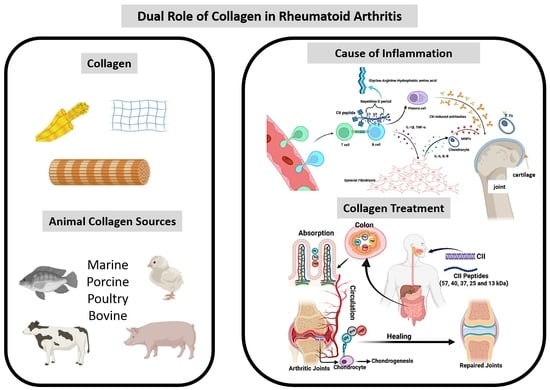
Paradoxical Duel Role of Collagen in Rheumatoid Arthritis: Cause of Inflammation and Treatment
Abstract
1. Introduction
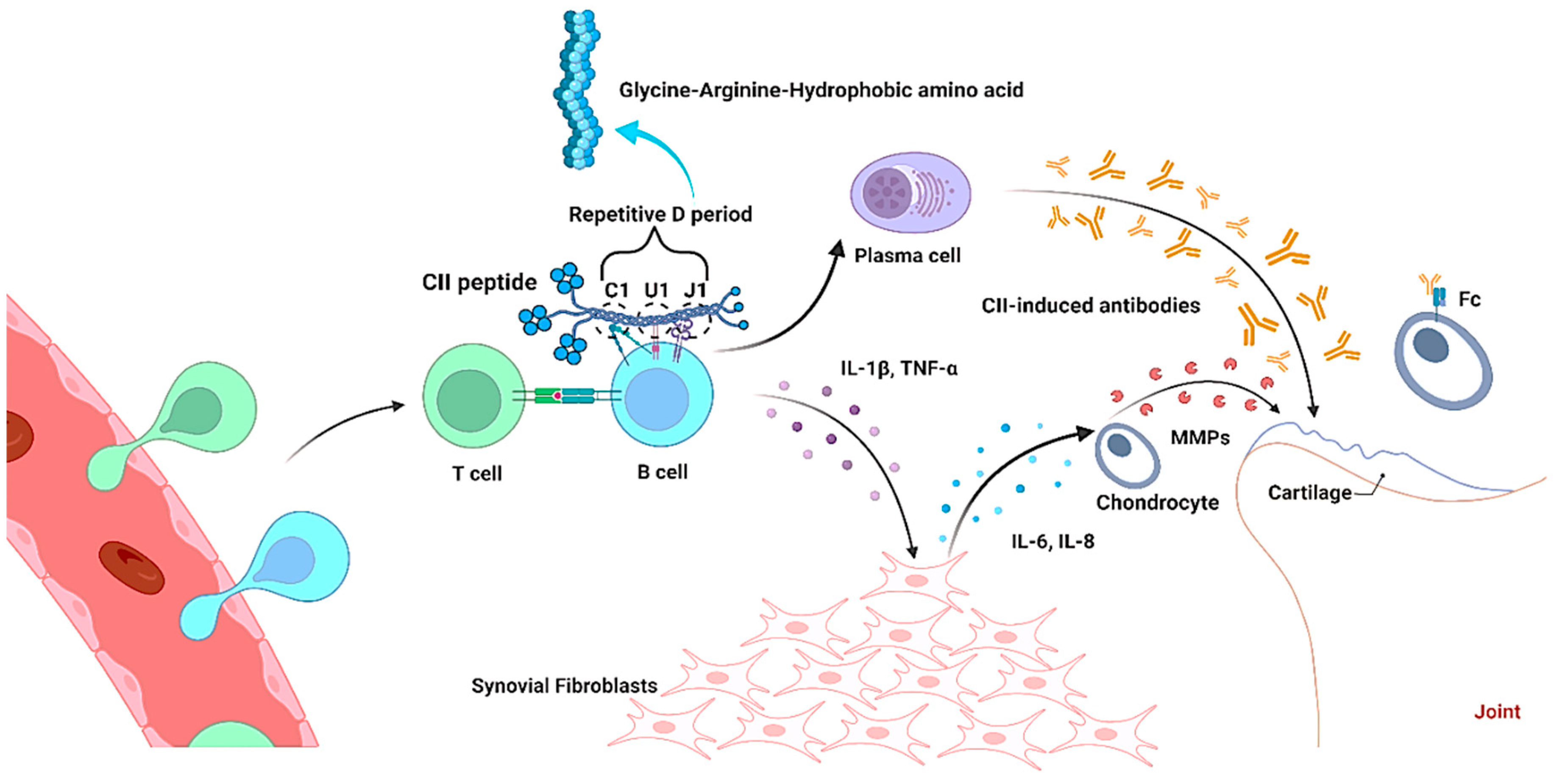
2. Mechanism of Collagen in Arthritis Induction
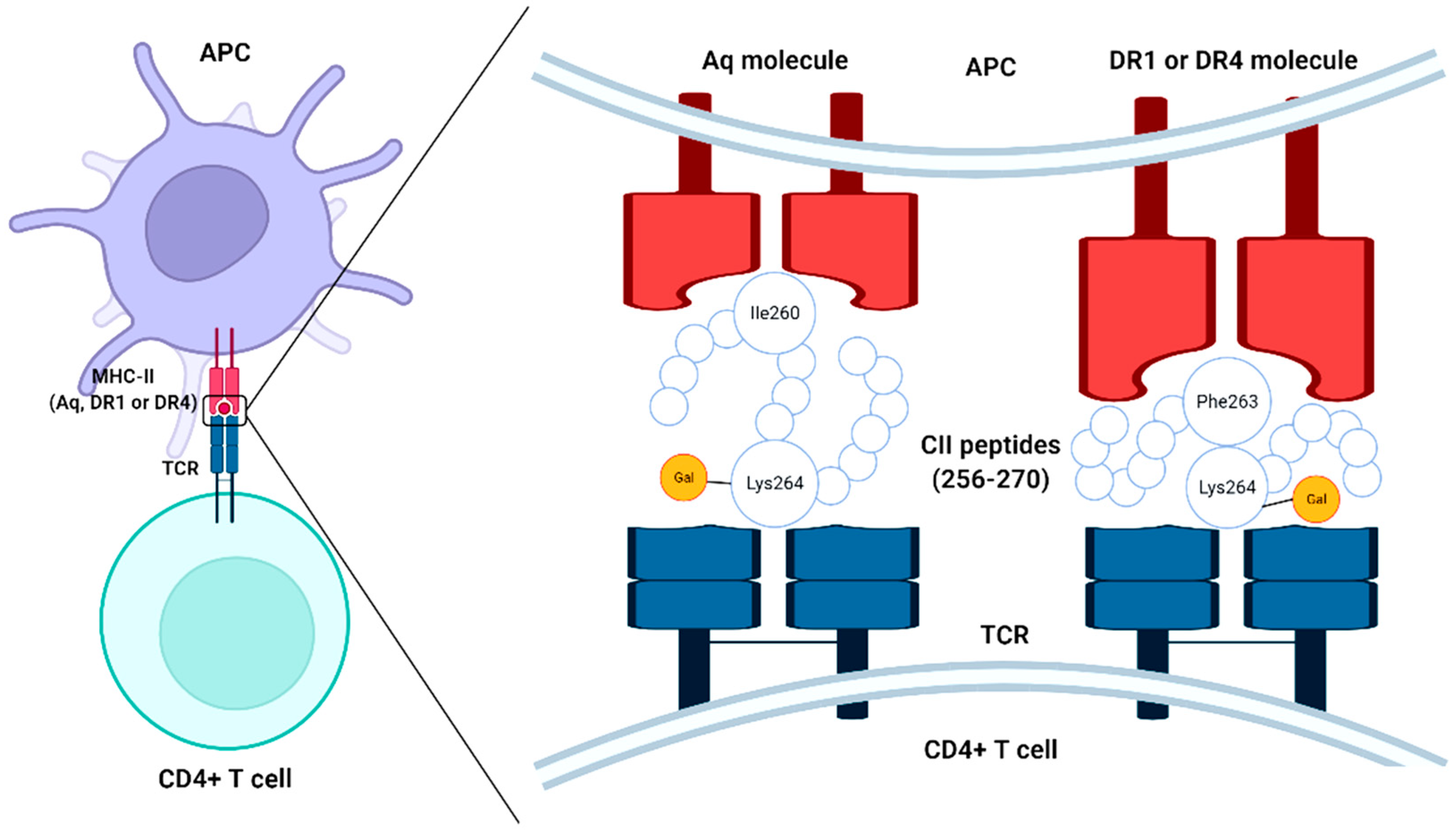
2.1. Interaction of Collagen with T Cells
2.2. Interaction of Collagen with B Cell
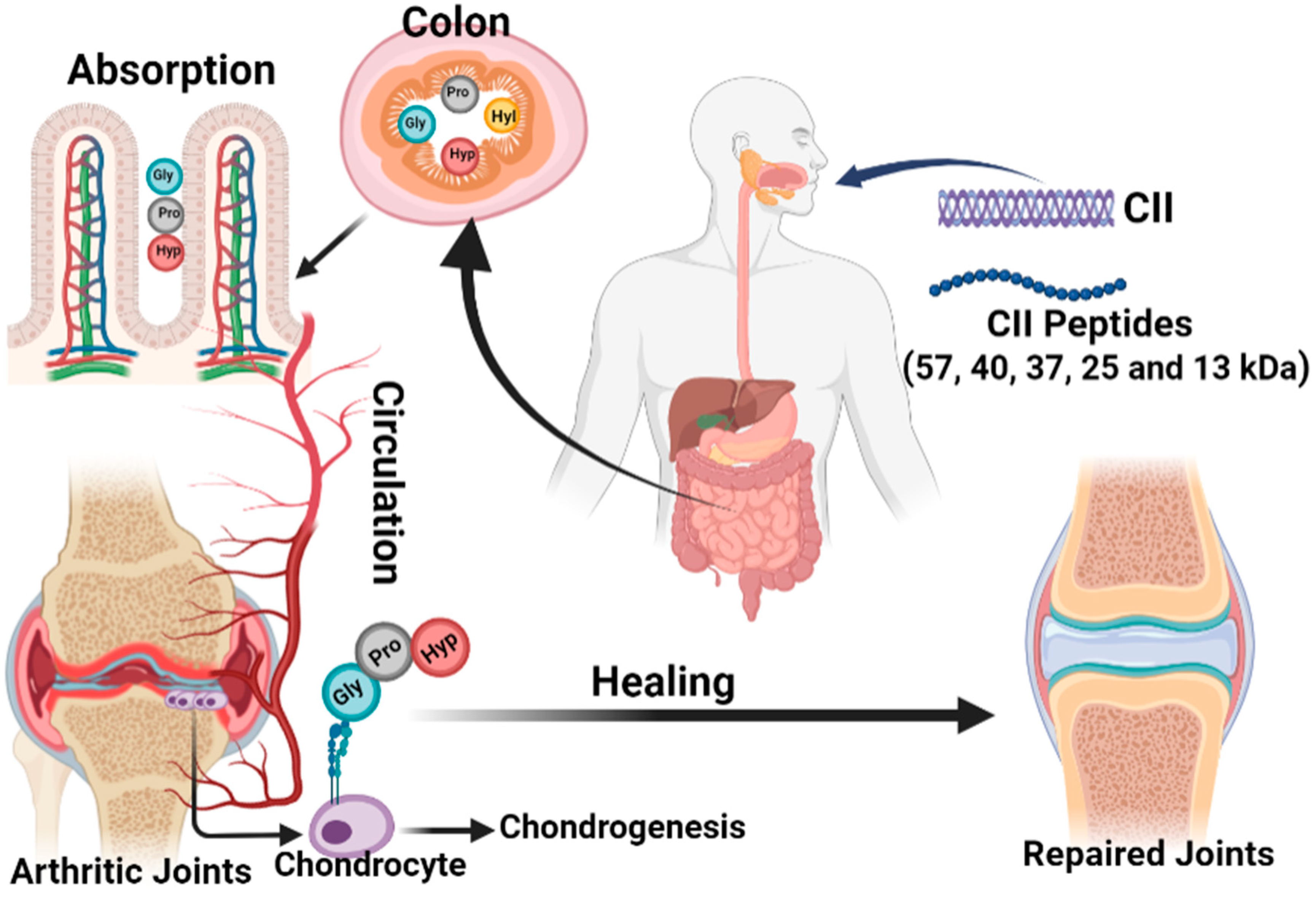
3. Collagen Treatment Strategy in Arthritis
3.1. Collagen Supports for Arthritis Treatment
| Study Type | Type of Collagen and Molecular Weight | Year | Outcome | Reference |
|---|---|---|---|---|
| in vitro/in vivo | CII 37 KDa | 2015 | Antioxidant | [113] |
| in vitro | CII peptide | 2019 | Antioxidant | [114] |
| in vitro | CI peptide | 2017 | Immunologic tolerance | [115] |
| in vitro | CII (25, 40 and 57 KDa) | 2016 | Suppress immune response | [116] |
| in vivo | Collagen peptide | 2019 | Maintaining amino acid balance | [117] |
| Clinical trials | Collagen Hydrolyzates | 2021 | Support joint health and anti-osteoarthritis (OA) | [121] |
| Clinical trials | Collagen hydrolyzates | 2019 | Increases the postprandial plasma concentration of aminoacids | [122] |
| Clinical trials | CII | 2022 | Preventing joint inflammation of OA and RA | [131] |
| in vitro/in vivo | Collagen hydrolyzates | 2010 | Increasing osteoblastogenesis and improve bone metabolism | [136] |
| in vivo | CII hydrolyzates | 2012 | Improve OA related symptoms | [137] |
| in vivo | Collagen peptide | 2018 | Relief OA symptoms | [139] |
| Clinical trials | CII hydrolyzates | 2008 | Support joint health | [140] |
| Clinical trials | Collagen hydrolyzates | 2011 | Patient exhibited changes in proteoglycan content in knee | [143] |
3.2. Negative Impact of Collagen in Arthritis
3.3. Molecular Mechanism of Collagen for Cartilage Homeostasis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CI | Type I collagen |
| CII | Type II collagen |
| MMPs | Matrix metalloproteinases |
| NK cells | Natural Killer cells |
| DCs | Dendritic cells |
| CIA | Collagen-induced arthritis model |
| RA | Rheumatoid arthritis |
| OA | Osteoarthritis |
| SFs | Synovial fibroblasts |
| mAbs | Monoclonal antibodies |
| TCR | T cell receptor |
| LPS | Lipopolysaccharide |
| RUNX2 | Runt-related transcription factor 2 |
| ALP | Alkaline Phosphatase |
| OPN | Osteopontin |
| OCN | Osteocalcin |
| TNF-α | Tumor necrosis factor-α |
| IL | Interleukin |
| TGF | β- Transforming growth factor β |
| ECM | Extracellular matrix |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular signal-regulated kinases |
| Sox9 | SRY-related high-mobility group-box gene 9 |
References
- Gentile, P.; De Angelis, B.; Agovino, A.; Orlandi, F.; Migner, A.; Di Pasquali, C.; Cervelli, V. Use of platelet rich plasma and hyaluronic acid in the treatment of complications of achilles tendon reconstruction. World J. Plast. Surg. 2016, 5, 124–132. [Google Scholar] [PubMed]
- Deng, Z.; Lin, Z.; Zhong, Q.; Lu, M.; Fang, H.; Liu, J.; Duan, L.; Chen, L.; Wang, L.; Wang, D.; et al. Interleukin 1 beta-induced chloride currents are important in osteoarthritis onset: An in vitro study. Acta Biochim. Biophys. Sin. 2021, 53, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β (lymphotoxin α) can activate the inflammatory environment in human chondrocytes. Arthritis Res. Ther. 2013, 15, R202. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-kB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef]
- Pattappa, G.; Schewior, R.; Hofmeister, I.; Seja, J.; Zellner, J.; Johnstone, B.; Docheva, D.; Angele, P. Physioxia has a beneficial effect on cartilage matrix production in interleukin-1 beta-inhibited mesenchymal stem cell chondrogenesis. Cells 2019, 8, 936. [Google Scholar] [CrossRef]
- Kour, S.; Garimella, M.G.; Shiroor, D.A.; Mhaske, S.T.; Joshi, S.R.; Singh, K.; Pal, S.; Mittal, M.; Krishnan, H.B.; Chattopadhyay, N.; et al. IL-3 decreases cartilage degeneration by downregulating matrix metalloproteinases and reduces joint destruction in osteoarthritic mice. J. Immunol. 2016, 196, 5024–5035. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Wiegertjes, R.; van de Loo, F.A.; Blaney Davidson, E.N. A roadmap to target interleukin-6 in osteoarthritis. Rheumatology 2020, 59, 2681–2694. [Google Scholar] [CrossRef]
- Li, C.; Wang, Q.; Wang, J.F. Transforming growth factor-β (TGF-β) induces the expression of chondrogenesis-related genes through TGF-β receptor II (TGFRII)–AKT–mTOR signaling in primary cultured mouse precartilaginous stem cells. Biochem. Biophys. Res. Commun. 2014, 450, 646–651. [Google Scholar] [CrossRef]
- Wiegertjes, R.; Van Caam, A.; Van Beuningen, H.; Koenders, M.; Van Lent, P.; Van Der Kraan, P.; van de Loo, F.; Davidson, E.B. TGF-β dampens IL-6 signaling in articular chondrocytes by decreasing IL-6 receptor expression. Osteoarthr. Cartil. 2019, 27, 1197–1207. [Google Scholar] [CrossRef]
- Tarkowski, A.; Carlsten, H.; Herberts, P.; Klareskog, L.; Koopman, W.J. Secretion of antibodies to types I and II collagen by synovial tissue cells in patients with rheumatoid arthritis. Arthritis Rheum. 1989, 32, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Rudolphi, U.; Rzepka, R.; Batsford, S.; Kaufmann, S.H.; von der Mark, K.; Peter, H.H.; Melchers, I. The B cell repertoire of patients with rheumatoid arthritis. II. Increased frequencies of IgG+ and IgA+ B cells specific for mycobacterial heat-shock protein 60 or human type II collagen in synovial fluid and tissue. Arthritis Rheum. 1997, 40, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Londei, M.; Savill, C.M.; Verhoef, A.; Brennan, F.; Leech, Z.A.; Duance, V.; Maini, R.N.; Feldmann, M. Persistence of collagen type II-specific T-cell clones in the synovial membrane of a patient with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 1989, 86, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, W.U.; Cho, M.L.; Lee, S.K.; Youn, J.; Kim, S.I.; Yoo, W.H.; Park, J.H.; Min, J.K.; Lee, S.H.; et al. Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255–274) in patients with rheumatoid arthritis. Arthritis Rheum. 1999, 42, 2085–2093. [Google Scholar] [CrossRef]
- Cook, A.D.; Stockman, A.; Brand, C.A.; Tait, B.D.; Mackay, I.R.; Muirden, K.D.; Bernard, C.C.; Rowley, M.J. Antibodies to type II collagen and HLA disease susceptibility markers in rheumatoid arthritis. Arthritis Rheum. 1999, 42, 2569–2576. [Google Scholar] [CrossRef]
- Burkhardt, H.; Koller, T.; Engström, Å.; Nandakumar, K.S.; Turnay, J.; Kraetsch, H.G.; Kalden, J.R.; Holmdahl, R. Epitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouse. Arthritis Rheum. 2002, 46, 2339–2348. [Google Scholar] [CrossRef]
- Roudier, J.; Rhodes, G.; Petersen, J.; Vaughan, J.; Carson, D. The epstein-barr virus glycoprotein gp110, a molecular link between HLA DR4, HLA DR1, and rheumatoid arthritis. Scand. J. Immunol. 1988, 27, 367–371. [Google Scholar] [CrossRef]
- Fujinami, R.; Nelson, J.; Walker, L.; Oldstone, M. Sequence homology and immunologic cross-reactivity of human cytomegalovirus with HLA-DR beta chain: A means for graft rejection and immunosuppression. J. Virol. 1988, 62, 100–105. [Google Scholar] [CrossRef]
- Yoo, T.; Kim, S.-Y.; Stuart, J.; Floyd, R.; Olson, G.; Cremer, M.; Kang, A. Induction of arthritis in monkeys by immunization with type II collagen. J. Exp. Med. 1988, 168, 777–782. [Google Scholar] [CrossRef]
- Courtenay, J.; Dallman, M.J.; Dayan, A.; Martin, A.; Mosedale, B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature 1980, 283, 666–668. [Google Scholar] [CrossRef]
- Trentham, D.E.; Townes, A.S.; Kang, A.H. Autoimmunity to type II collagen an experimental model of arthritis. J. Exp. Med. 1977, 146, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Luthra, H.; Moore, S.; O’Fallon, W. Serum IgG anti-native type II collagen antibodies in rheumatoid arthritis: Association with HLA DR4 and lack of clinical correlation. Clin. Exp. Rheumatol. 1988, 6, 373–380. [Google Scholar] [PubMed]
- Stuart, J.M.; Huffstutter, E.H.; Townes, A.S.; Kang, A.H. Incidence and specificity of antibodies to types I, II, III, IV, and V collagen in rheumatoid arthritis and other rheumatic diseases as measured by 125I-radioimmunoassay. Arthritis Rheum. 1983, 26, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Watson, W.C.; Tooms, R.E.; Carnesale, P.G.; Dutkowsky, J.P. A case of germinal center formation by CD45RO T and CD20 B lymphocytes in rheumatoid arthritic subchondral bone: Proposal for a two-compartment model of immune-mediated disease with implications for immunotherapeutic strategies. Clin. Immunol. Immunopathol. 1994, 73, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J.M.; Dixon, F.J. Serum transfer of collagen-induced arthritis in mice. J. Exp. Med. 1983, 158, 378–392. [Google Scholar] [CrossRef]
- Wooley, P.H.; Luthra, H.S.; Singh, S.K.; Huse, A.R.; Stuart, J.M.; David, C.S. Passive Transfer of Arthritis to Mice by Injection of Human Anti-Type II Collagen Antibody. Mayo Clin. Proc. 1984, 59, 737–743. [Google Scholar] [CrossRef]
- Myers, L.; Tang, B.; Stuart, J.; Kang, A. The role of IL-4 in regulation of murine collagen-induced arthritis. Clin. Immunol. 2002, 102, 185–191. [Google Scholar] [CrossRef]
- Katsumata, K.; Ishihara, J.; Mansurov, A.; Ishihara, A.; Raczy, M.M.; Yuba, E.; Hubbell, J.A. Targeting inflammatory sites through collagen affinity enhances the therapeutic efficacy of anti-inflammatory antibodies. Sci. Adv. 2019, 5, eaay1971. [Google Scholar] [CrossRef]
- Devasia, S.; Joseph, J.T.; Stephena, P.S.; Koizumi, S.; Himeno, A.; Gayathri, V.; Madhavan, S. Bioactive collagen peptides ameliorate monoidoacetic acid induced osteoarthritis in rats. J. Orthop. Res. Ther. 2021, 6, 1205. [Google Scholar] [CrossRef]
- Hakuta, A.; Yamaguchi, Y.; Okawa, T.; Yamamoto, S.; Sakai, Y.; Aihara, M. Anti-inflammatory effect of collagen tripeptide in atopic dermatitis. J. Dermatol. Sci. 2017, 88, 357–364. [Google Scholar] [CrossRef]
- Zinkernagel, R.M.; Callahan, G.; Althage, A.; Cooper, S.; Klein, P.; Klein, J. On the thymus in the differentiation of “H-2 self-recognition” by T cells: Evidence for dual recognition? J. Exp. Med. 1978, 147, 882–896. [Google Scholar] [CrossRef]
- Kappler, J.W.; Staerz, U.; White, J.; Marrack, P.C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature 1988, 332, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Rooke, R.; Waltzinger, C.; Benoist, C.; Mathis, D. Targeted complementation of MHC class II deficiency by intrathymic delivery of recombinant adenoviruses. Immunity 1997, 7, 123–134. [Google Scholar] [CrossRef][Green Version]
- Brunsberg, U.; Gustafsson, K.; Jansson, L.; Michaëlsson, E.; Ährlund-Richter, L.; Pettersson, S.; Mattsson, R.; Holmdahl, R. Expression of a transgenic class II Ab gene confers susceptibility to collagen-induced arthritis. Eur. J. Immunol. 1994, 24, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, E.; Andersson, M.; Holmdahl, R.; Engström, Å. Identification of an immunodominant type-II collagen peptide recognized by T cells in H-2q mice: Self tolerance at the level of determinant selection. Eur. J. Immunol. 1992, 22, 1819–1825. [Google Scholar] [CrossRef]
- Brand, D.D.; Myers, L.K.; Terato, K.; Whittington, K.B.; Stuart, J.M.; Kang, A.H.; Rosloniec, E.F. Characterization of the T cell determinants in the induction of autoimmune arthritis by bovine alpha 1 (II)-CB11 in H-2q mice. J. Immunol. 1994, 152, 3088–3097. [Google Scholar]
- Gregersen, P.K.; Silver, J.; Winchester, R.J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987, 30, 1205–1213. [Google Scholar] [CrossRef]
- Källberg, H.; Padyukov, L.; Plenge, R.M.; Rönnelid, J.; Gregersen, P.K.; Mil, A.H.V.D.H.-V.; Toes, R.E.; Huizinga, T.W.; Klareskog, L.; Alfredsson, L. Gene-gene and gene-environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am. J. Hum. Genet. 2007, 80, 867–875. [Google Scholar] [CrossRef]
- Vingsbo, C.; Sahlstrand, P.; Brun, J.G.; Jonsson, R.; Saxne, T.; Holmdahl, R. Pristane-induced arthritis in rats: A new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am. J. Pathol. 1996, 149, 1675. [Google Scholar]
- Kawahito, Y.; Cannon, G.W.; Gulko, P.S.; Remmers, E.F.; Longman, R.E.; Reese, V.R.; Wang, J.; Griffiths, M.M.; Wilder, R.L. Localization of quantitative trait loci regulating adjuvant-induced arthritis in rats: Evidence for genetic factors common to multiple autoimmune diseases. J. Immunol. 1998, 161, 4411–4419. [Google Scholar]
- Matsumoto, I.; Staub, A.; Benoist, C.; Mathis, D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science 1999, 286, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Tosu, M.; Yoshida, E.; Takiguchi, M.; Sato, K.; Kitajima, I.; Nishioka, K.; Yamamoto, K.; Takeda, T.; Hatanaka, M.; et al. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science 1991, 253, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Ikeda, H.; Ishizu, A.; Nakamaru, Y.; Sugaya, T.; Kikuchi, K.; Yamada, S.; Wakisaka, A.; Kasai, N.; Koike, T. A wide spectrum of collagen vascular and autoimmune diseases in transgenic rats carrying the env-pX gene of human T lymphocyte virus type I. Int. Immunol. 1997, 9, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; McCarthy, T.G.; Goronzy, J.J. Correlation between disease phenotype and genetic heterogeneity in rheumatoid arthritis. J. Clin. Investig. 1995, 95, 2120–2126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holmdahl, R.; Andersson, M.; Goldschmidt, T.J.; Gustafsson, K.E.; Jansson, L.; Mo, J.A. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol. Rev. 1990, 118, 193–232. [Google Scholar] [CrossRef]
- Kotzin, B.L.; Falta, M.T.; Crawford, F.; Rosloniec, E.F.; Bill, J.; Marrack, P.; Kappler, J. Use of soluble peptide–DR4 tetramers to detect synovial T cells specific for cartilage antigens in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 291–296. [Google Scholar] [CrossRef]
- Stastny, P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N. Engl. J. Med. 1978, 298, 869–871. [Google Scholar] [CrossRef]
- Fugger, L.; Rothbard, J.B.; Sonderstrup-McDevitt, G. Specificity of an HLA-DRB1*0401-restricted T cell response to type II collagen. Eur. J. Immunol. 1996, 26, 928–933. [Google Scholar] [CrossRef]
- Dessen, A.; Lawrence, C.M.; Cupo, S.; Zaller, D.M.; Wiley, D.C. X-ray crystal structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity 1997, 7, 473–481. [Google Scholar] [CrossRef]
- Metsäranta, M.; Toman, D.; de Crombrugghe, B.; Vuorio, E. Mouse type II collagen gene. Complete nucleotide sequence, exon structure, and alternative splicing. J. Biol. Chem. 1991, 266, 16862–16869. [Google Scholar] [CrossRef]
- Malmström, V.; Kjellén, P.; Holmdahl, R. Type II collagen in cartilage evokes peptide-specific tolerance and skews the immune response. J. Autoimmun. 1998, 11, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Corthay, A.; Bäcklund, J.; Holmdahl, R. Role of glycopeptide-specific T cells in collagen-induced arthritis: An example how post-translational modification of proteins may be involved in autoimmune disease. Ann. Med. 2001, 33, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Michaëlssony, E.; Holmdahl, M.; Engström, A.; Burkhardt, H.; Scheynius, A.; Holmdahl, R. Macrophages, but not dendritic cells, present collagen to T cells. Eur. J. Immunol. 1995, 25, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Manoury-Schwartz, B.; Chiocchia, G.; Fournier, C. Processing and presentation of type II collagen, a fibrillar autoantigen, by H-2q antigen-presenting cells. Eur. J. Immunol. 1995, 25, 3235–3242. [Google Scholar] [CrossRef]
- Malmström, V.; Michaëlsson, E.; Burkhardt, H.; Mattsson, R.; Vuorio, E.; Holmdahl, R. Systemic versus cartilage-specific expression of a type II collagen-specific T-cell epitope determines the level of tolerance and susceptibility to arthritis. Proc. Natl. Acad. Sci. USA 1996, 93, 4480–4485. [Google Scholar] [CrossRef]
- Nishikimi, A.; Koyama, Y.I.; Ishihara, S.; Kobayashi, S.; Tometsuka, C.; Kusubata, M.; Kuwaba, K.; Hayashida, O.; Hattori, S.; Katagiri, K. Collagen-derived peptides modulate CD4 + T-cell differentiation and suppress allergic responses in mice. Immun. Inflamm. Diseas 2018, 6, 245–255. [Google Scholar] [CrossRef]
- Iwamoto, I.; Nakajima, H.; Endo, H.; Yoshida, S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J. Exp. Med. 1993, 177, 573–576. [Google Scholar] [CrossRef]
- Zhao, S.T.; Wang, C.Z. Regulatory T cells and asthma. J. Zhejiang Univ. Sci. B 2018, 19, 663–673. [Google Scholar] [CrossRef]
- Palomares, O.; Yaman, G.; Azkur, A.K.; Akkoc, T.; Akdis, M.; Akdis, C.A. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 2010, 40, 1232–1240. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Seyer, J.M.; Kang, A.H. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc. Natl. Acad. Sci. USA 1978, 75, 871–875. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Kang, A.H. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J. Exp. Med. 1976, 143, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Laskin, D.L.; Kimura, T.; Sakakibara, S.; Riley, D.J.; Berg, R.A. Chemotactic activity of collagen-like polypeptides for human peripheral blood neutrophils. J. Leukoc. Biol. 1986, 39, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.I.; Hayashida, O.; Kuwaba, K.; Takara, T.; Kusubata, M.; Tsukada, Y. Supplemental ingestion of collagen peptide improves T-cell-related human immune status:Placebo-controlled double-blind study. Jpn. Pharmacol. Ther. 2015, 43, 51–56. [Google Scholar]
- Ranges, G.; Sriram, S.; Cooper, S. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J. Exp. Med. 1985, 162, 1105–1110. [Google Scholar] [CrossRef]
- Goldschmidt, T.; Jansson, L.; Holmdahl, R. In vivo elimination of T cells expressing specific T-cell receptor V beta chains in mice susceptible to collagen-induced arthritis. Immunology 1990, 69, 508. [Google Scholar]
- Michaëlsson, E.; Malmström, V.; Reis, S.; Engström, Å.; Burkhardt, H.; Holmdahl, R. T cell recognition of carbohydrates on type II collagen. J. Exp. Med. 1994, 180, 745–749. [Google Scholar] [CrossRef]
- Michaëlsson, E.; Broddefalk, J.; Engström, Å.; Kihlberg, J.; Holmdahl, R. Antigen processing and presentation of a naturally glycosylated protein elicits major histocompatibility complex class II-restricted, carbohydrate-specific T cells. Eur. J. Immunol. 1996, 26, 1906–1910. [Google Scholar] [CrossRef]
- Kjellén, P. Characterization of the interaction between IA and the immunodominant collagen type II 256–270 peptide. Eur. J. Immunol. 1997, 28, 755–767. [Google Scholar] [CrossRef]
- Rosloniec, E.F.; Whittington, K.B.; Brand, D.D.; Myers, L.K.; Stuart, J.M. Identification of MHC class II and TCR binding residues in the type II collagen immunodominant determinant mediating collagen-induced arthritis. Cell. Immunol. 1996, 172, 21–28. [Google Scholar] [CrossRef]
- Kjellén, P.; Brunsberg, U.; Broddefalk, J.; Hansen, B.; Vestberg, M.; Ivarsson, I.; Engström, Å.; Svejgaard, A.; Kihlberg, J.; Fugger, L.; et al. The structural basis of MHC control of collagen-induced arthritis; binding of the immunodominant type II collagen 256–270 glycopeptide to H-2Aq and H-2Ap molecules. Eur. J. Immunol. 1998, 28, 755–766. [Google Scholar] [CrossRef]
- Brand, D.D.; Kang, A.H.; Rosloniec, E.F. Immunopathogenesis of Collagen Arthritis. Springer Semin. Immunopathol. 2003, 25, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Da, R.-R.; Qin, Y.; Baeten, D.; Zhang, Y. B cell clonal expansion and somatic hypermutation of Ig variable heavy chain genes in the synovial membrane of patients with osteoarthritis. J. Immunol. 2007, 178, 557–565. [Google Scholar] [CrossRef] [PubMed]
- John, T.; Kohl, B.; Mobasheri, A.; Ertel, W.; Shakibaei, M. Interleukin-18 induces apoptosis in human articular chondrocytes. Histol. Histopathol. 2007, 22, 469–482. [Google Scholar]
- Zheng, Z.; Liu, T.; Li, X.; Ding, J.; Feng, Y.; Miao, J.; Luo, X.; Wu, Z.; Zhu, P. Kinetic changes of regulatory B10 cells in collagen-induced arthritis could be regulated by cytokines IFN-γ and TGF-β1. Inflamm. Res. 2015, 64, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.T.; Sun, Y.H.; Zong, S.H.; Xiang, Y.B. Serum levels of il-6 and tnf-α may correlate with activity and severity of rheumatoid arthritis. Med. Sci. Monit. 2015, 21, 4030–4038. [Google Scholar] [CrossRef]
- Achudhan, D.; Liu, S.C.; Lin, Y.Y.; Huang, C.C.; Tsai, C.H.; Ko, C.Y.; Chiang, I.P.; Kuo, Y.H.; Tang, C.H. Antcin K inhibits tnf-α, il-1β and il-8 expression in synovial fibroblasts and ameliorates cartilage degradation: Implications for the treatment of rheumatoid arthritis. Front. Immunol. 2021, 12, 790925. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Groote, D.D.; Labasse, A.H.; Gaspar, S.E.; Zheng, S.X.; Geenen, V.G.; Reginster, J.Y.L. Effects of exogenous il-1β, tnfα, il-6, il-8 and lif on cytokine production by human articular chondrocytes. Osteoarthr. Cartil. 1996, 4, 163–173. [Google Scholar] [CrossRef]
- Störch, H.; Zimmermann, B.; Resch, B.; Tykocinski, L.-O.; Moradi, B.; Horn, P.; Kaya, Z.; Blank, N.; Rehart, S.; Thomsen, M.; et al. Activated human b cells induce inflammatory fibroblasts with cartilage-destructive properties and become functionally suppressed in return. Ann. Rheum. Dis. 2016, 75, 924–932. [Google Scholar] [CrossRef]
- Terato, K.; Hasty, K.A.; Reife, R.A.; Cremer, M.A.; Kang, A.; Stuart, J. Induction of arthritis with monoclonal antibodies to collagen. J. Immunol. 1992, 148, 2103–2108. [Google Scholar]
- Nandakumar, K.S.; Svensson, L.; Holmdahl, R. Collagen type ii-specific monoclonal antibody-induced arthritis in mice: Description of the disease and the influence of age, sex, and genes. Am. J. Pathol. 2003, 163, 1827–1837. [Google Scholar] [CrossRef]
- Watson, W.; Brown, P.; Pitcock, J.; Townes, A. Passive transfer studies with type ii collagen antibody in b10. d2/old and new line and c57b1/6 normal and beige (chediak-higashi) strains: Evidence of important roles for c5 and multiple inflammatory cell types in the development of erosive arthritis. Arthritis Rheum. 1987, 30, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Bajtner, E.; Nandakumar, K.S.; Engström, Å.; Holmdahl, R. Chronic development of collagen-induced arthritis is associated with arthritogenic antibodies against specific epitopes on type ii collagen. Arthritis Res. Ther. 2005, 7, R1148–R1157. [Google Scholar] [CrossRef] [PubMed]
- Wernhoff, P.; Unger, C.; Bajtner, E.; Burkhardt, H.; Holmdahl, R. Identification of conformation-dependent epitopes and v gene selection in the b cell response to type ii collagen in the da rat. Int. Immunol. 2001, 13, 909–919. [Google Scholar] [CrossRef]
- Tsark, E.C.; Wang, w.; Teng, Y.-C.; Arkfeld, D.; Dodge, G.R.; Kovats, S. Differential mhc class ii-mediated presentation of rheumatoid arthritis autoantigens by human dendritic cells and macrophages. J. Immunol. 2002, 169, 6625–6633. [Google Scholar] [CrossRef]
- Schulte, S.; Unger, C.; Mo, J.A.; Wendler, O.; Bauer, E.; Frischholz, S.; Von der mark, K.; Kalden, J.R.; Holmdahl, R.; Burkhardt, H. Arthritis-related b cell epitopes in collagen ii are conformation-dependent and sterically privileged in accessible sites of cartilage collagen fibrils. J. Biol. Chem. 1998, 273, 1551–1561. [Google Scholar] [CrossRef]
- Svensson, L.; Jirholt, J.; Holmdahl, R.; Jansson, L. B cell-deficient mice do not develop type ii collagen-induced arthritis (cia). Clin. Exp. Immunol. 1998, 111, 521–526. [Google Scholar] [CrossRef]
- Holmdahl, R.; Rubin, K.; Klareskog, L.; Larsson, E.; Wigzell, H. Characterization of the antibody response in mice with type ii collagen–induced arthritis, using monoclonal anti–type ii collagen antibodies. Arthritis Rheum. 1986, 29, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Dzhambazov, B.; Bockermann, R.; Blom, T.; Holmdahl, R. A transient post-translationally modified form of cartilage type ii collagen is ignored by self-reactive t cells. J. Immunol. 2004, 173, 4729–4735. [Google Scholar] [CrossRef]
- Dzhambazov, B.; Holmdahl, M.; Yamada, H.; Lu, S.; Vestberg, M.; Holm, B.; Johnell, O.; Kihlberg, J.; Holmdahl, R. The major t cell epitope on type ii collagen is glycosylated in normal cartilage but modified by arthritis in both rats and humans. Eur. J. Immunol. 2005, 35, 357–366. [Google Scholar] [CrossRef]
- Terato, K.; Harper, D.S.; Griffiths, M.M.; Hasty, D.L.; Ye, X.J.; Cremer, M.A.; Seyer, J.M. Collagen-induced arthritis in mice: Synergistic effect of e. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type ii collagen. Autoimmunity 1995, 22, 137–147. [Google Scholar] [CrossRef]
- Bäcklund, J.; Carlsen, S.; Höger, T.; Holm, B.; Fugger, L.; Kihlberg, J.; Burkhardt, H.; Holmdahl, R. Predominant selection of t cells specific for the glycosylated collagen type ii epitope (263–270) in humanized transgenic mice and in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2002, 99, 9960–9965. [Google Scholar] [CrossRef] [PubMed]
- Terato, K.; Hasty, K.A.; Cremer, M.A.; Stuart, J.M.; Townes, A.S.; Kang, A. Collagen-induced arthritis in mice. localization of an arthritogenic determinant to a fragment of the type ii collagen molecule. J. Exp. Med. 1985, 162, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Holmdahl, R.; Mo, J.A.; Jonsson, R.; Karlstrom, K.; Scheynius, A. Multiple epitopes on cartilage type ii collagen are accessible for antibody binding in vivo. Autoimmunity 1991, 10, 27–34. [Google Scholar] [CrossRef]
- Holmdahl, R.; Jansson, L.; Larsson, A.; Jonsson, R. Arthritis in dba/1 mice induced with passively transferred type ii collagen immune serum. Scand. J. Immunol. 1990, 31, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Helfgott, S.M.; Bazin, H.; Dessein, A.; Trentham, D.E. Suppressive effects of anti-μ serum on the development of collagen arthritis in rats. Clin. Immunol. Immunopathol. 1984, 31, 403–411. [Google Scholar] [CrossRef]
- Jansson, L.; Holmdahl, R. Genes on the x chromosome affect development of collagen-induced arthritis in mice. Clin. Exp. Immunol. 1993, 94, 459–465. [Google Scholar] [CrossRef]
- Worthington, J.; Brass, A.; Morgan, K. Identification of antibody epitopes within the cb-11 peptide of type ii collagen. i: Detection of antibody binding sites by epitope scanning. Autoimmunity 1991, 10, 201–207. [Google Scholar] [CrossRef]
- Brand, D.D.; Marion, T.N.; Myers, L.K.; Rosloniec, E.F.; Watson, W.C.; Stuart, J.M.; Kang, A.H. Autoantibodies to murine type ii collagen in collagen-induced arthritis: A comparison of susceptible and nonsusceptible strains. J. Immunol. 1996, 157, 5178–5184. [Google Scholar]
- Hedbom, E.; Heinegård, D. Interaction of a 59-kda connective tissue matrix protein with collagen i and collagen ii. J. Biol. Chem. 1989, 264, 6898–6905. [Google Scholar] [CrossRef]
- Hedbom, E.; Heinegård, D. Binding of fibromodulin and decorin to separate sites on fibrillar collagens. J. Biol. Chem. 1993, 268, 27307–27312. [Google Scholar] [CrossRef]
- Wu, J.-J.; Eyre, D.R. Structural analysis of cross-linking domains in cartilage type xi collagen: Insights on polymeric assembly. J. Biol. Chem. 1995, 270, 18865–18870. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, L.; Mendler, M.; Huber, S.; Bruckner, P.; Winterhalter, K.H.; Irwin, M.I.; Mayne, R. D-periodic distribution of collagen type ix along cartilage fibrils. J. Cell Biol. 1988, 106, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Woods, P.E.; Eyre, D.R. Identification of cross-linking sites in bovine cartilage type IX collagen reveals an antiparallel type II-type IX molecular relationship and type IX to type IX bonding. J. Biol. Chem. 1992, 267, 23007–23014. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Hengartner, H.; Zinkernagel, R.M. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: Role of antigen patterns in B cell induction? Eur. J. Immunol. 1995, 25, 3445–3451. [Google Scholar] [CrossRef]
- Kouskoff, V.; Korganow, A.S.; Duchatelle, V.; Degott, C.; Benoist, C.; Mathis, D. Organ-specific disease provoked by systemic autoimmunity. Cell 1996, 87, 811–822. [Google Scholar] [CrossRef]
- Leandro, M.J.; Edwards, J.C.; Cambridge, G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann. Rheum. Dis. 2002, 61, 883–888. [Google Scholar] [CrossRef]
- Edwards, J.C.; Szczepanski, L.; Szechinski, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M.; Shaw, T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004, 350, 2572–2581. [Google Scholar] [CrossRef]
- Martin, F.; Chan, A.C. Pathogenic roles of B cells in human autoimmunity: Insights from the clinic. Immunity 2004, 20, 517–527. [Google Scholar] [CrossRef]
- Patel, D.D. B cell-ablative therapy for the treatment of autoimmune diseases. Arthritis Rheum. 2002, 46, 1984–1995. [Google Scholar] [CrossRef]
- Dunussi-Joannopoulos, K.; Hancock, G.E.; Kunz, A.; Hegen, M.; Zhou, X.X.; Sheppard, B.J.; Lamothe, J.; Li, E.; Ma, H.L.; Hamann, P.R.; et al. B-cell depletion inhibits arthritis in a collagen-induced arthritis (CIA) model, but does not adversely affect humoral responses in a respiratory syncytial virus (RSV) vaccination model. Blood 2005, 106, 2235–2243. [Google Scholar] [CrossRef]
- Holmdahl, R.; Bailey, C.; Enander, I.; Mayer, R.; Klareskog, L.; Moran, T.; Bona, C. Origin of the autoreactive anti-type II collagen response. II. Specificities, antibody isotypes and usage of V gene families of anti-type II collagen B cells. J. Immunol. 1989, 142, 1881–1886. [Google Scholar] [PubMed]
- Jeevithan, E.; Bao, B.; Zhang, J.; Hong, S.; Wu, W. Purification, characterization and antioxidant properties of low molecular weight collagenous polypeptide (37 kDa) prepared from whale shark cartilage (Rhincodon typus). J. Food Sci. Technol. 2015, 52, 6312–6322. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Ou, A.; Liu, S.; Elango, J.; Wang, S.; Henriques da Silva, T.; Wu, W.; Robinson, J.; Bao, B. Effects of ion concentrations on the hydroxyl radical scavenging rate and reducing power of fish collagen peptides. J. Food Biochem. 2019, 43, e12789. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Elango, J.; Zhang, J.; Bao, B.; Guo, R.; Palaniyandi, K.; Robinson, J.S.; Geevaretnam, J.; Regenstein, J.M.; Wu, W. Immunological effects of collagen and collagen peptide from blue shark cartilage on 6T-CEM cells. Process Biochem. 2017, 57, 219–227. [Google Scholar] [CrossRef]
- Jeevithan, E.; Jingyi, Z.; Bao, B.; Shujun, W.; Jeyashakila, R.; Wu, W. Biocompatibility assessment of type-II collagen and its polypeptide for tissue engineering: Effect of collagen’s molecular weight and glycoprotein content on tumor necrosis factor (Fas/Apo-1) receptor activation in human acute T-lymphocyte leukemia cell line. RSC Adv. 2016, 6, 14236–14246. [Google Scholar]
- Paul, C.; Leser, S.; Oesser, S. Significant amounts of functional collagen peptides can be incorporated in the diet while maintaining indispensable amino acid balance. Nutrients 2019, 11, 1079. [Google Scholar] [CrossRef]
- Trentham, D.E.; Dynesius-Trentham, R.A.; Orav, E.J.; Combitchi, D.; Lorenzo, C.; Sewell, K.L.; Hafler, D.A.; Weiner, H.L. Effects of oral administration of type II collagen on rheumatoid arthritis. Science 1993, 261, 1727–1730. [Google Scholar] [CrossRef]
- Castro-Sánchez, P.; Martín-Villa, J.M. Gut immune system and oral tolerance. Br. J. Nutr. 2013, 109 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef]
- Worbs, T.; Bode, U.; Yan, S.; Hoffmann, M.W.; Hintzen, G.; Bernhardt, G.; Förster, R.; Pabst, O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 2006, 203, 519–527. [Google Scholar] [CrossRef]
- Mobasheri, A.; Mahmoudian, A.; Kalvaityte, U.; Uzieliene, I.; Larder, C.E.; Iskandar, M.M.; Kubow, S.; Hamdan, P.C.; de Almeida, C.S., Jr.; Favazzo, L.J.; et al. A white paper on collagen hydrolyzates and ultrahydrolyzates: Potential supplements to support joint health in osteoarthritis? Curr. Rheumatol. Rep. 2021, 23, 78. [Google Scholar] [CrossRef]
- Skov, K.; Oxfeldt, M.; Thøgersen, R.; Hansen, M.; Bertram, H.C. Enzymatic hydrolysis of a collagen hydrolysate enhances postprandial absorption rate—A randomized controlled trial. Nutrients 2019, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Low dose native type II collagen prevents pain in a rat osteoarthritis model. BMC Musculoskelet. Disord. 2013, 14, 228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jeevithan, E.; Bao, B.; Wang, S.; Gao, K.; Zhang, C.; Wu, W. Structural characterization, in vivo acute systemic toxicity assessment and in vitro intestinal absorption properties of tilapia (Oreochromis niloticus) skin acid and pepsin solublilized type I collagen. Process Biochem. 2016, 51, 2017–2025. [Google Scholar] [CrossRef]
- Jeevithan, E.; Jingyi, Z.; Wang, N.; He, L.; Bao, B.; Wu, W. Physico-chemical, antioxidant and intestinal absorption properties of whale shark type-II collagen based on its solubility with acid and pepsin. Process Biochem. 2015, 50, 463–472. [Google Scholar] [CrossRef]
- Oesser, S.; Adam, M.; Babel, W.; Seifert, J. Oral administration of (14)C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J. Nutr. 1999, 129, 1891–1895. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Liang, Q.; He, Y.; Wang, Z.; He, S.; Xu, J.; Ma, H. Determination of bioavailability and identification of collagen peptide in blood after oral ingestion of gelatin. J. Sci. Food Agric. 2015, 95, 2712–2717. [Google Scholar] [CrossRef]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef]
- Siebert, H.C.; Burg-Roderfeld, M.; Eckert, T.; Stötzel, S.; Kirch, U.; Diercks, T.; Humphries, M.J.; Frank, M.; Wechselberger, R.; Tajkhorshid, E.; et al. Interaction of the α2A domain of integrin with small collagen fragments. Protein Cell 2010, 1, 393–405. [Google Scholar] [CrossRef]
- Stötzel, S.; Schurink, M.; Wienk, H.; Siebler, U.; Burg-Roderfeld, M.; Eckert, T.; Kulik, B.; Wechselberger, R.; Sewing, J.; Steinmeyer, J.; et al. Molecular organization of various collagen fragments as revealed by atomic force microscopy and diffusion-ordered NMR spectroscopy. Chemphyschem 2012, 13, 3117–3125. [Google Scholar] [CrossRef]
- Bagchi, D.; Misner, B.; Bagchi, M.; Kothari, S.C.; Downs, B.W.; Fafard, R.D.; Preuss, H.G. Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: A mechanistic exploration. Int. J. Clin. Pharmacol. 2002, 22, 101–110. [Google Scholar]
- Pabst, O.; Mowat, A.M. Oral tolerance to food protein. Mucosal Immunol. 2012, 5, 232–239. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed collagen—Sources and applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.E.; Oesser, S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: A review of the literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Seifert, J. Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Res. 2003, 311, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Guillerminet, F.; Beaupied, H.; Fabien-Soulé, V.; Tomé, D.; Benhamou, C.L.; Roux, C.; Blais, A. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: An in vitro and in vivo study. Bone 2010, 46, 827–834. [Google Scholar] [CrossRef]
- Schauss, A.G.; Stenehjem, J.; Park, J.; Endres, J.R.; Clewell, A. Effect of the novel low molecular weight hydrolyzed chicken sternal cartilage extract, BioCell Collagen, on improving osteoarthritis-related symptoms: A randomized, double-blind, placebo-controlled trial. J. Agric. Food Chem. 2012, 60, 4096–4101. [Google Scholar] [CrossRef]
- van de Water, E.; Oosterlinck, M.; Dumoulin, M.; Korthagen, N.M.; van Weeren, P.R.; van den Broek, J.; Everts, H.; Pille, F.; van Doorn, D.A. The preventive effects of two nutraceuticals on experimentally induced acute synovitis. Equine Vet. J. 2017, 49, 532–538. [Google Scholar] [CrossRef]
- Dobenecker, B.; Reese, S.; Jahn, W.; Schunck, M.; Hugenberg, J.; Louton, H.; Oesser, S. Specific bioactive collagen peptides (PETAGILE®) as supplement for horses with osteoarthritis: A two-centred study. J. Anim. Physiol. Anim. Nutr. 2018, 102 (Suppl. S1), 16–23. [Google Scholar] [CrossRef]
- Clark, K.L.; Sebastianelli, W.; Flechsenhar, K.R.; Aukermann, D.F.; Meza, F.; Millard, R.L.; Deitch, J.R.; Sherbondy, P.S.; Albert, A. 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr. Med. Res. Opin. 2008, 24, 1485–1496. [Google Scholar] [CrossRef]
- Walrand, S.; Chiotelli, E.; Noirt, F.; Mwewa, S.; Lassel, T. Consumption of a functional fermented milk containing collagen hydrolysate improves the concentration of collagen-specific amino acids in plasma. J. Agric. Food Chem. 2008, 56, 7790–7795. [Google Scholar] [CrossRef]
- Benito-Ruiz, P.; Camacho-Zambrano, M.M.; Carrillo-Arcentales, J.N.; Mestanza-Peralta, M.A.; Vallejo-Flores, C.A.; Vargas-López, S.V.; Villacís-Tamayo, R.A.; Zurita-Gavilanes, L.A. A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S2), 99–113. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Nuite, M.; Krishnan, N.; Ruthazer, R.; Price, L.L.; Burstein, D.; Griffith, J.; Flechsenhar, K. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: A pilot randomized controlled trial. Osteoarthr. Cartil. 2011, 19, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Van Vijven, J.P.; Luijsterburg, P.A.; Verhagen, A.P.; van Osch, G.J.; Kloppenburg, M.; Bierma-Zeinstra, S.M. Symptomatic and chondroprotective treatment with collagen derivatives in osteoarthritis: A systematic review. Osteoarthr. Cartil. 2012, 20, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Schadow, S.; Simons, V.S.; Lochnit, G.; Kordelle, J.; Gazova, Z.; Siebert, H.C.; Steinmeyer, J. Metabolic response of human osteoarthritic cartilage to biochemically characterized collagen hydrolysates. Int. J. Mol. Sci. 2017, 18, 207. [Google Scholar] [CrossRef]
- Raabe, O.; Reich, C.; Wenisch, S.; Hild, A.; Burg-Roderfeld, M.; Siebert, H.C.; Arnhold, S. Hydrolyzed fish collagen induced chondrogenic differentiation of equine adipose tissue-derived stromal cells. Histochem. Cell Biol. 2010, 134, 545–554. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Pustlauk, W.; Paul, B.; Gelinsky, M.; Bernhardt, A. Jellyfish collagen and alginate: Combined marine materials for superior chondrogenesis of hMSC. Mater. Sci. Eng. C 2016, 64, 190–198. [Google Scholar] [CrossRef]
- Wang, J.; He, C.; Cheng, N.; Yang, Q.; Chen, M.; You, L.; Zhang, Q. Bone marrow stem cells response to collagen/single-wall carbon nanotubes-coohs nanocomposite films with transforming growth factor beta 1. J. Nanosci. Nanotechnol. 2015, 15, 4844–4850. [Google Scholar] [CrossRef]
- Diogo, G.S.; Carneiro, F.; Freitas-Ribeiro, S.; Sotelo, C.G.; Pérez-Martín, R.I.; Pirraco, R.P.; Reis, R.L.; Silva, T.H. Prionace glauca skin collagen bioengineered constructs as a promising approach to trigger cartilage regeneration. Mater. Sci. Eng. C 2021, 120, 111587. [Google Scholar] [CrossRef]
- Hsu, H.H.; Uemura, T.; Yamaguchi, I.; Ikoma, T.; Tanaka, J. Chondrogenic differentiation of human mesenchymal stem cells on fish scale collagen. J. Biosci. Bioeng. 2016, 122, 219–225. [Google Scholar] [CrossRef]
- Pugliano, M.; Vanbellinghen, X.; Schwinté, P.; Benkirane-Jessel, N.; Keller, L. Combined jellyfish collagen type II, human stem cells and Tgf-²3 as a therapeutic implant for cartilage repair. J. Stem Cell Res. Ther. 2017, 7, 1–9. [Google Scholar]
- Liu, C.; Sun, J. Potential application of hydrolyzed fish collagen for inducing the multidirectional differentiation of rat bone marrow mesenchymal stem cells. Biomacromolecules 2014, 15, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, S. The Signaling pathways involved in chondrocyte differentiation and hypertrophic differentiation. Stem Cells Int. 2016, 2016, 2470351. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elango, J.; Zamora-Ledezma, C.; Ge, B.; Hou, C.; Pan, Z.; Bao, B.; Pérez Albacete Martínez, C.; Granero Marín, J.M.; de Val, J.E.M.S.; Bao, C.; et al. Paradoxical Duel Role of Collagen in Rheumatoid Arthritis: Cause of Inflammation and Treatment. Bioengineering 2022, 9, 321. https://doi.org/10.3390/bioengineering9070321
Elango J, Zamora-Ledezma C, Ge B, Hou C, Pan Z, Bao B, Pérez Albacete Martínez C, Granero Marín JM, de Val JEMS, Bao C, et al. Paradoxical Duel Role of Collagen in Rheumatoid Arthritis: Cause of Inflammation and Treatment. Bioengineering. 2022; 9(7):321. https://doi.org/10.3390/bioengineering9070321
Chicago/Turabian StyleElango, Jeevithan, Camilo Zamora-Ledezma, Baolin Ge, Chunyu Hou, Zhilin Pan, Bin Bao, Carlos Pérez Albacete Martínez, José Manuel Granero Marín, José Eduardo Maté Sánchez de Val, Chunling Bao, and et al. 2022. "Paradoxical Duel Role of Collagen in Rheumatoid Arthritis: Cause of Inflammation and Treatment" Bioengineering 9, no. 7: 321. https://doi.org/10.3390/bioengineering9070321
APA StyleElango, J., Zamora-Ledezma, C., Ge, B., Hou, C., Pan, Z., Bao, B., Pérez Albacete Martínez, C., Granero Marín, J. M., de Val, J. E. M. S., Bao, C., & Wu, W. (2022). Paradoxical Duel Role of Collagen in Rheumatoid Arthritis: Cause of Inflammation and Treatment. Bioengineering, 9(7), 321. https://doi.org/10.3390/bioengineering9070321