Phenotypes of Motor Deficit and Pain after Experimental Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
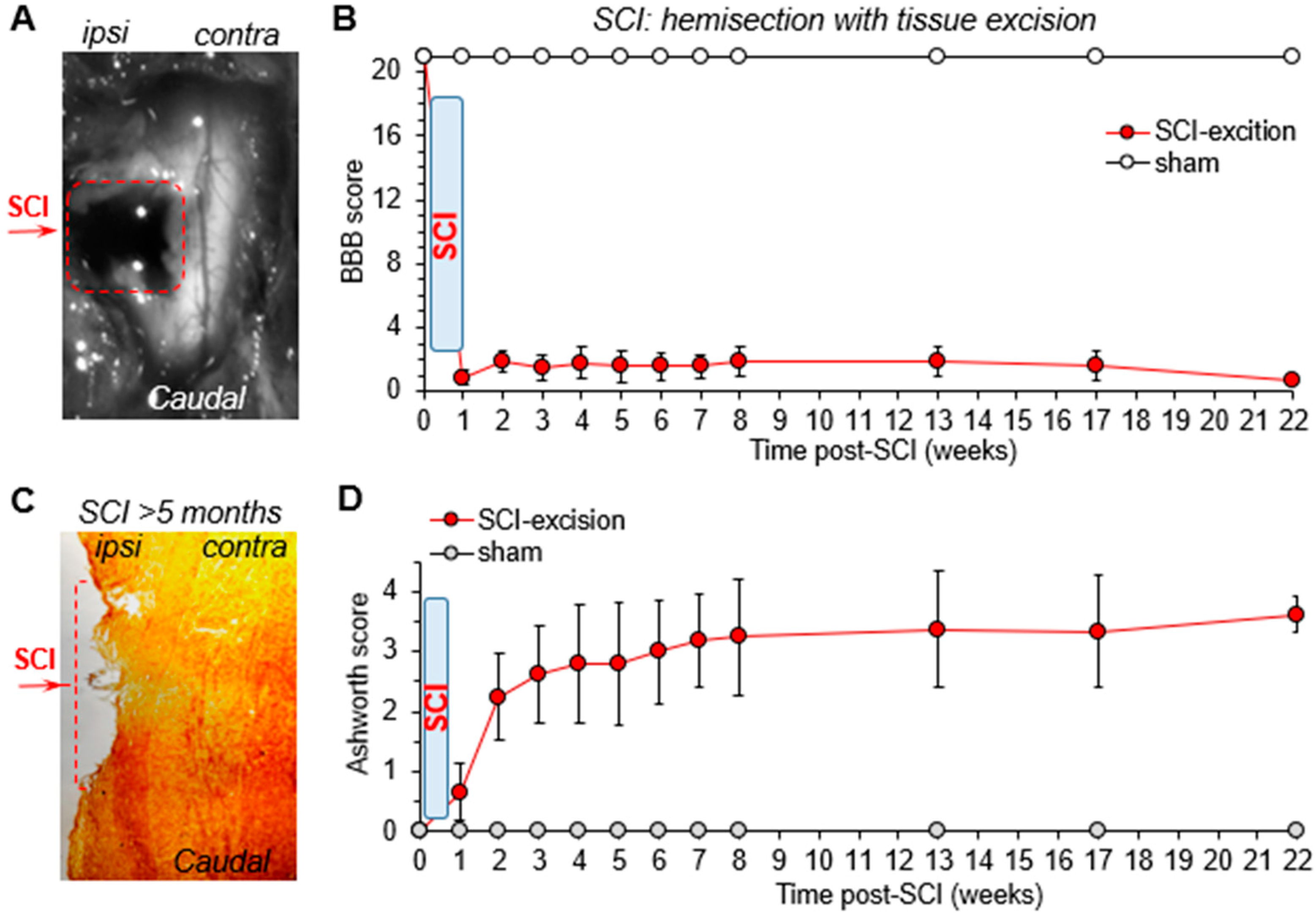
2.2. Experimental SCI for Severe Disability: Hemisection with Tissue Excision
2.3. Experimental SCI for Moderate Motor Dysfunction: Hemisection with Tissue Incision
2.4. Spinal Cord Tissue Post-Hoc
2.5. Basso, Beattie, Bresnahan (BBB), and Ashworth Scoring of Motor Deficit: The Open-Field Test
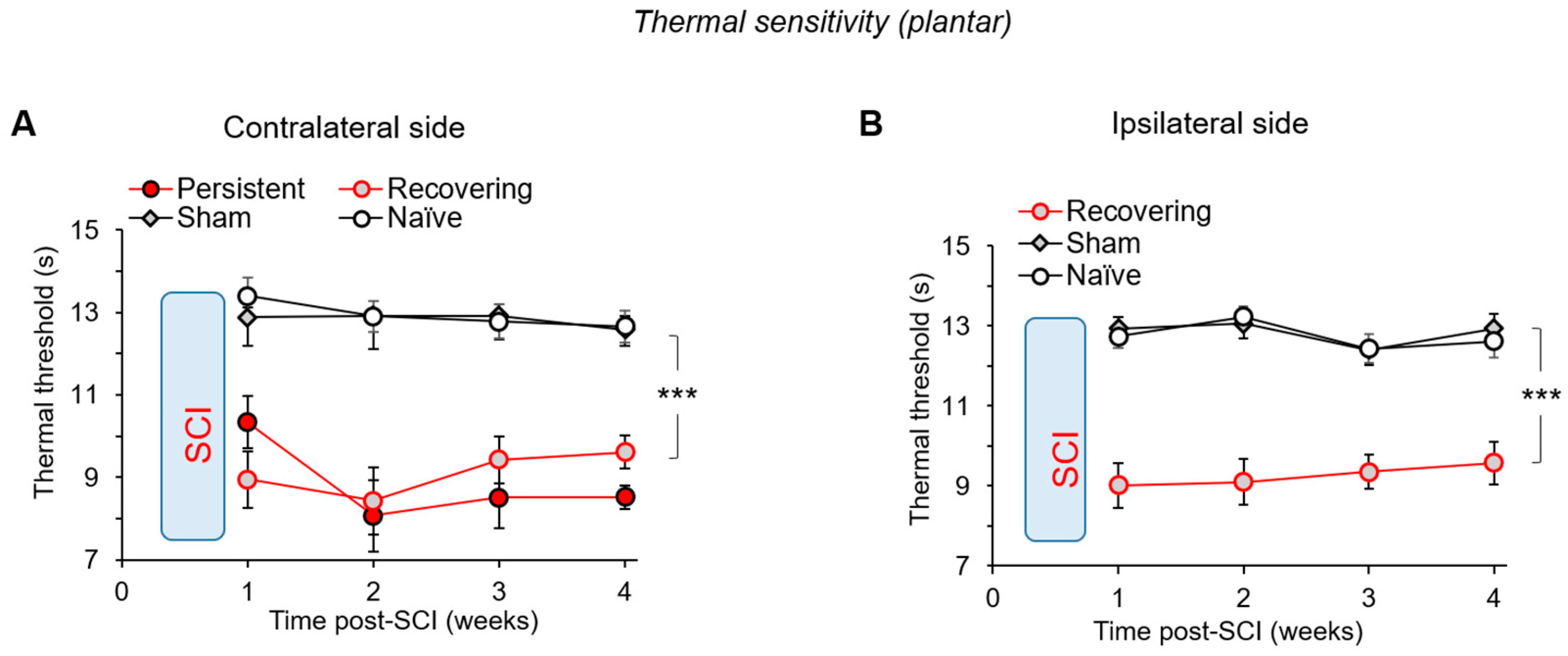
2.6. Nociceptive Threshold of Thermal Modality: The Hargreaves Plantar Test
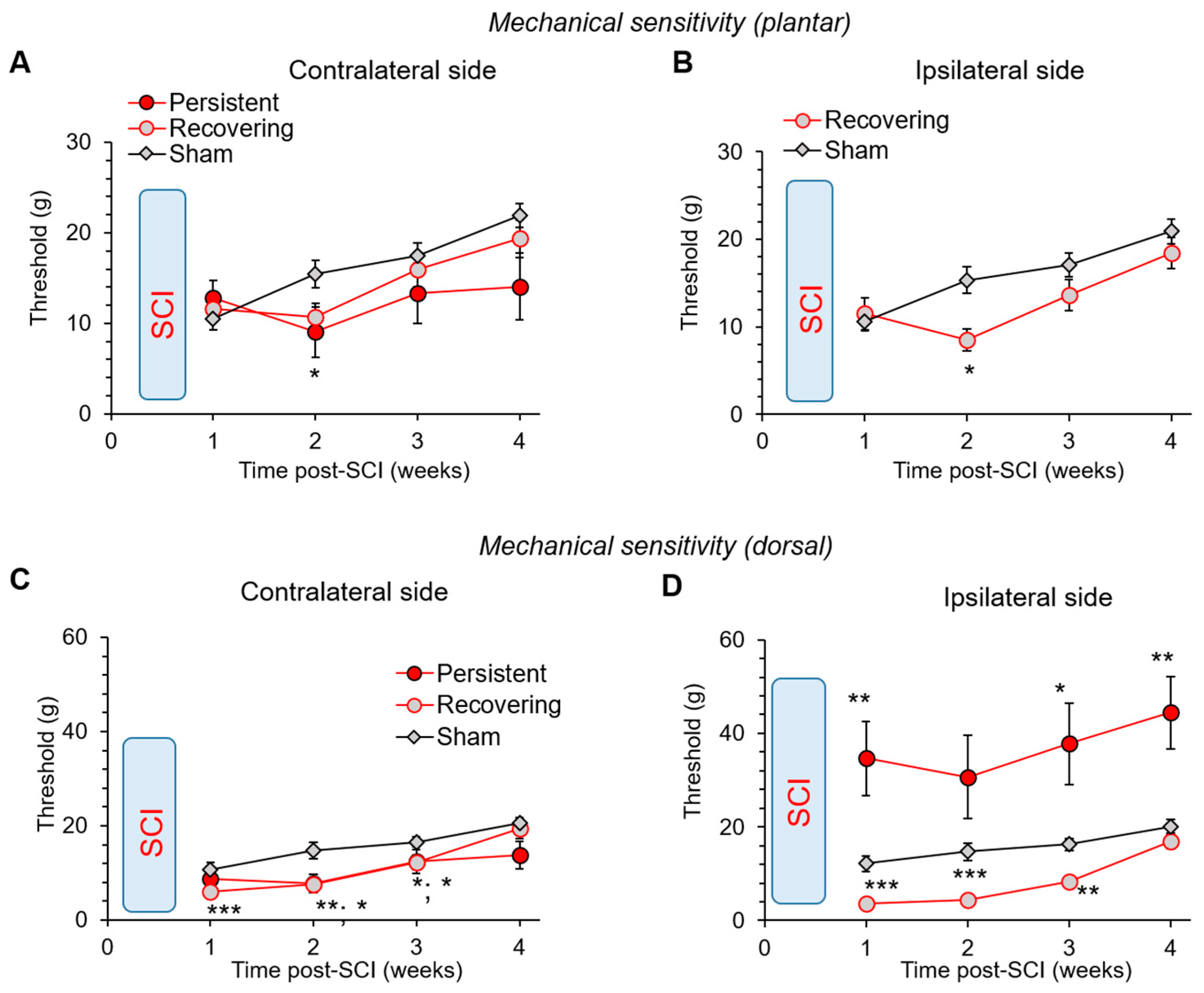
2.7. Nociceptive Threshold of Mechanical Modality: Plantar von Frey Test
2.8. Nociceptive Mechanical Threshold: Dorsal von Frey Test
2.9. Nociceptive Mechanical Threshold at the Spinal Level: Spinal von Frey Test
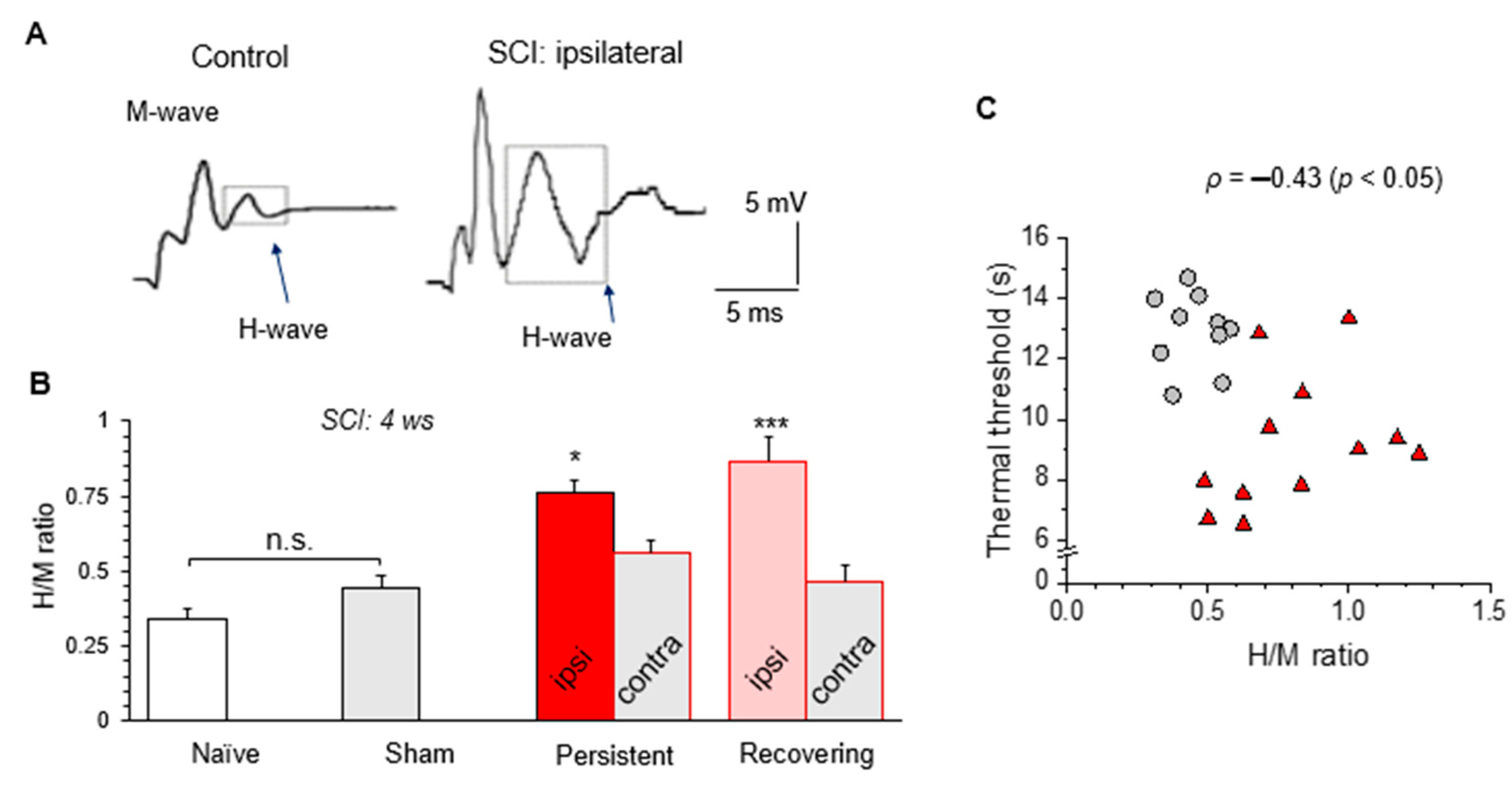
2.10. Hoffman (H)-Reflex
2.11. Statistical Analysis
3. Results
3.1. The Time Course of Motor Deficit Depends on the Severity of Neurotrauma
3.2. The Recovery of Motor Function Depends on the Age at the Time of Injury
3.3. Young Animals Reveal Two Populations: Those with Persistent or Recovering Motor Deficit after SCI
3.4. Changes in the Locomotive Capability after a Mild SCI
3.5. Thermal Pain Hypersensitivity after a Mild SCI
3.6. Changes in the Mechanical Sensitivity: Allodynia vs. Lost Sensation after a Mild SCI
3.7. H-Reflex Recordings Confirm the Impaired Sensory-Motor Integration after a Mild SCI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Score | Criteria |
|---|---|
| 0 | No hind limb movement |
| 1 | Slight (less than half movement) of one or two joints (the hip and/or the knee) |
| 2 | Extensive movement of one joint (more than half of the normal range) |
| 3 | Extensive movement of two joints |
| 4 | Slight movement of all three joints |
| 5 | Slight movement of two joints and extensive movement of the third |
| 6 | Extensive movement of two joints and slight movement of the third |
| 7 | Extensive or full movement of all joints |
| 8 | Sweeping with no weight support or plantar placement of the paw with no weight support |
| 9 | Weight support with plantar placement of the paw with stationary position or occasional, frequent, or consistent weight supported dorsal stepping and no plantar stepping |
| 10 | Occasional weight supported plantar steps |
| 11 | Frequent to consistent weight supported plantar steps |
| 12 | Frequent to consistent weight supported plantar steps and occasional coordination between fore and hind limbs |
| 13 | Frequent to consistent weight supported plantar steps and frequent between fore and hind limbs |
| 14 | Consistent weight supported plantar steps, consistent coordination |
| 15 | Consistent plantar stepping and consistent coordination; and no toe clearance or occasional toe clearance during forward limb advancement; predominant paw position is parallel to the body at initial contact |
| 16 | Consistent plantar stepping and consistent coordination during gait; and toe clearance occurs frequently during forward limb advancement; predominant paw position is rotated when raising and parallel at contact with the surface |
| 17 | Parallel positioning of the paw relative to the rostrocaudal axis when raising and at contact with the surface |
| Score | Criteria |
|---|---|
| 0 | No increase in muscle tone |
| 1 | Slight increase in tone when limb is moved in flexion or extension |
| 2 | More marked increase in tone, with limb still easily flexed |
| 3 | Considerable strength of tone with difficult passive movement |
| 4 | Limb rigid in flexion or extension |
| BBB Score | Weight/Age | Model | Reference |
|---|---|---|---|
| 18 | 200–250 g | Hemisection | [18] |
| 15–17 | 100–125 g | Hemisection | [19] |
| 15 | 250–320 g | Hemisection | [36] |
| 10–15 | 165–550 g | Hemisection | [43] |
| 11–13 | 210 g | Hemisection | [46] |
| 12–14 | 8 weeks | Hemisection | [29] |
| 12 | 220–250 g | Hemisection | [63] |
| 6–10 | 8 weeks | Hemisection | (Figure 2A) |
| 2–5 | 5 months | Hemisection | (Figure 2A) |
| 4–5 | 200–220 g | Hemisection with excision | [30] |
| 4–5 | 180–200 g | Hemisection with excision | [31] |
| 1–3 | 8 weeks | Hemisection with excision | (Figure 1A) |
| 3–7 | 260–280 g | Transection | [64] |
| 3 | 225–250 g | Transection | [65] |
References
- James, S.L.; Bannick, M.S.; Montjoy-Venning, W.C.; Lucchesi, L.R.; Dandona, L.; Dandona, R.; Hawley, C.; Hay, S.I.; Jakovljevic, M.; Khalil, I.; et al. Global, Regional, and National Burden of Traumatic Brain Injury and Spinal Cord Injury, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Dietz, V.; Fouad, K. Restoration of Sensorimotor Functions after Spinal Cord Injury. Brain 2014, 137, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Dragojlovic, N.; Romanoski, N.L.; Verduzco-Gutierrez, M.; Francisco, G.E. Prevalence and Treatment Characteristics of Spastic Hypertonia on First-Time Admission to Acute Inpatient Rehabilitation. Am. J. Phys. Med. Rehabil. 2022, 101, 348–352. [Google Scholar] [CrossRef]
- Skoog, B.; Jakobsson, K.-E. Prevalence of Spasticity and Below-Level Neuropathic Pain Related to Spinal Cord Injury Level and Damage to the Lower Spinal Segments. J. Rehabil. Med. Clin. Commun. 2020, 3, 1000039. [Google Scholar] [CrossRef]
- Mills, P.B.; Holtz, K.A.; Szefer, E.; Noonan, V.K.; Kwon, B.K. Early Predictors of Developing Problematic Spasticity Following Traumatic Spinal Cord Injury: A Prospective Cohort Study. J. Spinal Cord Med. 2020, 43, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Holtz, K.A.; Lipson, R.; Noonan, V.K.; Kwon, B.K.; Mills, P.B. Prevalence and Effect of Problematic Spasticity after Traumatic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2017, 98, 1132–1138. [Google Scholar] [CrossRef]
- Finnerup, N.B. Pain in Patients with Spinal Cord Injury. Pain 2013, 154 (Suppl. 1), S71–S76. [Google Scholar] [CrossRef]
- Burke, D.; Fullen, B.M.; Stokes, D.; Lennon, O. Neuropathic Pain Prevalence Following Spinal Cord Injury: A Systematic Review and Meta-Analysis. Eur. J. Pain 2017, 21, 29–44. [Google Scholar] [CrossRef]
- Bresnahan, J.J.; Scoblionko, B.R.; Zorn, D.; Graves, D.E.; Viscusi, E.R. The Demographics of Pain after Spinal Cord Injury: A Survey of Our Model System. Spinal Cord Ser. Cases 2022, 8, 14. [Google Scholar] [CrossRef]
- Felix, E.R.; Cardenas, D.D.; Bryce, T.N.; Charlifue, S.; Lee, T.K.; MacIntyre, B.; Mulroy, S.; Taylor, H. Prevalence and Impact of Neuropathic and Nonneuropathic Pain in Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2022, 103, 729–737. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.J.; Kim, T.L.; Kim, E.Y.; Ham, D.; Lee, J.; Kim, T.; Shin, J.W.; Son, M.; Sung, J.H.; et al. Prevalence and Characteristics of Neuropathic Pain in Patients with Spinal Cord Injury Referred to a Rehabilitation Center. Ann. Rehabil. Med. 2020, 44, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Johannesen, I.L.; Sindrup, S.H.; Bach, F.W.; Jensen, T.S. Pain and Dysesthesia in Patients with Spinal Cord Injury: A Postal Survey. Spinal Cord 2001, 39, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.M.; Hicks, A.L. Spasticity after Spinal Cord Injury. Spinal Cord 2005, 43, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-Regulation of the Potassium-Chloride Cotransporter KCC2 Contributes to Spasticity after Spinal Cord Injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef]
- Bedi, S.S.; Yang, Q.; Crook, R.J.; Du, J.; Wu, Z.; Fishman, H.M.; Grill, R.J.; Carlton, S.M.; Walters, E.T. Chronic Spontaneous Activity Generated in the Somata of Primary Nociceptors Is Associated with Pain-Related Behavior after Spinal Cord Injury. J. Neurosci. 2010, 30, 14870–14882. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Hulsebosch, C.E. Neuronal Hyperexcitability: A Substrate for Central Neuropathic Pain after Spinal Cord Injury. Curr. Pain Headache Rep. 2011, 15, 215–222. [Google Scholar] [CrossRef]
- Kopach, O.; Medvediev, V.; Krotov, V.; Borisyuk, A.; Tsymbaliuk, V.; Voitenko, N. Opposite, Bidirectional Shifts in Excitation and Inhibition in Specific Types of Dorsal Horn Interneurons Are Associated with Spasticity and Pain Post-SCI. Sci. Rep. 2017, 7, 5884. [Google Scholar] [CrossRef]
- Christensen, M.D.; Everhart, A.W.; Pickelman, J.T.; Hulsebosch, C.E. Mechanical and Thermal Allodynia in Chronic Central Pain Following Spinal Cord Injury. Pain 1996, 68, 97–107. [Google Scholar] [CrossRef]
- Mills, C.D.; Hains, B.C.; Johnson, K.M.; Hulsebosch, C.E. Strain and Model Differences in Behavioral Outcomes after Spinal Cord Injury in Rat. J. Neurotrauma 2001, 18, 743–756. [Google Scholar] [CrossRef]
- Detloff, M.R.; Fisher, L.C.; Deibert, R.J.; Basso, D.M. Acute and Chronic Tactile Sensory Testing after Spinal Cord Injury in Rats. J. Vis. Exp. 2012, 62, e3247. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Norrbrink, C.; Trok, K.; Piehl, F.; Johannesen, I.L.; Sørensen, J.C.; Jensen, T.S.; Werhagen, L. Phenotypes and Predictors of Pain Following Traumatic Spinal Cord Injury: A Prospective Study. J. Pain Off. J. Am. Pain Soc. 2014, 15, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.D.; Moriña, D.; Rodríguez, N.; Saurí, J.; Vidal, J.; Navarro, A.; Navarro, X. Sensory Symptom Profiles of Patients with Neuropathic Pain After Spinal Cord Injury. Clin. J. Pain 2017, 33, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Kiwerski, J.E. Application of Dexamethasone in the Treatment of Acute Spinal Cord Injury. Injury 1993, 24, 457–460. [Google Scholar] [CrossRef]
- Kopach, O.; Krotov, V.; Goncharenko, J.; Voitenko, N. Inhibition of Spinal Ca2+-Permeable AMPA Receptors with Dicationic Compounds Alleviates Persistent Inflammatory Pain without Adverse Effects. Front. Cell. Neurosci. 2016, 10, 50. [Google Scholar] [CrossRef][Green Version]
- Kopach, O.; Zheng, K.; Dong, L.; Sapelkin, A.; Voitenko, N.; Sukhorukov, G.B.; Rusakov, D.A. Nano-Engineered Microcapsules Boost the Treatment of Persistent Pain. Drug Deliv. 2018, 25, 435–447. [Google Scholar] [CrossRef]
- Kopach, O.; Krotov, V.; Shysh, A.; Sotnic, A.; Viatchenko-Karpinski, V.; Dosenko, V.; Voitenko, N. Spinal PKCα Inhibition and Gene-Silencing for Pain Relief: AMPAR Trafficking at the Synapses between Primary Afferents and Sensory Interneurons. Sci. Rep. 2018, 8, 10285. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Tsai, J.Y.; Wu, Y.N.; Hwang, I.S.; Chen, T.I.; Chen, J.J.J. Time Course Quantification of Spastic Hypertonia Following Spinal Hemisection in Rats. Neuroscience 2010, 167, 185–198. [Google Scholar] [CrossRef]
- Majczyński, H.; Sławińska, U. Locomotor Recovery after Thoracic Spinal Cord Lesions in Cats, Rats and Humans. Acta Neurobiol. Exp. (Wars) 2007, 67, 235–257. [Google Scholar]
- Pertici, V.; Amendola, J.; Laurin, J.; Gigmes, D.; Madaschi, L.; Carelli, S.; Marqueste, T.; Gorio, A.; Decherchi, P. The Use of Poly(N-[2-Hydroxypropyl]-Methacrylamide) Hydrogel to Repair a T10 Spinal Cord Hemisection in Rat: A Behavioural, Electrophysiological and Anatomical Examination. ASN Neuro 2013, 5, AN20120082. [Google Scholar] [CrossRef]
- Jian, R.; Yixu, Y.; Sheyu, L.; Jianhong, S.; Yaohua, Y.; Xing, S.; Qingfeng, H.; Xiaojian, L.; Lei, Z.; Yan, Z.; et al. Repair of Spinal Cord Injury by Chitosan Scaffold with Glioma ECM and SB216763 Implantation in Adult Rats. J. Biomed. Mater. Res. Part A 2015, 103, 3259–3272. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.; You, R.; Kaplan, D.L.; Liu, Y.; Qu, J.; Li, X.; Li, M.; Wang, X. Multichannel Silk Protein/Laminin Grafts for Spinal Cord Injury Repair. J. Biomed. Mater. Res. A 2016, 104, 3045–3057. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Song, B.; Roy, R.R.; Zhong, H.; Herrmann, J.E.; Ao, Y.; Qi, J.; Edgerton, V.R.; Sofroniew, M.V. Recovery of Supraspinal Control of Stepping via Indirect Propriospinal Relay Connections after Spinal Cord Injury. Nat. Med. 2008, 14, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Ropper, A.E.; Thakor, D.K.; Han, I.; Yu, D.; Zeng, X.; Anderson, J.E.; Aljuboori, Z.; Kim, S.-W.; Wang, H.; Sidman, R.L.; et al. Defining Recovery Neurobiology of Injured Spinal Cord by Synthetic Matrix-Assisted HMSC Implantation. Proc. Natl. Acad. Sci. USA 2017, 114, E820–E829. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Dergham, P.; Ellezam, B.; Essagian, C.; Avedissian, H.; Lubell, W.D.; McKerracher, L. Rho Signaling Pathway Targeted to Promote Spinal Cord Repair. J. Neurosci. 2002, 22, 6570–6577. [Google Scholar] [CrossRef]
- Webb, A.A.; Muir, G.D. Compensatory Locomotor Adjustments of Rats with Cervical or Thoracic Spinal Cord Hemisections. J. Neurotrauma 2002, 19, 239–256. [Google Scholar] [CrossRef]
- Martinez, M.; Brezun, J.-M.; Bonnier, L.; Xerri, C. A New Rating Scale for Open-Field Evaluation of Behavioral Recovery after Cervical Spinal Cord Injury in Rats. J. Neurotrauma 2009, 26, 1043–1053. [Google Scholar] [CrossRef]
- Taylor, J.S.; Friedman, R.F.; Munson, J.B.; Vierck, C.J. Stretch Hyperreflexia of Triceps Surae Muscles in the Conscious Cat after Dorsolateral Spinal Lesions. J. Neurosci. 1997, 17, 5004–5015. [Google Scholar] [CrossRef][Green Version]
- Hahm, S.-C.; Yoon, Y.W.; Kim, J. High-Frequency Transcutaneous Electrical Nerve Stimulation Alleviates Spasticity after Spinal Contusion by Inhibiting Activated Microglia in Rats. Neurorehabil. Neural Repair. 2015, 29, 370–381. [Google Scholar] [CrossRef]
- Haas, B.M.; Bergström, E.; Jamous, A.; Bennie, A. The Inter Rater Reliability of the Original and of the Modified Ashworth Scale for the Assessment of Spasticity in Patients with Spinal Cord Injury. Spinal Cord 1996, 34, 560–564. [Google Scholar] [CrossRef]
- Baunsgaard, C.B.; Nissen, U.V.; Christensen, K.B.; Biering-Sørensen, F. Modified Ashworth Scale and Spasm Frequency Score in Spinal Cord Injury: Reliability and Correlation. Spinal Cord 2016, 54, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-W.; Wang, L.-H.; Zhang, M.; Han, J.-S. Decreased Dynorphin A (1-17) in the Spinal Cord of Spastic Rats after the Compressive Injury. Brain Res. Bull 2005, 67, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Hains, B.C.; Johnson, K.M.; Hulsebosch, C.E. Effect of Age at Time of Spinal Cord Injury on Behavioral Outcomes in Rat. J. Neurotrauma 2004, 21, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Kloos, A.D.; Fisher, L.C.; Detloff, M.R.; Hassenzahl, D.L.; Basso, D.M. Stepwise Motor and All-or-None Sensory Recovery Is Associated with Nonlinear Sparing after Incremental Spinal Cord Injury in Rats. Exp. Neurol. 2005, 191, 251–265. [Google Scholar] [CrossRef]
- You, S.-W.; Chen, B.-Y.; Liu, H.-L.; Lang, B.; Xia, J.-L.; Jiao, X.-Y.; Ju, G. Spontaneous Recovery of Locomotion Induced by Remaining Fibers after Spinal Cord Transection in Adult Rats. Restor. Neurol. Neurosci. 2003, 21, 39–45. [Google Scholar]
- Arvanian, V.L.; Liang, L.; Tesfa, A.; Fahmy, M.; Petrosyan, H.A. Buprenorphine, a Partial Opioid Agonist, Prevents Modulation of H-Reflex Induced by Pulsed Electromagnetic Stimulation in Spinal Cord Injured Rats. Neurosci. Lett. 2022, 777, 136583. [Google Scholar] [CrossRef]
- Li, X.; Song, X.; Fang, L.; Ding, J.; Qi, L.; Wang, Q.; Dong, C.; Wang, S.; Wu, J.; Wang, T.; et al. Body Weight-Supported Treadmill Training Ameliorates Motoneuronal Hyperexcitability by Increasing GAD-65/67 and KCC2 Expression via TrkB Signaling in Rats with Incomplete Spinal Cord Injury. Neurochem. Res. 2022, 47, 1679–1691. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Crown, E.D.; Unabia, G.C.; Hulsebosch, C.E. Propentofylline Attenuates Allodynia, Glial Activation and Modulates GABAergic Tone after Spinal Cord Injury in the Rat. Pain 2008, 138, 410–422. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, Y.W.; Hong, S.K.; Na, H.S. Cold and Mechanical Allodynia in Both Hindpaws and Tail Following Thoracic Spinal Cord Hemisection in Rats: Time Courses and Their Correlates. Neurosci. Lett. 2003, 343, 200–204. [Google Scholar] [CrossRef]
- Hogan, Q.; Sapunar, D.; Modric-Jednacak, K.; McCallum, J.B. Detection of Neuropathic Pain in a Rat Model of Peripheral Nerve Injury. Anesthesiology 2004, 101, 476–487. [Google Scholar] [CrossRef]
- Sherman, K.; Woyach, V.; Eisenach, J.C.; Hopp, F.A.; Cao, F.; Hogan, Q.H.; Dean, C. Heterogeneity in Patterns of Pain Development after Nerve Injury in Rats and the Influence of Sex. Neurobiol. Pain 2021, 10, 100069. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, C.A.; Houle, T.T.; Gutierrez, S.; Eisenach, J.C. Modeling Individual Recovery after Peripheral Nerve Injury in Rats and the Effects of Parturition. Anesthesiology 2014, 121, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Kopach, O.; Krotov, V.; Belan, P.; Voitenko, N. Inflammatory-Induced Changes in Synaptic Drive and Postsynaptic AMPARs in Lamina II Dorsal Horn Neurons Are Cell-Type Specific. Pain 2015, 156, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.C.; Mathews, K.J.; Mao, Y.; Nguyen, T.; Gorrie, C.A. Differences in the Cellular Response to Acute Spinal Cord Injury between Developing and Mature Rats Highlights the Potential Significance of the Inflammatory Response. Front. Cell. Neurosci. 2017, 10, 310. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success after Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- D’Amico, J.M.; Condliffe, E.G.; Martins, K.J.B.; Bennett, D.J.; Gorassini, M.A. Recovery of Neuronal and Network Excitability after Spinal Cord Injury and Implications for Spasticity. Front. Integr. Neurosci. 2014, 8, 36. [Google Scholar] [CrossRef]
- Norton, J.A.; Bennett, D.J.; Knash, M.E.; Murray, K.C.; Gorassini, M.A. Changes in Sensory-Evoked Synaptic Activation of Motoneurons after Spinal Cord Injury in Man. Brain 2008, 131, 1478–1491. [Google Scholar] [CrossRef]
- Chen, L.M.; Mishra, A.; Yang, P.-F.; Wang, F.; Gore, J.C. Injury Alters Intrinsic Functional Connectivity within the Primate Spinal Cord. Proc. Natl. Acad. Sci. USA 2015, 112, 5991–5996. [Google Scholar] [CrossRef]
- Bareyre, F.M.; Kerschensteiner, M.; Raineteau, O.; Mettenleiter, T.C.; Weinmann, O.; Schwab, M.E. The Injured Spinal Cord Spontaneously Forms a New Intraspinal Circuit in Adult Rats. Nat. Neurosci. 2004, 7, 269–277. [Google Scholar] [CrossRef]
- Deumens, R.; Koopmans, G.C.; Joosten, E.A.J. Regeneration of Descending Axon Tracts after Spinal Cord Injury. Prog. Neurobiol. 2005, 77, 57–89. [Google Scholar] [CrossRef]
- Bannatyne, B.A.; Edgley, S.A.; Hammar, I.; Jankowska, E.; Maxwell, D.J. Differential Projections of Excitatory and Inhibitory Dorsal Horn Interneurons Relaying Information from Group II Muscle Afferents in the Cat Spinal Cord. J. Neurosci. 2006, 26, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Harvey, P.J.; Bennett, D.J. Effects of Baclofen on Spinal Reflexes and Persistent Inward Currents in Motoneurons of Chronic Spinal Rats with Spasticity. J. Neurophysiol. 2004, 92, 2694–2703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Yuan, Y.; Chen, Y.; Jiang, L.; Liao, R.J.; Wang, L.; Zhang, X.N.; Ohtsu, H.; Hu, W.W.; Chen, Z. Histamine Promotes Locomotion Recovery After Spinal Cord Hemisection via Inhibiting Astrocytic Scar Formation. CNS Neurosci. Ther. 2015, 21, 454–462. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.J.; Wang, Q.Y.; Wang, Q.; Wei, X.M.; Li, N.; Guo, W.P.; Dou, Z.L. Correlation between Pathological Characteristics and Young’s Modulus Value of Spastic Gastrocnemius in a Spinal Cord Injury Rat Model. BioMed Res. Int. 2017, 2017, 5387948. [Google Scholar] [CrossRef] [PubMed]
- All, A.H.; Al-Nashash, H. Comparative Analysis of Functional Assessment for Contusion and Transection Models of Spinal Cord Injury. Spinal Cord 2021, 59, 1206–1209. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krotov, V.; Medvediev, V.; Abdallah, I.; Bozhenko, A.; Tatarchuk, M.; Ishchenko, Y.; Pichkur, L.; Savosko, S.; Tsymbaliuk, V.; Kopach, O.; et al. Phenotypes of Motor Deficit and Pain after Experimental Spinal Cord Injury. Bioengineering 2022, 9, 262. https://doi.org/10.3390/bioengineering9060262
Krotov V, Medvediev V, Abdallah I, Bozhenko A, Tatarchuk M, Ishchenko Y, Pichkur L, Savosko S, Tsymbaliuk V, Kopach O, et al. Phenotypes of Motor Deficit and Pain after Experimental Spinal Cord Injury. Bioengineering. 2022; 9(6):262. https://doi.org/10.3390/bioengineering9060262
Chicago/Turabian StyleKrotov, Volodymyr, Volodymyr Medvediev, Ibrahim Abdallah, Arseniy Bozhenko, Mykhailo Tatarchuk, Yevheniia Ishchenko, Leonid Pichkur, Serhii Savosko, Vitaliy Tsymbaliuk, Olga Kopach, and et al. 2022. "Phenotypes of Motor Deficit and Pain after Experimental Spinal Cord Injury" Bioengineering 9, no. 6: 262. https://doi.org/10.3390/bioengineering9060262
APA StyleKrotov, V., Medvediev, V., Abdallah, I., Bozhenko, A., Tatarchuk, M., Ishchenko, Y., Pichkur, L., Savosko, S., Tsymbaliuk, V., Kopach, O., & Voitenko, N. (2022). Phenotypes of Motor Deficit and Pain after Experimental Spinal Cord Injury. Bioengineering, 9(6), 262. https://doi.org/10.3390/bioengineering9060262







