Construction and In Vitro Evaluation of a Tumor Acidic pH-Targeting Drug Delivery System Based on Escherichia coli Nissle 1917 Bacterial Ghosts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids, Bacterial Strains, and Cell Culture
2.2. Effect of Lysis Protein E in Bacteria
2.3. Western Blot of Lysis Protein E
2.4. Identification of the Protein Display System of the Engineered EcN Using SDS-PAGE and Immunofluorescence
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Transmission Electron Microscopy (TEM) Analysis
2.7. Drug Packaging
2.8. Drug Release Profile of BGs
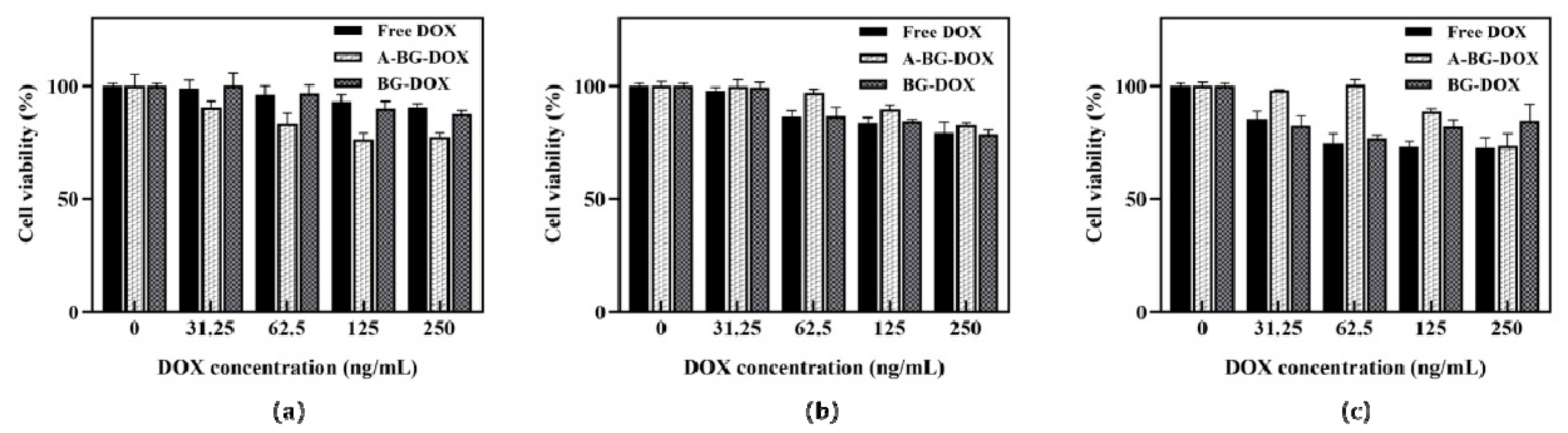
2.9. Cytotoxicity of BGs against HepG2 Cancer Cells
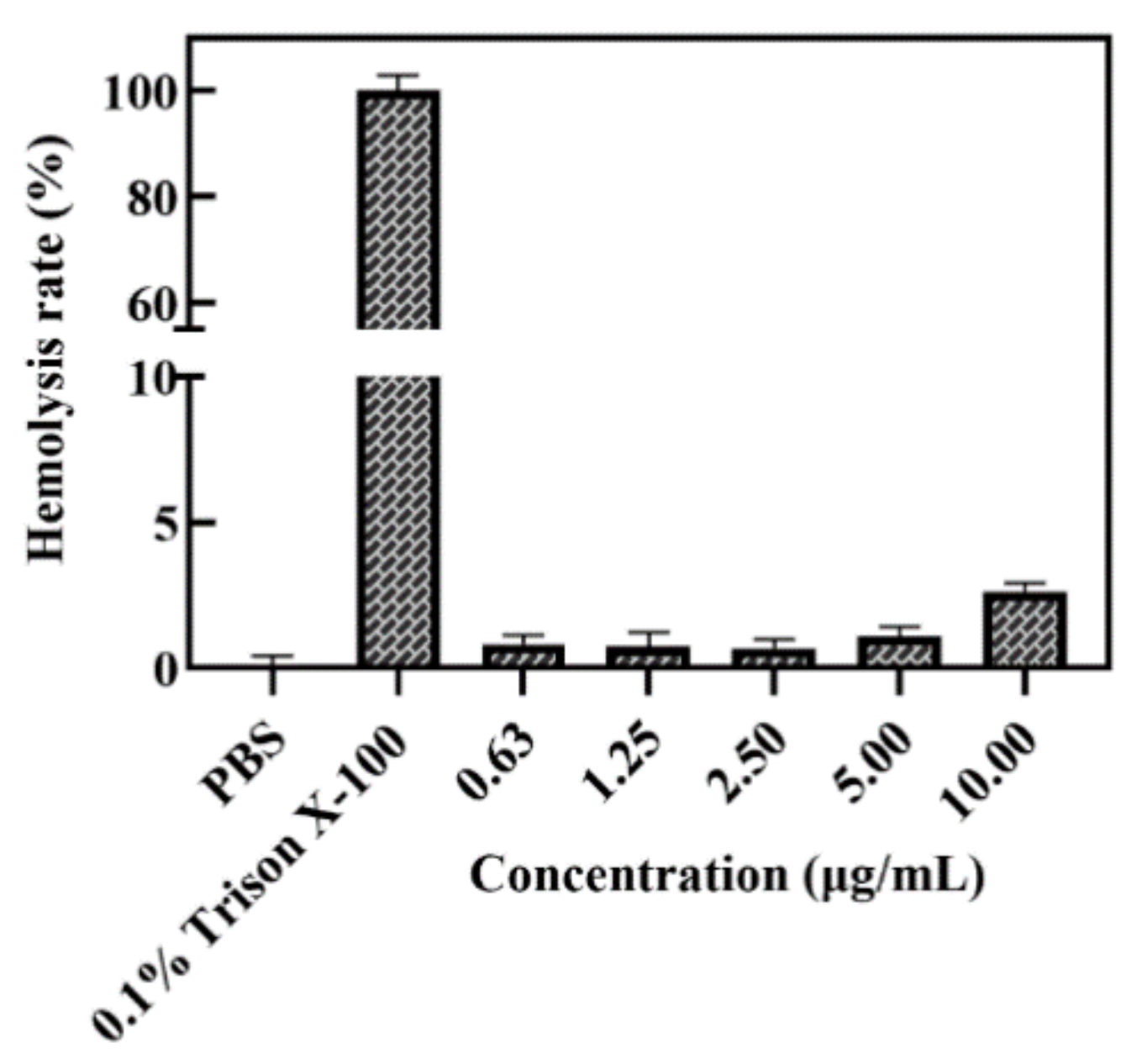
2.10. Hemolysis Assay of A-BG-DOX
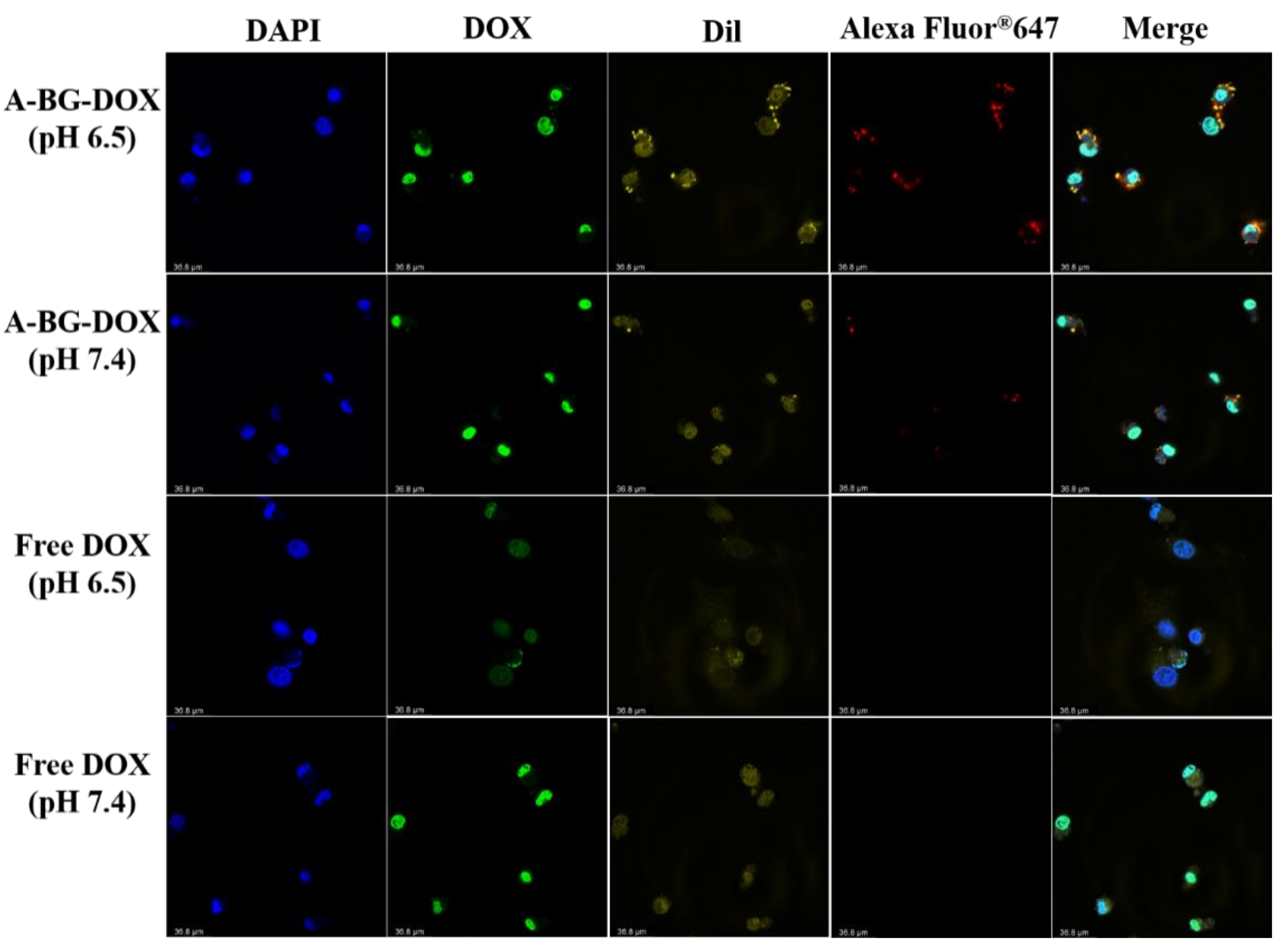
2.11. In Vitro Cellular Uptake of A-BG-DOX Analysis by Confocal Fluorescence Microscopy
2.12. Flow Cytometry Analysis of Cell Apoptosis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Construction of the E. coli Bacterial Ghosts Using Protein E
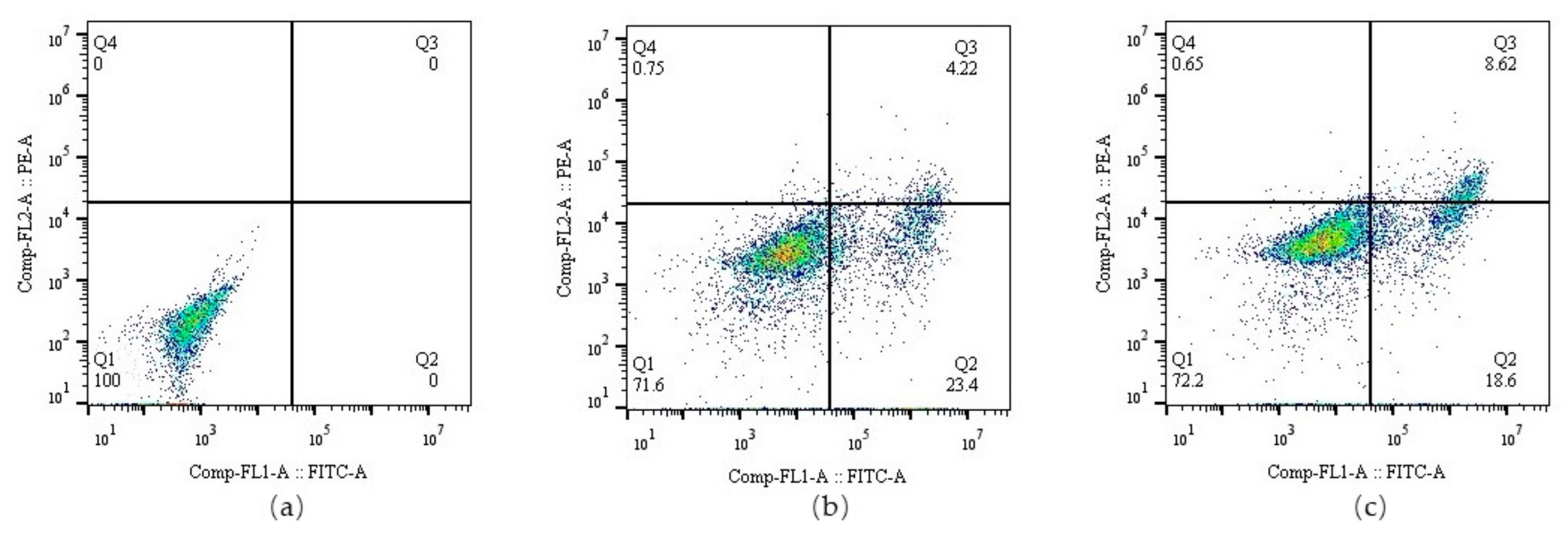
3.2. ATRAM Protein Surface Expression on an Lpp-OmpA Anchor in EcN
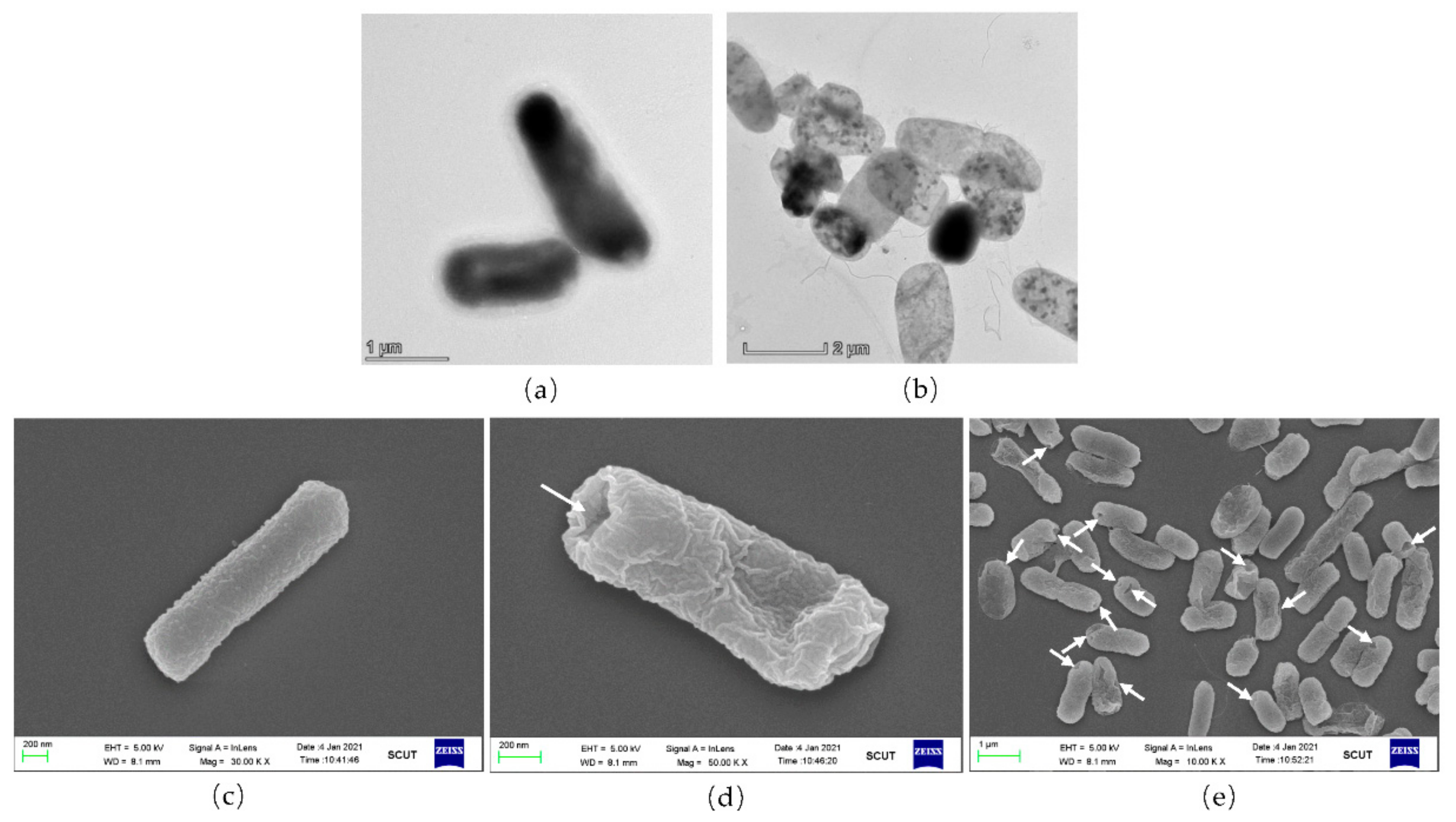
3.3. Morphology Assessment of EcN Ghosts Expressing Lpp-OmpA-ATRAM
3.4. DOX Packaging and Release Assays
3.5. Lpp-OMPA-ATRAM Protein Expression on Bacterial Ghosts Enhances Cancer Therapy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Todorova, V.K.; Wei, J.Y.; Makhoul, I. Subclinical doxorubicin-induced cardiotoxicity update: Role of neutrophils and endothelium. Am. J. Cancer Res. 2021, 11, 4070–4091. [Google Scholar]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Wu, L.; Ni, C.; Zhang, L.; Shi, G. Preparation of pH-sensitive zwitterionic nano micelles and drug controlled release for enhancing cellular uptake. J. Biomater. Sci. Polym. Ed. 2016, 27, 643–656. [Google Scholar] [CrossRef]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Chen, F.; Zang, Z.; Chen, Z.; Cui, L.; Chang, Z.; Ma, A.; Yin, T.; Liang, R.; Han, Y.; Wu, Z.; et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials 2019, 214, 119226. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Qin, M.; Zhang, X.; Zhang, Z.; Sun, X.; Gu, Z. Bacteria-Driven Hypoxia Targeting for Combined Biotherapy and Photothermal Therapy. ACS Nano 2018, 12, 5995–6005. [Google Scholar] [CrossRef]
- Nie, D.; Dai, Z.; Li, J.; Yang, Y.; Xi, Z.; Wang, J.; Zhang, W.; Qian, K.; Guo, S.; Zhu, C.; et al. Cancer-Cell-Membrane-Coated Nanoparticles with a Yolk-Shell Structure Augment Cancer Chemotherapy. Nano Lett. 2020, 20, 936–946. [Google Scholar] [CrossRef]
- Lee, J.Y.; Vyas, C.K.; Kim, G.G.; Choi, P.S.; Hur, M.G.; Yang, S.D.; Kong, Y.B.; Lee, E.J.; Park, J.H. Red Blood Cell Membrane Bioengineered Zr-89 Labelled Hollow Mesoporous Silica Nanosphere for Overcoming Phagocytosis. Sci. Rep. 2019, 9, 7419. [Google Scholar] [CrossRef]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Yoo, J.W.; Irvine, D.J.; Discher, D.E.; Mitragotri, S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011, 10, 521–535. [Google Scholar] [CrossRef]
- Moghimipour, E.; Abedishirehjin, S.; Baghbadorani, M.A.; Handali, S. Bacteria and Archaea: A new era of cancer therapy. J. Control Release 2021, 338, 1–7. [Google Scholar] [CrossRef]
- Zheng, J.H.; Nguyen, V.H.; Jiang, S.N.; Park, S.H.; Tan, W.; Hong, S.H.; Shin, M.G.; Chung, I.J.; Hong, Y.; Bom, H.S.; et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci. Transl. Med. 2017, 9, eaak9537. [Google Scholar] [CrossRef]
- Suh, S.; Jo, A.; Traore, M.A.; Zhan, Y.; Coutermarsh-Ott, S.L.; Ringel-Scaia, V.M.; Allen, I.C.; Davis, R.M.; Behkam, B. Nanoscale Bacteria-Enabled Autonomous Drug Delivery System (NanoBEADS) Enhances Intratumoral Transport of Nanomedicine. Adv. Sci. 2019, 6, 1801309. [Google Scholar] [CrossRef]
- Fan, C.; Davison, P.A.; Habgood, R.; Zeng, H.; Decker, C.M.; Gesell Salazar, M.; Lueangwattanapong, K.; Townley, H.E.; Yang, A.; Thompson, I.P.; et al. Chromosome-free bacterial cells are safe and programmable platforms for synthetic biology. Proc. Natl. Acad. Sci. USA 2020, 117, 6752–6761. [Google Scholar] [CrossRef]
- Yu, X.; Lin, C.; Yu, J.; Qi, Q.; Wang, Q. Bioengineered Escherichia coli Nissle 1917 for tumour-targeting therapy. Microb. Biotechnol. 2020, 13, 629–636. [Google Scholar] [CrossRef]
- Kudela, P.; Koller, V.J.; Lubitz, W. Bacterial ghosts (BGs)—Advanced antigen and drug delivery system. Vaccine 2010, 28, 5760–5767. [Google Scholar] [CrossRef]
- Langemann, T.; Koller, V.J.; Muhammad, A.; Kudela, P.; Mayr, U.B.; Lubitz, W. The Bacterial Ghost platform system: Production and applications. Bioeng. Bugs 2010, 1, 326–336. [Google Scholar] [CrossRef]
- Muhammad, A.; Champeimont, J.; Mayr, U.B.; Lubitz, W.; Kudela, P. Bacterial ghosts as carriers of protein subunit and DNA-encoded antigens for vaccine applications. Expert Rev. Vaccines 2012, 11, 97–116. [Google Scholar] [CrossRef]
- Montanaro, J.; Inic-Kanada, A.; Ladurner, A.; Stein, E.; Belij, S.; Bintner, N.; Schlacher, S.; Schuerer, N.; Mayr, U.B.; Lubitz, W.; et al. Escherichia coli Nissle 1917 bacterial ghosts retain crucial surface properties and express chlamydial antigen: An imaging study of a delivery system for the ocular surface. Drug Des. Devel Ther. 2015, 9, 3741–3754. [Google Scholar] [CrossRef][Green Version]
- Xie, S.; Li, S.; Zhang, Z.; Chen, M.; Ran, P.; Li, X. Bacterial ghosts for targeting delivery and subsequent responsive release of ciprofloxacin to destruct intracellular bacteria. Chem. Eng. J. 2020, 399, 125700. [Google Scholar] [CrossRef]
- Zhu, W.; Hao, L.; Liu, X.; Borras-Hidalgo, O.; Zhang, Y. Enhanced anti-proliferative efficacy of epothilone B loaded with Escherichia coli Nissle 1917 bacterial ghosts on the HeLa cells by mitochondrial pathway of apoptosis. Drug Dev. Ind. Pharm. 2018, 44, 1328–1335. [Google Scholar] [CrossRef]
- Riedmann, E.M.; Lubitz, W.; McGrath, J.; Kyd, J.M.; Cripps, A.W. Effectiveness of engineering the nontypeable Haemophilus influenzae antigen Omp26 as an S-layer fusion in bacterial ghosts as a mucosal vaccine delivery. Hum. Vaccines 2011, 7 (Suppl. S1), 99–107. [Google Scholar] [CrossRef]
- Zhou, P.; Wu, H.; Chen, S.; Bai, Q.; Chen, X.; Chen, L.; Zeng, X.; Liu, L.; Chen, L. MOMP and MIP DNA-loaded bacterial ghosts reduce the severity of lung lesions in mice after Chlamydia psittaci respiratory tract infection. Immunobiology 2019, 224, 739–746. [Google Scholar] [CrossRef]
- Paukner, S.; Kohl, G.; Lubitz, W. Bacterial ghosts as novel advanced drug delivery systems: Antiproliferative activity of loaded doxorubicin in human Caco-2 cells. J. Control Release 2004, 94, 63–74. [Google Scholar] [CrossRef]
- Youssof, A.M.E.; Alanazi, F.K.; Salem-Bekhit, M.M.; Shakeel, F.; Haq, N. Bacterial Ghosts Carrying 5-Fluorouracil: A Novel Biological Carrier for Targeting Colorectal Cancer. AAPS PharmSciTech 2019, 20, 48. [Google Scholar] [CrossRef]
- Weerakkody, D.; Moshnikova, A.; Thakur, M.S.; Moshnikova, V.; Daniels, J.; Engelman, D.M.; Andreev, O.A.; Reshetnyak, Y.K. Family of pH (low) insertion peptides for tumor targeting. Proc. Natl. Acad. Sci. USA 2013, 110, 5834–5839. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Alves, D.S.; Scott, H.L.; Davis, F.L.; Barrera, F.N. A Novel Soluble Peptide with pH-Responsive Membrane Insertion. Biochemistry 2015, 54, 6567–6575. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, L.; Wang, M.; Sun, Q.; Liu, K.; Wang, J. A Novel and Efficient High-Yield Method for Preparing Bacterial Ghosts. Toxins 2021, 13, 420. [Google Scholar] [CrossRef]
- Rabea, S.; Alanazi, F.K.; Ashour, A.E.; Salem-Bekhit, M.M.; Yassin, A.S.; Moneib, N.A.; Hashem, A.E.M.; Haq, N. Salmonella-innovative targeting carrier: Loading with doxorubicin for cancer treatment. Saudi Pharm. J. 2020, 28, 1253–1262. [Google Scholar] [CrossRef]
- Calik, P.; Levent, H. Effects of pretreated beet molasses on benzaldehyde lyase production by recombinant Escherichia coli BL21(DE3)pLySs. J. Appl. Microbiol. 2009, 107, 1536–1541. [Google Scholar] [CrossRef]
- Li, R.; Xie, L.; Zhu, Z.; Liu, Q.; Hu, Y.; Jiang, X.; Yu, L.; Qian, X.; Guo, W.; Ding, Y.; et al. Reversion of pH-induced physiological drug resistance: A novel function of copolymeric nanoparticles. PLoS ONE 2011, 6, e24172. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Palanikumar, L.; Kennel, S.J.; Alves, D.S.; Ye, Y.; Wall, J.S.; Magzoub, M.; Barrera, F.N. Mechanistic insights into the pH-dependent membrane peptide ATRAM. J. Control Release 2019, 298, 142–153. [Google Scholar] [CrossRef]
- Danino, T.; Prindle, A.; Kwong, G.A.; Skalak, M.; Li, H.; Allen, K.; Hasty, J.; Bhatia, S.N. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 2015, 7, 289ra84. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Liu, Q.; Hu, A.; Jiang, S.; Wang, S.; Liu, R.; Han, K.; Wang, J. Construction and In Vitro Evaluation of a Tumor Acidic pH-Targeting Drug Delivery System Based on Escherichia coli Nissle 1917 Bacterial Ghosts. Bioengineering 2022, 9, 433. https://doi.org/10.3390/bioengineering9090433
Ma Y, Liu Q, Hu A, Jiang S, Wang S, Liu R, Han K, Wang J. Construction and In Vitro Evaluation of a Tumor Acidic pH-Targeting Drug Delivery System Based on Escherichia coli Nissle 1917 Bacterial Ghosts. Bioengineering. 2022; 9(9):433. https://doi.org/10.3390/bioengineering9090433
Chicago/Turabian StyleMa, Yi, Qiying Liu, Aihua Hu, Shoujin Jiang, Sijia Wang, Ran Liu, Kun Han, and Jufang Wang. 2022. "Construction and In Vitro Evaluation of a Tumor Acidic pH-Targeting Drug Delivery System Based on Escherichia coli Nissle 1917 Bacterial Ghosts" Bioengineering 9, no. 9: 433. https://doi.org/10.3390/bioengineering9090433
APA StyleMa, Y., Liu, Q., Hu, A., Jiang, S., Wang, S., Liu, R., Han, K., & Wang, J. (2022). Construction and In Vitro Evaluation of a Tumor Acidic pH-Targeting Drug Delivery System Based on Escherichia coli Nissle 1917 Bacterial Ghosts. Bioengineering, 9(9), 433. https://doi.org/10.3390/bioengineering9090433





