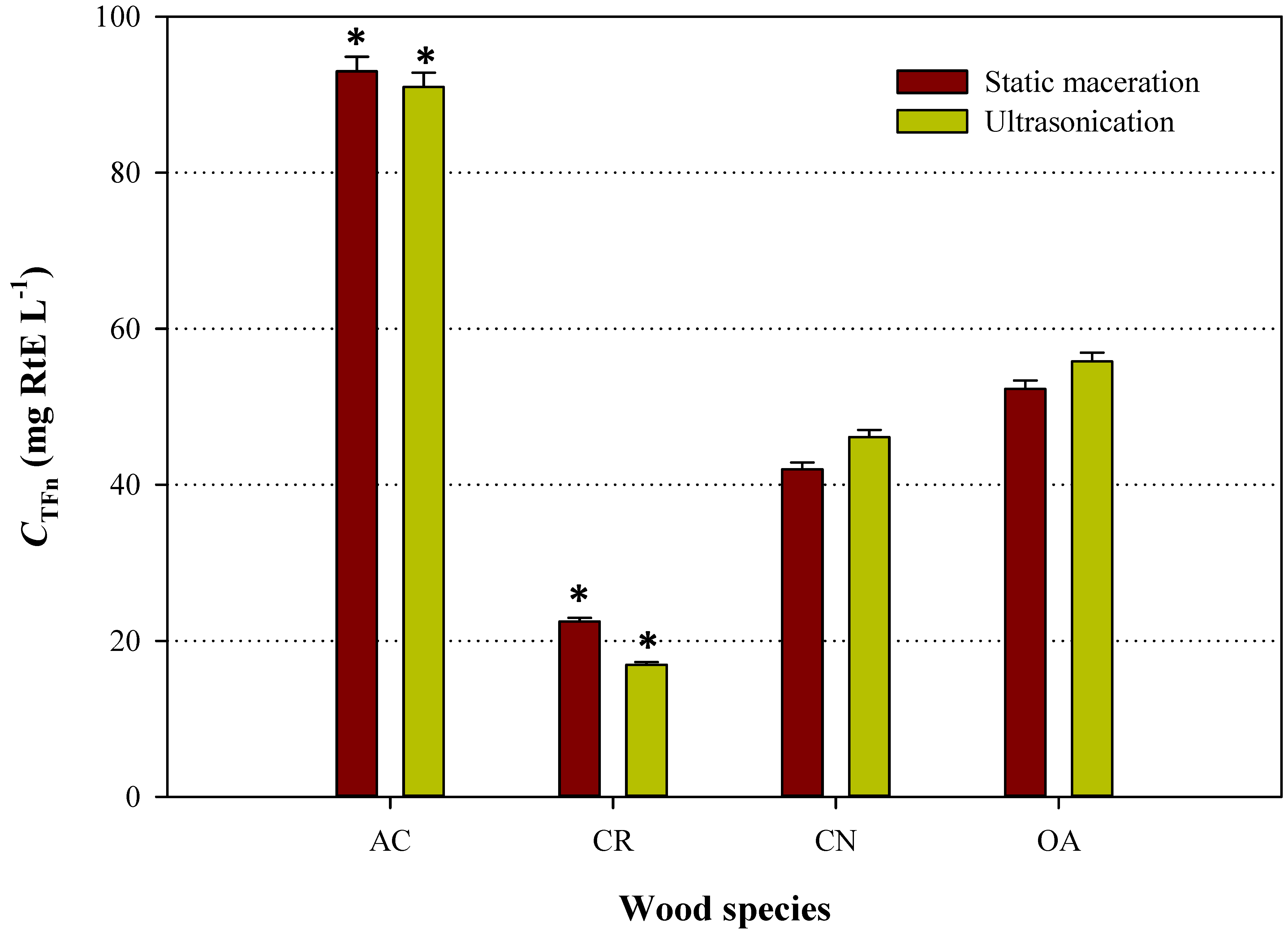
3.2. Antioxidant Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| AAR | antiradical activity (mM DPPH) |
| CTP | total polyphenol concentration (mg·GAE·L−1) |
| CTFn | total flavonoid concentration (mg·RtE·L−1) |
| PR | reducing power (mM AAE) |
Abbreviations
| AAE | ascorbic acid equivalents |
| DPPH• | 2,2-diphenyl-picrylhydrazyl radical |
| GAE | gallic acid equivalents |
| RtE | rutin (quercetin 3-O-rutinoside) equivalents |
| SM | static maceration |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| US | ultrasonication |
References
- Mosedale, J.; Puech, J.-L. Wood maturation of distilled beverages. Trends Food Sci. Technol. 1998, 9, 95–101. [Google Scholar] [CrossRef]
- Tao, Y.; García, J.F.; Sun, D.-W. Advances in wine aging technologies for enhancing wine quality and accelerating wine aging process. Crit. Rev. Food Sci. Nutr. 2014, 54, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.C. Study of ultrasonic wave treatments for accelerating the aging process in a rice alcoholic beverage. Food Chem. 2005, 92, 337–342. [Google Scholar] [CrossRef]
- Martín, J.F.G.; Sun, D.-W. Ultrasound and electric fields as novel techniques for assisting the wine aging process: The state-of-the-art research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Kana, K.; Kanellaki, M.; Papadimitriou, A.; Koutinas, A. Cause of and methods to reduce methanol content of Tsicoudia, Tsipouro and Ouzo. Int. J. Food Sci. Technol. 1991, 26, 241–247. [Google Scholar] [CrossRef]
- Apostolopoulou, A.; Flouros, A.; Demertzis, P.; Akrida-Demertzi, K. Differences in concentration of principal volatile constituents in traditional Greek distillates. Food Control 2005, 16, 157–164. [Google Scholar] [CrossRef]
- Geroyannaki, M.; Komaitis, M.E.; Stavrakas, D.E.; Polysiou, M.; Athanasopoulos, P.E.; Spanos, M. Evaluation of acetaldehyde and methanol in Greek traditional alcoholic beverages from varietal fermented grape pomaces (Vitis vinifera L.). Food Control 2007, 18, 988–995. [Google Scholar] [CrossRef]
- Flouros, A.; Apostolopoulou, A.; Demertzis, P.; Akrida-Demertzi, K. Note: Influence of the packaging material on the major volatile compounds of tsipouro, a traditional Greek distillate. Food Sci. Technol. Int. 2003, 9, 371–378. [Google Scholar] [CrossRef]
- Fotakis, C.; Christodouleas, D.; Kokkotou, K.; Zervou, M.; Zoumpoulakis, P.; Moulos, P.; Liouni, M.; Calokerinos, A. NMR metabolite profiling of Greek grape marc spirits. Food Chem. 2013, 138, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Zervou, M. NMR metabolic fingerprinting and chemometrics driven authentication of Greek grape marc spirits. Food Chem. 2016, 196, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Psarra, C.; Gortzi, O.; Makris, D.P. Kinetics of polyphenol extraction from wood chips in wine model solutions: Effect of chip amount and botanical species. J. Inst. Brew. 2015, 121, 207–212. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: A comparison with conventional eco-friendly solvents. Recycling 2016, 1, 194–204. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kefalas, P.; Moutounet, M. Extraction of phenolics in liquid model matrices containing oak chips: Kinetics, liquid chromatography-mass spectroscopy characterisation and association with in vitro antiradical activity. Food Chem. 2008, 110, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, Z.; Sun, D.-W. Experimental and modeling studies of ultrasound-assisted release of phenolics from oak chips into model wine. Ultrason. Sonochem. 2014, 21, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P. Kinetics of ultrasound-assisted flavonoid extraction from agri-food solid wastes using water/glycerol mixtures. Resources 2016, 5, 7. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications: A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Ye, X.; Sun, Y.; Ying, J.; Shen, Y.; Chen, J. Sonochemical effects on free phenolic acids under ultrasound treatment in a model system. Ultrason. Sonochem. 2013, 20, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.; Garcia, R.; Freitas, A.M.C.; Cabrita, M.J. Phenolic compounds released from oak, cherry, chestnut and Robinia chips into a syntethic wine: Influence of toasting level. Ciênc. Téc. Vitiv. 2012, 27, 17–26. [Google Scholar]
- Schwarz, M.; Rodríguez, M.; Martínez, C.; Bosquet, V.; Guillén, D.; Barroso, C.G. Antioxidant activity of Brandy de Jerez and other aged distillates, and correlation with their polyphenolic content. Food Chem. 2009, 116, 29–33. [Google Scholar] [CrossRef]
- Alonso, A.M.; Castro, R.; Rodrıguez, M.C.; Guillén, D.A.; Barroso, C.G. Study of the antioxidant power of brandies and vinegars derived from Sherry wines and correlation with their content in polyphenols. Food Res. Int. 2004, 37, 715–721. [Google Scholar] [CrossRef]
- Aoshima, H.; Tsunoue, H.; Koda, H.; Kiso, Y. Aging of whiskey increases 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity. J. Agric. Food Chem. 2004, 52, 5240–5244. [Google Scholar] [CrossRef] [PubMed]
- Canas, S.; Casanova, V.; Belchior, A.P. Antioxidant activity and phenolic content of Portuguese wine aged brandies. J. Food Compos. Anal. 2008, 21, 626–633. [Google Scholar] [CrossRef]
- Kanakaki, E.; Siderakou, D.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. Effect of the degree of toasting on the extraction pattern and profile of antioxidant polyphenols leached from oak chips in model wine systems. Eur. Food Res. Technol. 2015, 240, 1065–1074. [Google Scholar] [CrossRef]
- Alañón, M.; Castro-Vázquez, L.; Díaz-Maroto, M.; Gordon, M.; Pérez-Coello, M. A study of the antioxidant capacity of oak wood used in wine aging and the correlation with polyphenol composition. Food Chem. 2011, 128, 997–1002. [Google Scholar] [CrossRef]
- Vivas, N.; de Vivas Gaulejac, N.; Vitry, C.; Mouche, C.; Kahn, N.; Nonier-Bourden, M.F.; Absalon, C. Impact of ethanol content on the scavenging activities of oak wood C-glycosidic ellagitannins. Application to the evaluation of the nutritional status of spirits. J. Inst. Brew. 2013, 119, 116–125. [Google Scholar]
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, A.N.M.; de Fernández Simón, B.G.; Hernández, T.; Estrella, I. Phenolic compounds in cherry (Prunus avium) heartwood with a view to their use in cooperage. J. Agric. Food Chem. 2010, 58, 4907–4914. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; de Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; Hernández, M.T.; Estrella, I. Polyphenolic profile as a useful tool to identify the wood used in wine aging. Anal. Chim. Acta 2012, 732, 33–45. [Google Scholar] [CrossRef] [PubMed]
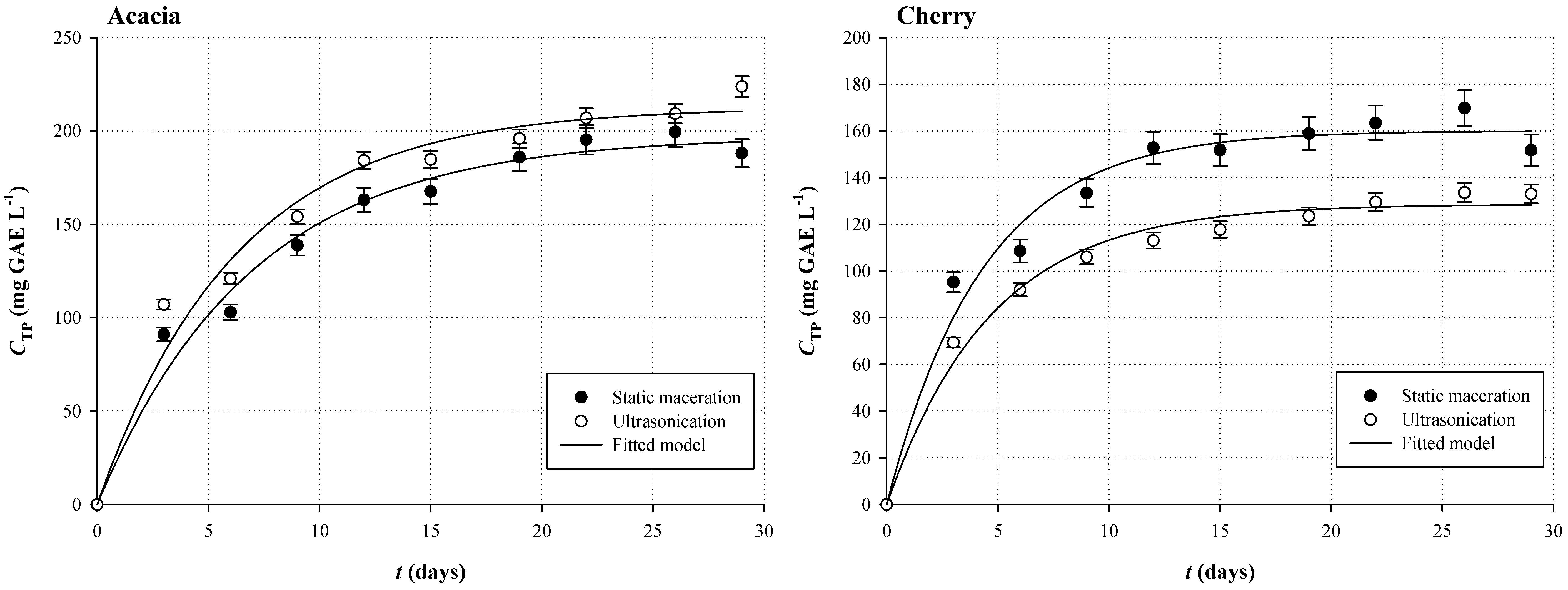
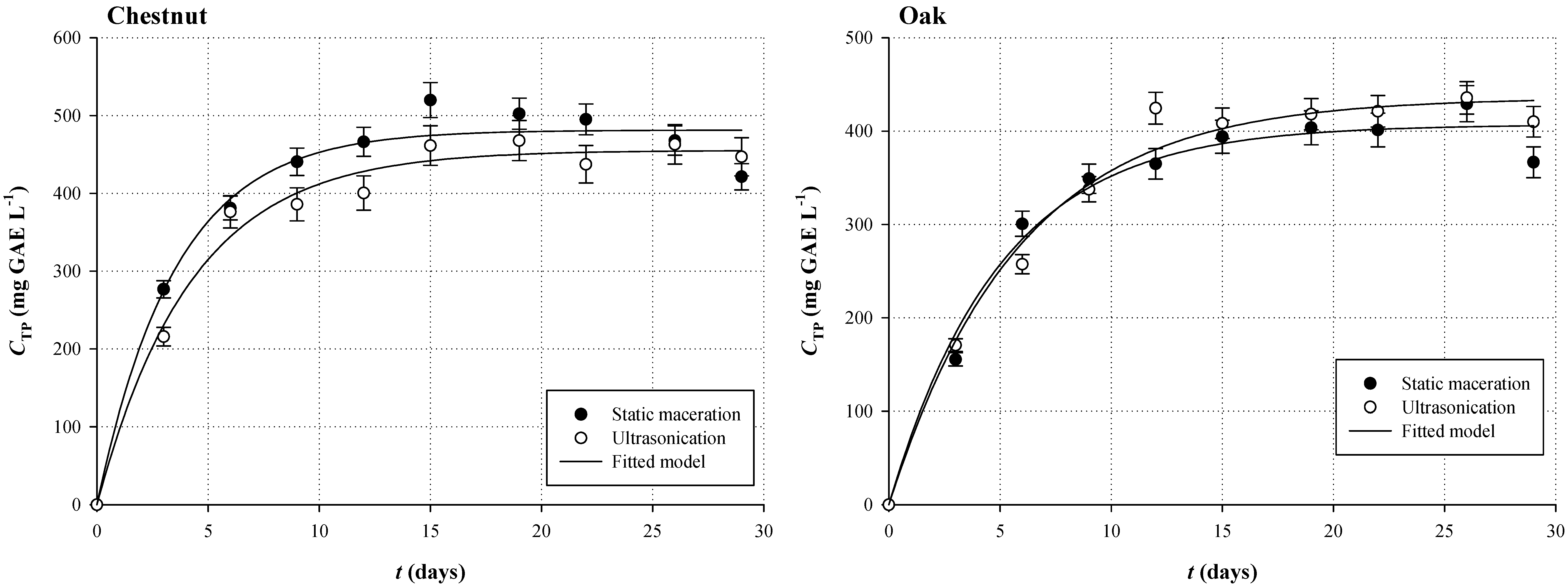

| Sample | Kinetic Parameters | |
|---|---|---|
| k (days−1) | CTP(s) (mg·GAE·L−1) | |
| Acacia | ||
| SM | 0.145 | 197.24 |
| US | 0.160 | 212.71 |
| Cherry | ||
| SM | 0.231 | 160.10 |
| US | 0.212 | 128.63 |
| Chestnut | ||
| SM | 0.282 | 481.47 |
| US | 0.235 | 455.39 |
| Oak | ||
| SM | 0.200 | 406.96 |
| US | 0.172 | 435.84 |
| Variable | By Variable | Correlation | Lower 95% | Upper 95% | Significance |
|---|---|---|---|---|---|
| AAR | CTP | 0.9475 | 0.7306 | 0.9907 | 0.0003 |
| AAR | CTFn | 0.0357 | −0.6862 | 0.7222 | 0.9331 |
| PR | CTP | 0.9791 | 0.8851 | 0.9963 | <0.0001 |
| PR | CTFn | 0.0375 | −0.6853 | 0.7231 | 0.9297 |
| PR | AAR | 0.9741 | 0.8592 | 0.9955 | <0.0001 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taloumi, T.; Makris, D.P. Accelerated Aging of the Traditional Greek Distillate Tsipouro Using Wooden Chips. Part I: Effect of Static Maceration vs. Ultrasonication on the Polyphenol Extraction and Antioxidant Activity. Beverages 2017, 3, 5. https://doi.org/10.3390/beverages3010005
Taloumi T, Makris DP. Accelerated Aging of the Traditional Greek Distillate Tsipouro Using Wooden Chips. Part I: Effect of Static Maceration vs. Ultrasonication on the Polyphenol Extraction and Antioxidant Activity. Beverages. 2017; 3(1):5. https://doi.org/10.3390/beverages3010005
Chicago/Turabian StyleTaloumi, Theodora, and Dimitris P. Makris. 2017. "Accelerated Aging of the Traditional Greek Distillate Tsipouro Using Wooden Chips. Part I: Effect of Static Maceration vs. Ultrasonication on the Polyphenol Extraction and Antioxidant Activity" Beverages 3, no. 1: 5. https://doi.org/10.3390/beverages3010005
APA StyleTaloumi, T., & Makris, D. P. (2017). Accelerated Aging of the Traditional Greek Distillate Tsipouro Using Wooden Chips. Part I: Effect of Static Maceration vs. Ultrasonication on the Polyphenol Extraction and Antioxidant Activity. Beverages, 3(1), 5. https://doi.org/10.3390/beverages3010005







