Substantially Improved Electrofusion Efficiency of Hybridoma Cells: Based on the Combination of Nanosecond and Microsecond Pulses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Cell Culture
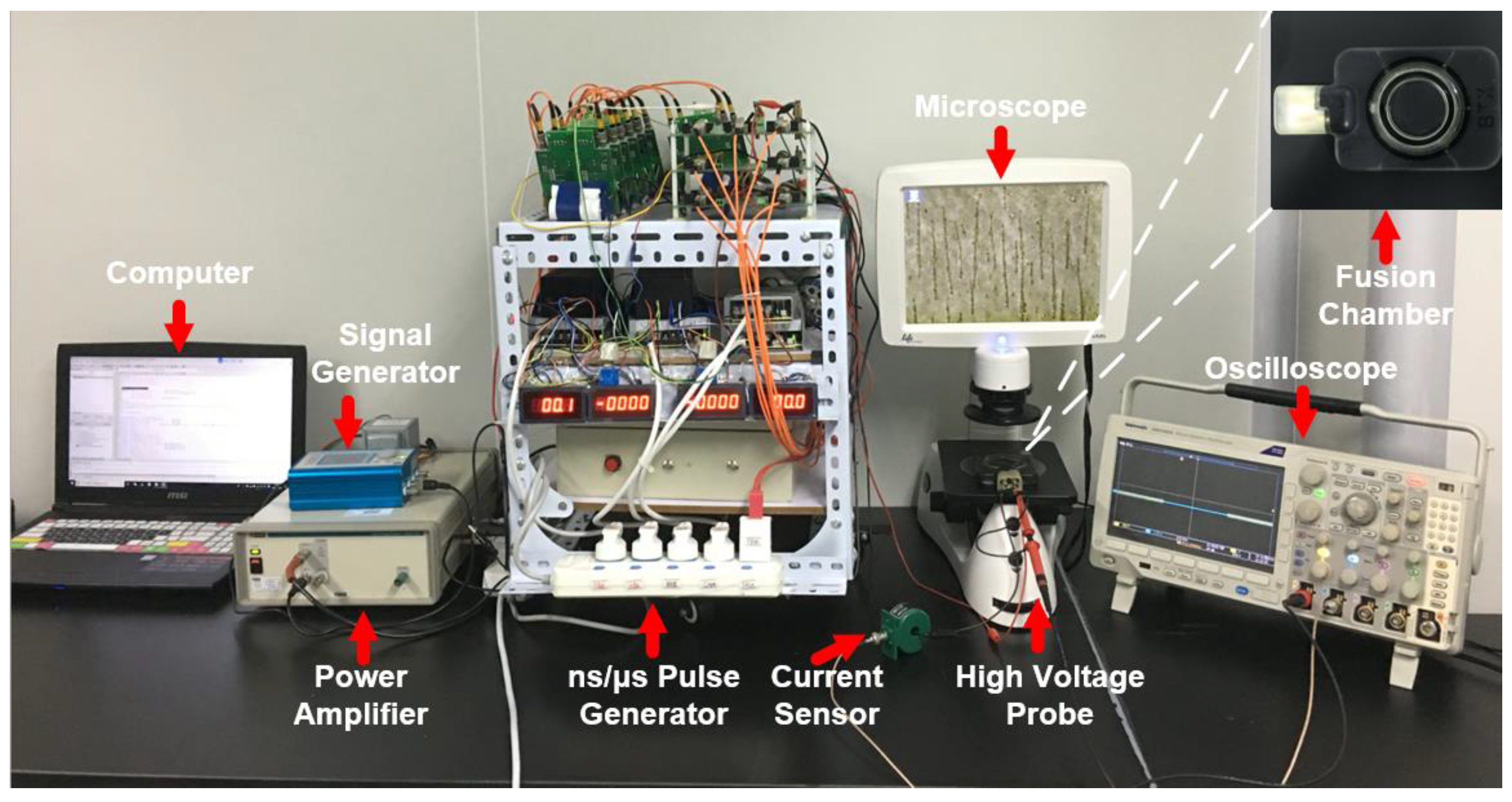
2.2. Cell Electrofusion Platform
2.3. Fluorescence Experiments
2.4. Hybridoma Culture Experiments
2.5. Definition of Cell Fusion Efficiency
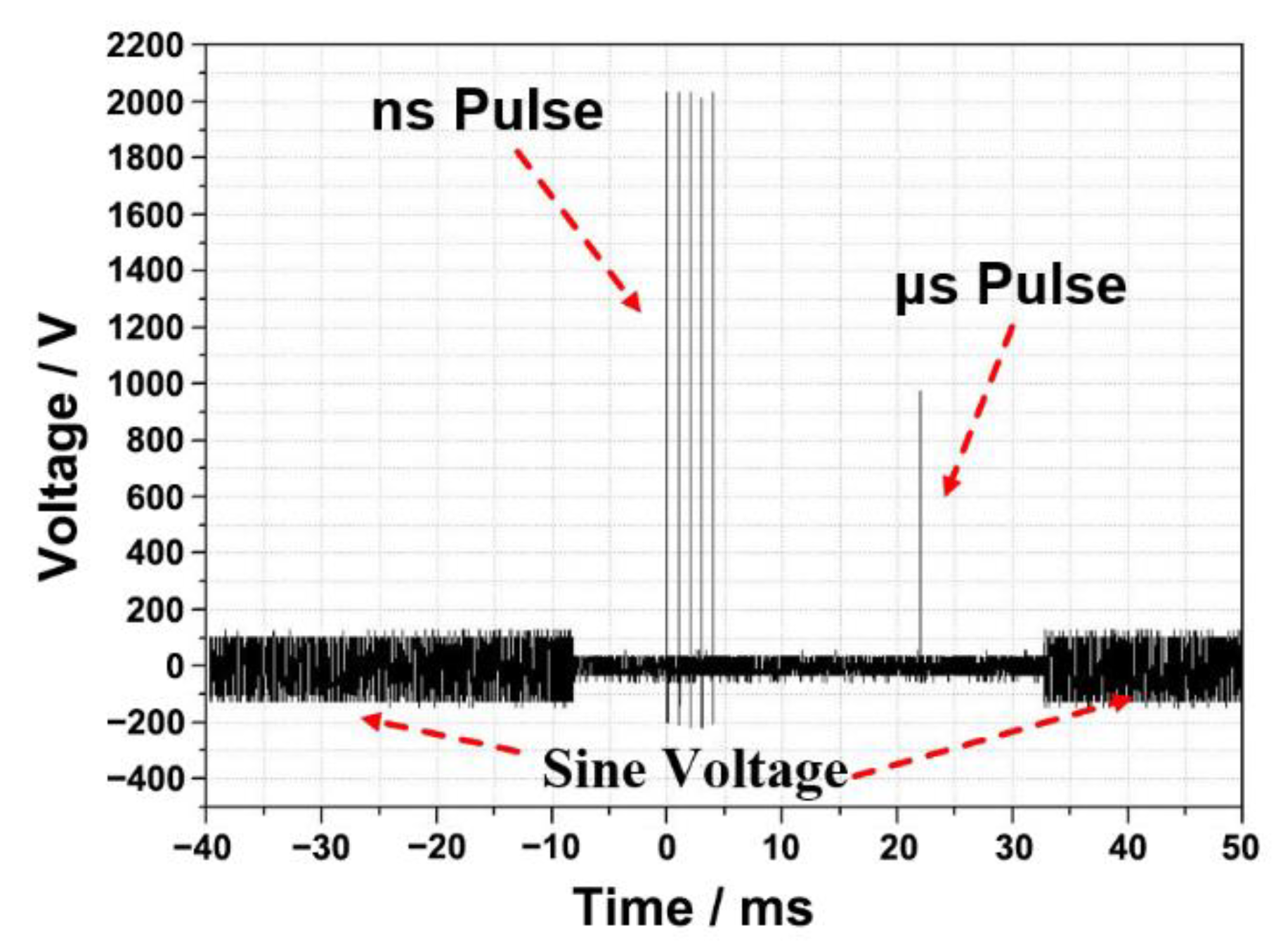
2.6. Electrofusion Protocols
2.7. Statistical Analysis
3. Results
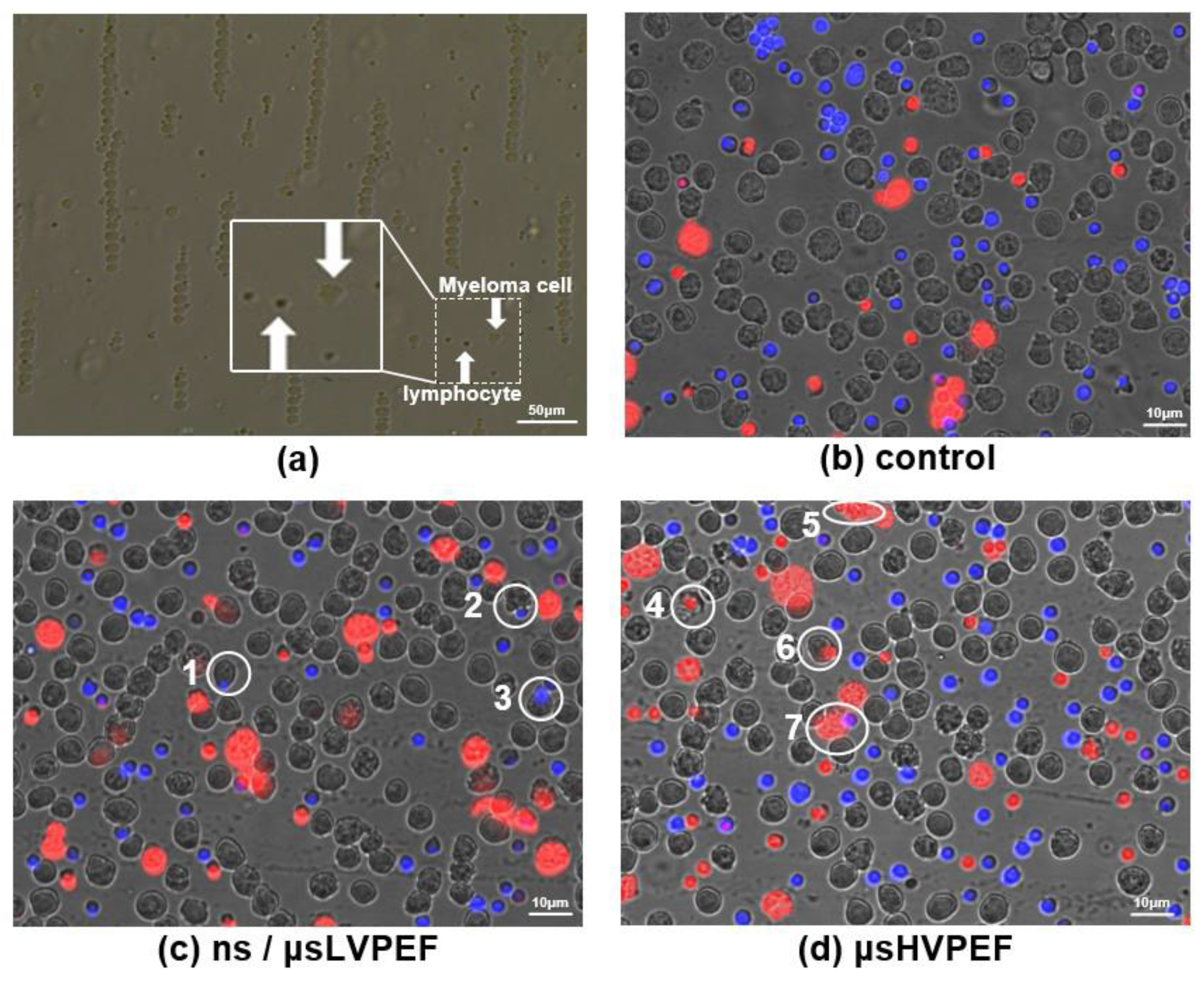
3.1. Fluorescence Experiments
3.2. Culture Experiments
4. Discussion
4.1. Characteristics of Cell Fusion Based on μsPEF (μsHVPEF or μsLVPEF)
4.2. Characteristics of Cell Fusion Based on nsPEF
4.3. Cell Fusion Based on ns/μsLVPEF
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, M.M.S.; Tsong, T.Y.; Conrad, M.K.; Strittmatter, S.M.; Hester, L.D.; Snyder, S.H. Monoclonal-Antibody Production by Receptor-Mediated Electrically Induced Cell-Fusion. Nature 1984, 310, 792–794. [Google Scholar] [CrossRef] [PubMed]
- vor dem Esche, U.; Huber, M.; Zgaga-Griesz, A.; Grunow, R.; Beyer, W.; Hahn, U.; Bessler, W.G. Passive vaccination with a human monoclonal antibody: Generation of antibodies and studies for efficacy in Bacillus anthracis infections. Immunobiology 2011, 216, 847–853. [Google Scholar] [CrossRef]
- Trontelj, K.; Rebersek, M.; Kanduser, M.; Serbec, V.C.; Sprohar, M.; Miklavcic, D. Optimization of bulk cell electrofusion in vitro for production of human-mouse heterohybridoma cells. Bioelectrochemistry 2008, 74, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Carroll, W.L.; Thielemans, K.; Dilley, J.; Levy, R. Mouse X Human Heterohybridomas as Fusion Partners with Human B-Cell Tumors. J. Immunol. Methods 1986, 89, 61–72. [Google Scholar] [CrossRef]
- Gong, J.L.; Koida, S.; Calderwood, S.K. Cell fusion: From hybridoma to dendritic cell-based vaccine. Expert Rev. Vaccines 2008, 7, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.Y.; Wang, L.; Cao, D.N.; Zou, S.Y.; Chen, C.Q.; Kang, H.M.; Song, Q.F.; Wang, H.; Tang, Y. A new method for rapid screening of hybridoma cell clones secreting paired antibodies using sandwich cell surface fluorescence immunosorbent assay. Anal. Chim. Acta 2021, 1163, 338493. [Google Scholar] [CrossRef]
- Parray, H.A.; Shukla, S.; Samal, S.; Shrivastava, T.; Ahmed, S.; Sharma, C.; Kumar, R. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int. Immunopharmacol. 2020, 85, 106639. [Google Scholar] [CrossRef]
- Le Gall, C.M.; van der Schoot, J.M.S.; Ramos-Tomillero, I.; Khalily, M.P.; van Dalen, F.J.; Wijfjes, Z.; Smeding, L.; van Dalen, D.; Cammarata, A.; Bonger, K.M.; et al. Dual Site-Specific Chemoenzymatic Antibody Fragment Conjugation Using CRISPR-Based Hybridoma Engineering. Bioconjugate Chem. 2021, 32, 301–310. [Google Scholar] [CrossRef]
- Tian, X.L.; Zhu, X.Y.; Song, W.P.; Yang, Z.L.; Wu, Y.L.; Ying, T.L. The prominent role of a CDR1 somatic hypermutation for convergent IGHV3-53/3-66 antibodies in binding to SARS-CoV-2. Emerg. Microbes Infect. 2022, 11, 1186–1190. [Google Scholar] [CrossRef]
- Chi, X.Y.; Li, Y.; Qiu, X.Y. V(D)J recombination, somatic hypermutation and class switch recombination of immunoglobulins: Mechanism and regulation. Immunology 2020, 160, 233–247. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Tomar, P.C. Hybridoma technology; advancements, clinical significance, and future aspects. J. Genet. Eng. Biotechnol. 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Satofuka, H.; Abe, S.; Moriwaki, T.; Okada, A.; Kazuki, K.; Tanaka, H.; Yamazaki, K.; Hichiwa, G.; Morimoto, K.; Takayama, H.; et al. Efficient human-like antibody repertoire and hybridoma production in trans-chromosomic mice carrying megabase-sized human immunoglobulin loci. Nat. Commun. 2022, 13, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; James, E.; Crowe, J. Use of Human Hybridoma Technology to Isolate Human Monoclonal Antibodies. Microbiol. Spectr. 2015, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Pourfathollah, A.A.; Moazzeni, S.M. Cephalin as an efficient fusogen in Hybridoma technology: Can it replace poly ethylene glycol? Hybridoma 2007, 26, 296–301. [Google Scholar] [CrossRef]
- Okada, Y.; Hashimoto, T.; Maeda, Y. Modification of Cell-Membranes with Viral Envelopes during Fusion of Cells with Hvj (Sendai Virus). 2. Effects of Pretreatment with a Small Number of Hvj. Exp. Cell Res. 1975, 93, 379–387. [Google Scholar] [CrossRef]
- Senda, M.; Takeda, J.; Abe, S.; Nakamura, T. Induction of Cell-Fusion of Plant-Protoplasts by Electrical-Stimulation. Plant Cell Physiol. 1979, 20, 1441–1443. [Google Scholar]
- Neumann, E.; Gerisch, G.; Opatz, K. Cell-Fusion Induced by High Electric Impulses Applied to Dictyostelium. Naturwissenschaften 1980, 67, 414–415. [Google Scholar] [CrossRef]
- Teissie, J.; Knutson, V.P.; Tsong, T.Y.; Lane, M.D. Electric Pulse-Induced Fusion of 3t3 Cells in Monolayer-Culture. Science 1982, 216, 537–538. [Google Scholar] [CrossRef]
- Zimmermann, U. Electric Field-Mediated Fusion and Related Electrical Phenomena. Biochim. et Biophys. Acta (BBA) Biomembr. 1982, 694, 227–277. [Google Scholar] [CrossRef]
- Hu, N.; Yang, J.; Joo, S.W.; Banerjee, A.N.; Qian, S.Z. Cell electrofusion in microfluidic devices: A review. Sens. Actuators B Chem. 2013, 178, 63–85. [Google Scholar] [CrossRef]
- Kemna, E.W.M. On chip electrofusion of single human B cells and mouse myeloma cells for efficient hybridoma generation (vol 32, pg 3138, 2011). Electrophoresis 2012, 33, 1225. [Google Scholar] [CrossRef]
- Zimmermann, U.; Vienken, J.; Pilwat, G.; Arnold, W.M. Electro-Fusion of Cells—Principles and Potential for the Future. Ciba Found. Symp. 1984, 103, 60–73. [Google Scholar] [PubMed]
- Vienken, J.; Zimmermann, U. An Improved Electrofusion Technique for Production of Mouse Hybridoma Cells. FEBS Lett. 1985, 182, 278–280. [Google Scholar] [CrossRef]
- Sixou, S.; Teissié, J. Specific electropermeabilization of leucocytes in a blood sample and application to large volumes of cells. Biochim. et Biophys. Acta (BBA) Biomembr. 1990, 1028, 154–160. [Google Scholar] [CrossRef]
- Ke, Q.; Li, C.X.; Wu, M.; Ge, L.P.; Yao, C.; Yao, C.G.; Mi, Y. Electrofusion by a bipolar pulsed electric field: Increased cell fusion efficiency for monoclonal antibody production. Bioelectrochemistry 2019, 127, 171–179. [Google Scholar] [CrossRef]
- Trontelj, K. Cell Fusion in vitro by Means of Electropermeabilization. Ph.D. Thesis, University of Ljubljana, Ljubljana, Slovenia, 2010. [Google Scholar]
- Pauly, H.; Schwan, H. Impendance of a suspension of ball-shaped particles with a shell; a model for the dielectric behavior of cell suspensions and protein solutions. Z. Fur Naturforschung. Teil B Chem. Biochem. Biophys. Biol. Und Verwandte Geb. 1959, 14, 125–131. [Google Scholar]
- Davalos, R.V.; Mir, L.M.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef]
- Li, C.; Ke, Q.; Yao, C.; Mi, Y.; Liu, H.; Lv, Y.; Yao, C. Cell electrofusion based on nanosecond/microsecond pulsed electric fields. PLoS ONE 2018, 13, e0197167. [Google Scholar] [CrossRef]
- Buescher, E.S.; Schoenbach, K.H. Effects of submicrosecond, high intensity pulsed electric fields on living cells-intracellular electromanipulation. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 788–794. [Google Scholar] [CrossRef]
- Schoenbach, K.H.; Hargrave, B.; Joshi, R.P.; Kolb, J.F.; Nuccitelli, R.; Osgood, C.; Pakhomov, A.; Stacey, M.; Swanson, R.J.; White, J.A.; et al. Bioelectric effects of intense nanosecond pulses. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1088–1109. [Google Scholar] [CrossRef]
- Beebe, S.J.; Fox, P.M.; Rec, L.J.; Somers, K.; Stark, R.H.; Schoenbach, K.H. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition. IEEE Trans. Plasma Sci. 2002, 30, 286–292. [Google Scholar] [CrossRef]
- Beebe, S.J.; Fox, P.M.; Rec, L.J.; Willis, L.K.; Schoenbach, K.H. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J. 2003, 17, 1493–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rems, L.; Usaj, M.; Kanduser, M.; Rebersek, M.; Miklavcic, D.; Pucihar, G. Cell electrofusion using nanosecond electric pulses. Sci. Rep. 2013, 3, 3382. [Google Scholar] [CrossRef]
- Li, C.X.; Ke, Q.; Yao, C.; Yao, C.G.; Mi, Y.; Wu, M.; Ge, L.P. Comparison of Bipolar and Unipolar Pulses in Cell Electrofusion: Simulation and Experimental Research. IEEE Trans. Biomed. Eng. 2019, 66, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Vernier, P.T.; Sun, Y.H.; Marcu, L.; Salemi, S.; Craft, C.M.; Gundersen, M.A. Calcium bursts induced by nanosecond electric pulses. Biochem. Biophys. Res. Commun. 2003, 310, 286–295. [Google Scholar] [CrossRef]
- Silve, A.; Leray, I.; Mir, L.M. Demonstration of cell membrane permeabilization to medium-sized molecules caused by a single 10 ns electric pulse. Bioelectrochemistry 2012, 87, 260–264. [Google Scholar] [CrossRef]
- Guo, S.Q.; Jackson, D.L.; Burcus, N.I.; Chen, Y.J.; Xiao, S.; Heller, R. Gene electrotransfer enhanced by nanosecond pulsed electric fields. Mol. Ther. Methods Clin. Dev. 2014, 1, 14043. [Google Scholar] [CrossRef]
- Sen, M.; Ino, K.; Ramon-Azcon, J.; Shiku, H.; Matsue, T. Cell pairing using a dielectrophoresis-based device with interdigitated array electrodes. Lab. Chip 2013, 13, 3650–3652. [Google Scholar] [CrossRef]
- Huang, L.; He, W.H.; Wang, W.H. A cell electro-rotation micro-device using polarized cells as electrodes. Electrophoresis 2019, 40, 784–791. [Google Scholar] [CrossRef]
- Sano, M.B.; Arena, C.B.; Bittleman, K.R.; DeWitt, M.R.; Cho, H.J.; Szot, C.S.; Saur, D.; Cissell, J.M.; Robertson, J.; Lee, Y.W.; et al. Bursts of Bipolar Microsecond Pulses Inhibit Tumor Growth. Sci. Rep. 2015, 5, 14999. [Google Scholar] [CrossRef]
- Yao, C.G.; Lv, Y.P.; Zhao, Y.J.; Dong, S.L.; Liu, H.M.; Ma, J.H. Synergistic combinations of short high-voltage pulses and long low-voltage pulses enhance irreversible electroporation efficacy. Sci. Rep. 2017, 7, 15123. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.N.; Constabel, F.; Michayluk, M.R.; Gamborg, O.L. Plant Protoplast Fusion and Growth of Intergeneric Hybrid Cells. Planta 1974, 120, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Suzuki, M.; Yasukawa, T. Selective retrieval of antibody-secreting hybridomas in cell arrays based on the dielectrophoresis. Biosens. Bioelectron. 2022, 209, 114250. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.P.; Ke, Y.B.; Yu, X.Z.; Yu, W.B.; Wen, K.; Shen, J.Z.; Wang, Z.H. Monoclonal Antibody Discovery Based on Precise Selection of Single Transgenic Hybridomas with an On-Cell-Surface and Antigen-Specific Anchor. ACS Appl. Mater. Interfaces 2022, 14, 17128–17141. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Q.; Yang, H.; Liu, C.; Tian, Y.C.; Wu, M.J.; Wu, Y.X.; Liu, Q. Bacterial coloration immunofluorescence strip for ultrasensitive rapid detection of bacterial antibodies and targeted antibody-secreting hybridomas. J. Immunol. Methods 2021, 501, 113208. [Google Scholar] [CrossRef]
- Shivarova, N.; Grigorova, R.; Forster, W.; Jacob, H.E.; Berg, H. Microbiological Implications of Electric-Field Effects.8. Fusion of Bacillus-Thuringiensis Protoplasts by High Electric-Field Pulses. Bioelectrochem. Bioenerg. 1983, 11, 181–185. [Google Scholar] [CrossRef]
- Karsten, U.; Stolley, P.; Walther, I.; Papsdorf, G.; Weber, S.; Conrad, K.; Pasternak, L.; Kopp, J. Direct Comparison of Electric Field-Mediated and Peg-Mediated Cell-Fusion for the Generation of Antibody-Producing Hybridomas. Hybridoma 1988, 7, 627–633. [Google Scholar] [CrossRef]
- Lewis, T.J. A model for bilayer membrane electroporation based on resultant electromechanical stress. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 769–777. [Google Scholar] [CrossRef]
- Saulis, G.; Saule, R. Size of the pores created by an electric pulse: Microsecond vs millisecond pulses. BBA Biomembr. 2012, 1818, 3032–3039. [Google Scholar] [CrossRef]
- Kiesel, M.; Reuss, R.; Endter, J.; Zimmermann, D.; Zimmermann, H.; Shirakashi, R.; Bamberg, E.; Zimmermann, U.; Sukhorukov, V.L. Swelling-activated pathways in human T-lymphocytes studied by cell volumetry and electrorotation. Biophys. J. 2006, 90, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.C.; McGraw, P.A.; House, F.S.; Crowe, J.E. An optimized electrofusion-based protocol for generating virus-specific human monoclonal antibodies. J. Immunol. Methods 2008, 336, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, V.L.; Reuss, R.; Zimmermann, D.; Held, C.; Muller, K.J.; Kiesel, M.; Gessner, P.; Steinbach, A.; Schenk, W.A.; Bamberg, E.; et al. Surviving high-intensity field pulses: Strategies for improving robustness and performance of electrotransfection and electrofusion. J. Membr. Biol. 2005, 206, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Pucihar, G.; Kotnik, T.; Teissie, J.; Miklavcic, D. Electropermeabilization of dense cell suspensions. Eur. Biophys. J. 2007, 36, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Gianulis, E.; Vernier, P.T.; Semenov, I.; Xiao, S.; Pakhomova, O.N. Multiple nanosecond electric pulses increase the number but not the size of long-lived nanopores in the cell membrane. Biochim. et Biophys. Acta (BBA) Biomembr. 2015, 1848, 958–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Group | Pulse Type | ns Electric Field (kV/cm) | ns Pulse Width (µs) | ns Number | μs Electric Field (kV/cm) | μs Pulse Width (µs) | μs Number | Total Dose (kV2μs/cm2) |
|---|---|---|---|---|---|---|---|---|
| 1 | ns/μsLVPEF | 6 | 0.2 | 5 | 2 | 40 | 1 | 36 + 160 |
| μsHVPEF | 0 | 0 | 0 | 2.2 | 40 | 1 | 196 | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | ns/μsLVPEF | 6 | 0.2 | 5 | 2.5 | 40 | 1 | 36 + 250 |
| μsHVPEF | 0 | 0 | 0 | 2.67 | 40 | 1 | 286 | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | ns/μsLVPEF | 6 | 0.2 | 5 | 3 | 40 | 1 | 36 + 360 |
| μsHVPEF | 0 | 0 | 0 | 3.15 | 40 | 1 | 396 | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Ke, Q.; Bi, J.; Li, X.; Huang, S.; Liu, Z.; Ge, L. Substantially Improved Electrofusion Efficiency of Hybridoma Cells: Based on the Combination of Nanosecond and Microsecond Pulses. Bioengineering 2022, 9, 450. https://doi.org/10.3390/bioengineering9090450
Wu M, Ke Q, Bi J, Li X, Huang S, Liu Z, Ge L. Substantially Improved Electrofusion Efficiency of Hybridoma Cells: Based on the Combination of Nanosecond and Microsecond Pulses. Bioengineering. 2022; 9(9):450. https://doi.org/10.3390/bioengineering9090450
Chicago/Turabian StyleWu, Meng, Qiang Ke, Jinhao Bi, Xinhao Li, Shuheng Huang, Zuohua Liu, and Liangpeng Ge. 2022. "Substantially Improved Electrofusion Efficiency of Hybridoma Cells: Based on the Combination of Nanosecond and Microsecond Pulses" Bioengineering 9, no. 9: 450. https://doi.org/10.3390/bioengineering9090450
APA StyleWu, M., Ke, Q., Bi, J., Li, X., Huang, S., Liu, Z., & Ge, L. (2022). Substantially Improved Electrofusion Efficiency of Hybridoma Cells: Based on the Combination of Nanosecond and Microsecond Pulses. Bioengineering, 9(9), 450. https://doi.org/10.3390/bioengineering9090450








