Enhancing Prednisone-Based Arthritis Therapy with Targeted IL-27 Gene Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vectors and Transgene Detection
2.2. Chemicals and Reagents
2.3. Experimental Animals & Sonodelivery of Plasmid DNA Expressing IL-27
2.4. Induction of Arthritis in Animals
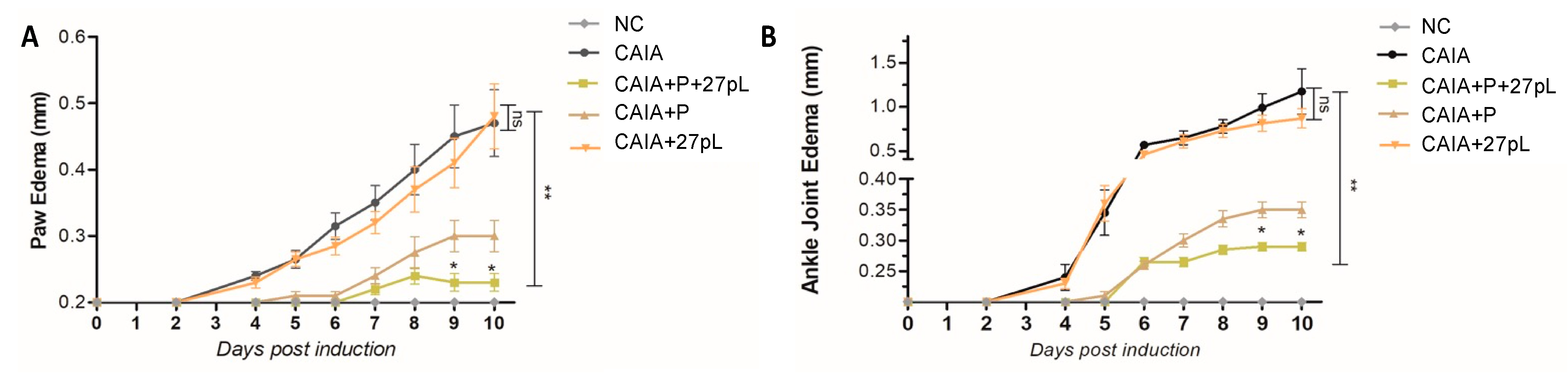
2.5. Assessment of Arthritis Severity
2.6. Measurement of Paw and Ankle Joint Thickness
2.7. Assessment of Bone Damage by Imaging Using an OsteoSense 750X Probe
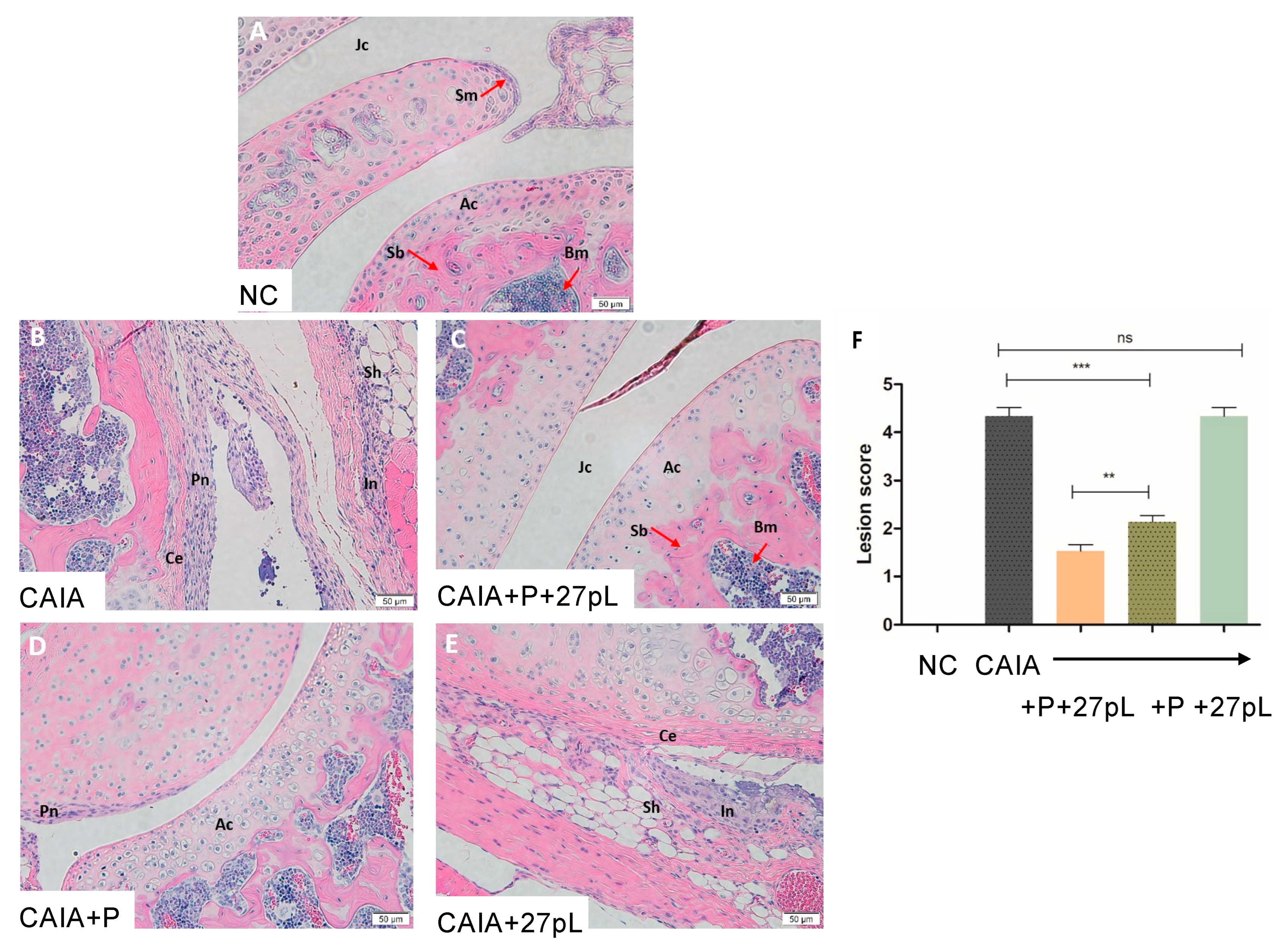
2.8. Histopathological Examination
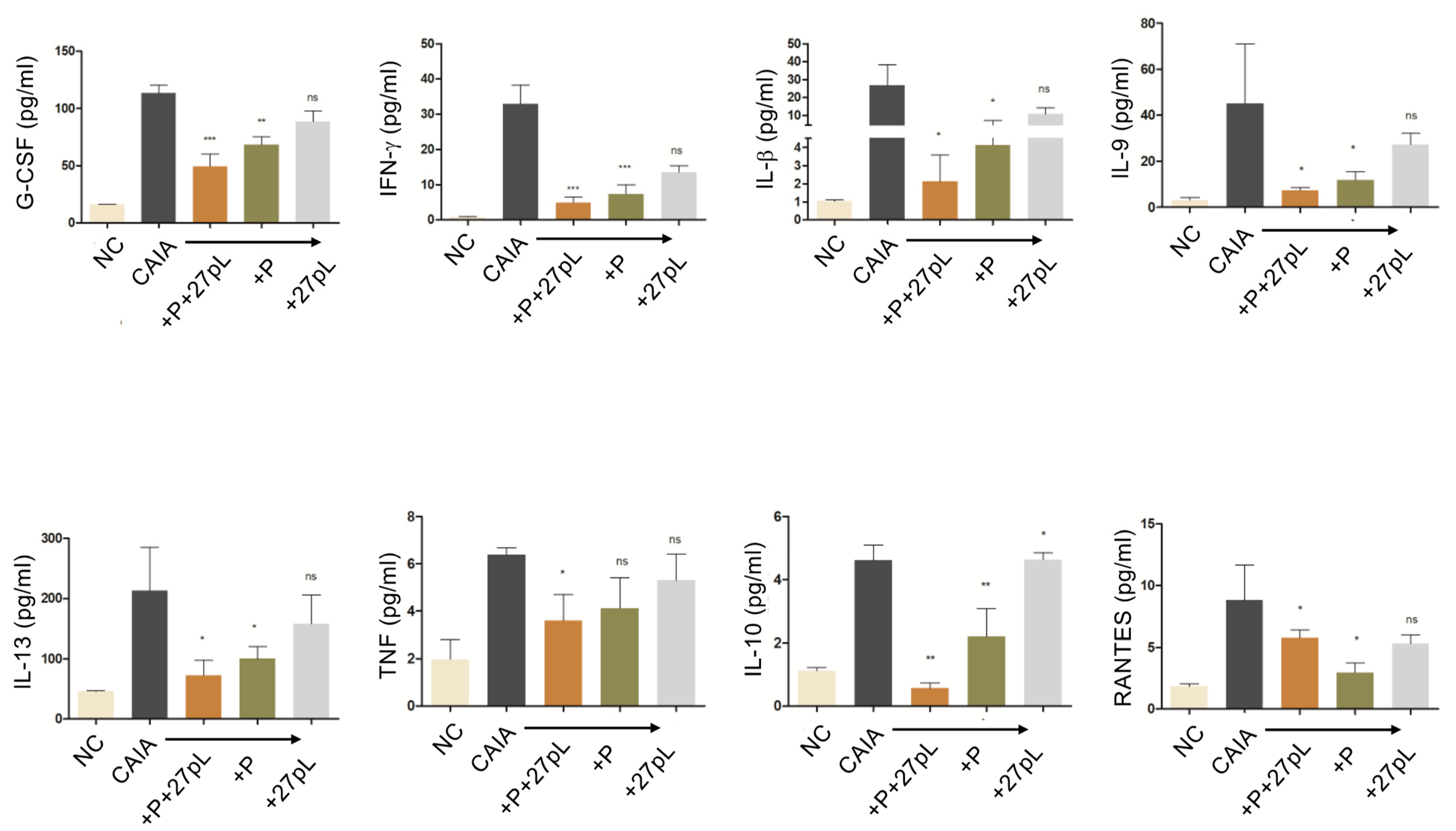
2.9. Multiplex Cytokine Assay
2.10. Statistical Analysis
3. Results
3.1. Generation of Targeted IL-27 Construct and Testing in Cells
3.2. 27pL and Prednisone (P) Therapeutic Application in the CAIA Model of Arthritis
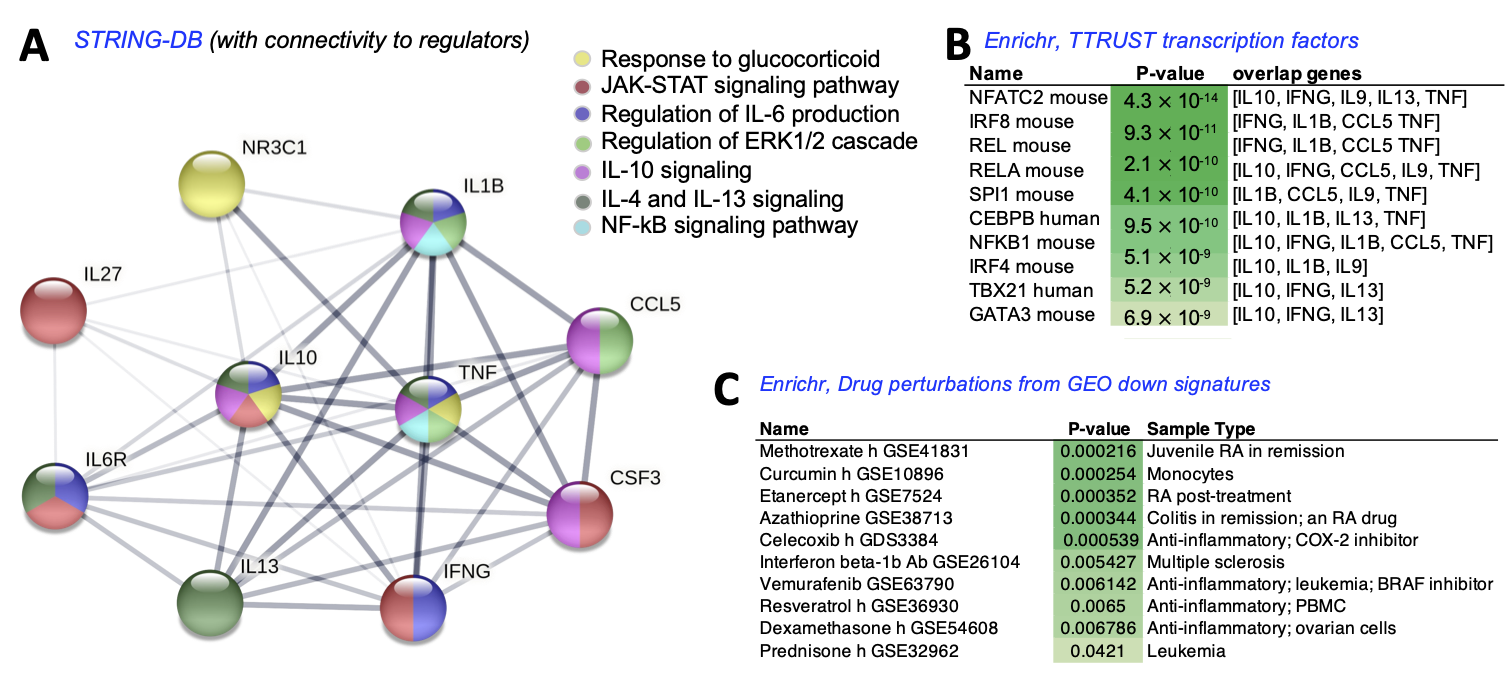
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.D.; Labranche, T.P.; Vasquez, K.O.; Kossodo, S.; Melton, M.; Rader, R.; Listello, J.T.; Abrams, M.A.; Misko, T.P. Optical tomographic imaging discriminates between disease-modifying anti-rheumatic drug (DMARD) and non-DMARD efficacy in collagen antibody-induced arthritis. Arthritis Res. Ther. 2010, 12, R105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khachigian, L.M. Collagen antibody-induced arthritis. Nat. Protoc. 2006, 1, 2512–2516. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Min, Y.; Lu, J.; Chen, W.; Zou, L.; Lv, X.; Cui, L.; Xu, B. Effect of prednisone treatment for 30 and 90 days on bone metabolism in collagen-induced arthritis (CIA) rats. J. Bone Miner. Metab. 2018, 36, 628–639. [Google Scholar] [CrossRef]
- Pickens, S.R.; Chamberlain, N.D.; Volin, M.V.; Mandelin, A.M.; Agrawal, H.; Matsui, M.; Yoshimoto, T.; Shahrara, S. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum. 2011, 63, 2289–2298. [Google Scholar] [CrossRef]
- Krasselt, M.; Baerwald, C. The current relevance and use of prednisone in rheumatoid arthritis. Expert Rev. Clin. Immunol. 2014, 10, 557–571. [Google Scholar] [CrossRef]
- Stumhofer, J.S.; Hunter, C.A. Advances in understanding the anti-inflammatory properties of IL-27. Immunol. Lett. 2008, 117, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Zolochevska, O.; Diaz-Quiñones, A.O.; Ellis, J.; Figueiredo, M.L. Interleukin-27 expression modifies prostate cancer cell crosstalk with bone and immune cells in vitro. J. Cell Physiol. 2013, 228, 1127–1136. [Google Scholar] [CrossRef]
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E.; et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002, 16, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Kalliolias, G.D.; Gordon, R.A.; Ivashkiv, L.B. Suppression of TNF-α and IL-1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL-27. J. Immunol. 2010, 185, 7047–7056. [Google Scholar] [CrossRef]
- Pradhan, A.; Lambert, Q.T.; Reuther, G.W. Transformation of hematopoietic cells and activation of JAK2-V617F by IL-27R, a component of a heterodimeric type I cytokine receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 18502–18507. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Chen, Z.; Yu, K.; Yan, J.; Li, T.; Ba, X.; Lin, W.; Huang, Y.; Shen, P.; Qin, K.; et al. Interleukin 27 signaling in rheumatoid arthritis patients: Good or evil? Front. Immunol. 2021, 12, 787252. [Google Scholar] [CrossRef] [PubMed]
- Rajaiah, R.; Puttabyatappa, M.; Polumuri, S.K.; Moudgil, K.D. Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. J. Biol. Chem. 2011, 286, 2817–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Zoheir, K.M.A.; Bakheet, S.A.; Alsaad, A.M.S.; Al-Shabanah, O.A.; Attia, S.M. STA-21, a STAT-3 inhibitor, attenuates the development and progression of inflammation in collagen antibody-induced arthritis. Immunobiology 2017, 222, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Meka, R.R.; Venkatesha, S.H.; Moudgil, K.D. Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis. J. Control Release 2018, 286, 279–288. [Google Scholar] [CrossRef]
- Fabbi, M.; Carbotti, G.; Ferrini, S. Dual roles of IL-27 in cancer biology and immunotherapy. Mediat. Inflamm. 2017, 2017, 3958069. [Google Scholar] [CrossRef] [Green Version]
- Oniki, S.; Nagai, H.; Horikawa, T.; Furukawa, J.; Belladonna, M.L.; Yoshimoto, T.; Hara, I.; Nishigori, C. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res. 2006, 66, 6395–6404. [Google Scholar] [CrossRef] [Green Version]
- Yoshimoto, T.; Morishima, N.; Mizoguchi, I.; Shimizu, M.; Nagai, H.; Oniki, S.; Oka, M.; Nishigori, C.; Mizuguchi, J. Antiproliferative activity of IL-27 on melanoma. J. Immunol. 2008, 180, 6527–6535. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Zhang, Y.; Li, Q. Therapeutic mechanisms of ibuprofen, prednisone and betamethasone in osteoarthritis. Mol. Med. Rep. 2017, 15, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, M.L.; Figueiredo Neto, M.; Salameh, J.W.; Decker, R.E.; Letteri, R.; Chan-Seng, D.; Emrick, T. Ligand-mediated targeting of cytokine interleukin-27 enhances its bioactivity. Mol. Ther. Methods Clin. Dev. 2020, 17, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Su, J.L.; Lai, K.P.; Chen, C.A.; Yang, C.Y.; Chen, P.S.; Chang, C.C.; Chou, C.H.; Hu, C.L.; Kuo, M.L.; Hsieh, C.Y.; et al. A novel peptide specifically binding to interleukin-6 receptor (gp80) inhibits angiogenesis and tumor growth. Cancer Res. 2005, 65, 4827–4835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumhofer, J.S.; Tait, E.D.; Quinn, W.J.; Hosken, N.; Spudy, B.; Goenka, R.; Fielding, C.A.; O’Hara, A.C.; Chen, Y.; Jones, M.L.; et al. A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat. Immunol. 2010, 11, 1119–1126. [Google Scholar] [CrossRef]
- Parelkar, S.S.; Letteri, R.; Chan-Seng, D.; Zolochevska, O.; Ellis, J.; Figueiredo, M.; Emrick, T. Polymer-peptide delivery platforms: Effect of oligopeptide orientation on polymer-based DNA delivery. Biomacromolecules 2014, 15, 1328–1336. [Google Scholar] [CrossRef] [Green Version]
- Balkrishna, A.; Sakat, S.S.; Joshi, K.; Paudel, S.; Joshi, D.; Joshi, K.; Ranjan, R.; Gupta, A.; Bhattacharya, K.; Varshney, A. Herbo-mineral formulation ‘Ashwashila’ attenuates rheumatoid arthritis symptoms in collagen-antibody-induced arthritis (CAIA) mice model. Sci. Rep. 2019, 9, 8025. [Google Scholar] [CrossRef] [Green Version]
- Balkrishna, A.; Sakat, S.S.; Joshi, K.; Paudel, S.; Joshi, D.; Ranjan, R.; Gupta, A.; Bhattacharya, K.; Varshney, A. Anti-inflammatory and anti-arthritic efficacies of an indian traditional herbo-mineral medicine “divya amvatari ras” in collagen antibody-induced arthritis (CAIA) mouse model through modulation of IL-6/IL-1β/TNF-α/NFκB signaling. Front. Pharmacol. 2019, 10, 659. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo Neto, M.; Figueiredo, M.L. Combination of interleukin-27 and MicroRNA for enhancing expression of anti-inflammatory and proosteogenic genes. Arthritis 2017, 2017, 6365857. [Google Scholar] [CrossRef]
- Figueiredo Neto, M.; Liu, S.; Salameh, J.W.; Yokota, H.; Figueiredo, M.L. Interleukin-27 gene delivery targeting IL-6Rα-expressing cells as a stress response therapy. Int. J. Mol. Sci. 2020, 21, 1108. [Google Scholar] [CrossRef] [Green Version]
- Salameh, J.W.; Kumar, S.; Rivera-Cruz, C.M.; Figueiredo, M.L. A second-generation nanoluc-IL27 fusion cytokine for targeted-gene-therapy applications. Bioengineering 2022, 9, 77. [Google Scholar] [CrossRef]
- PORF9-mcs Specification Sheet. Invivogen.com. Available online: http://101.200.202.226/files/prod/manuals/201304/25/532982002.pdf (accessed on 13 April 2022).
- Takebe, Y.; Seiki, M.; Fujisawa, J.; Hoy, P.; Yokota, K.; Arai, K.; Yoshida, M.; Arai, N. SR alpha promoter: An efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol. Cell Biol. 1988, 8, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Uetsuki, T.; Kaziro, Y.; Yamaguchi, N.; Sugano, S. Use of the human elongation factor 1 alpha promoter as a versatile and efficient expression system. Gene 1990, 91, 217–223. [Google Scholar] [CrossRef]
- Dharmapatni, A.A.; Cantley, M.D.; Marino, V.; Perilli, E.; Crotti, T.N.; Smith, M.D.; Haynes, D.R. The X-linked inhibitor of apoptosis protein inhibitor embelin suppresses inflammation and bone erosion in collagen antibody induced arthritis mice. Mediators Inflamm. 2015, 2015, 564042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, N.; Laverty, S.; Kraus, V.B.; Aigner, T. Basic methods in histopathology of joint tissues. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S113–S116. [Google Scholar] [CrossRef] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2020, 49, D605–D612. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene set knowledge discovery with enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Mahata, S.; Behera, S.K.; Kumar, S.; Sahoo, P.K.; Sarkar, S.; Fazil, M.H.U.T.; Nasare, V.D. In-silico and in-vitro investigation of STAT3-PIM1 heterodimeric complex: Its mechanism and inhibition by curcumin for cancer therapeutics. Int. J. Biol. Macromol. 2022, 208, 356–366. [Google Scholar] [CrossRef]
- Alqarni, A.M.; Zeidler, M.P. How does methotrexate work? Biochem. Soc. Trans. 2020, 48, 559–567. [Google Scholar] [CrossRef]
- Yang, M.Y.; Lee, H.T.; Chen, C.M.; Shen, C.C.; Ma, H.I. Celecoxib suppresses the phosphorylation of STAT3 protein and can enhance the radiosensitivity of medulloblastoma-derived cancer stem-like cells. Int. J. Mol. Sci. 2014, 15, 11013–11029. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Wang, Y.; Dong, L.; Li, M.; Cai, W. Anti-inflammatory effect of resveratrol through the suppression of NF-κB and JAK/STAT signaling pathways. Acta Biochim. Biophys. Sin. 2015, 47, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, M.L.; Letteri, R.; Chan-Seng, D.; Kumar, S.; Rivera-Cruz, C.M.; Emrick, T.S. Reengineering tumor microenvironment with sequential interleukin delivery. Bioengineering 2021, 8, 90. [Google Scholar] [CrossRef]
- Mulia, G.; Picanço-Castro, V.; Stavrou, E.F.; Athanassiadou, A.; Figueiredo, M.L. Advances in the development and the applications of non-viral, episomal vectors for gene therapy. Hum. Gene Ther. 2021, 32, 1076–1095. [Google Scholar] [CrossRef]
- Alexander, A.F.; Kelsey, I.; Forbes, H.; Miller-Jensen, K. Single-cell secretion analysis reveals a dual role for IL-10 in restraining and resolving the TLR4-induced inflammatory response. Cell Rep. 2021, 36, 109728. [Google Scholar] [CrossRef]
- Liu, F.D.; Kenngott, E.E.; Schröter, M.F.; Kühl, A.; Jennrich, S.; Watzlawick, R.; Hoffmann, U.; Wolff, T.; Norley, S.; Scheffold, A.; et al. Timed action of IL-27 protects from immunopathology while preserving defense in influenza. PLoS Pathog. 2014, 10, e1004110. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, A.A.; Decker, R.E.; Kumar, S.; Lamantia, Z.; Yokota, H.; Emrick, T.; Figueiredo, M.L. Enhancing Prednisone-Based Arthritis Therapy with Targeted IL-27 Gene Delivery. Bioengineering 2022, 9, 248. https://doi.org/10.3390/bioengineering9060248
Marin AA, Decker RE, Kumar S, Lamantia Z, Yokota H, Emrick T, Figueiredo ML. Enhancing Prednisone-Based Arthritis Therapy with Targeted IL-27 Gene Delivery. Bioengineering. 2022; 9(6):248. https://doi.org/10.3390/bioengineering9060248
Chicago/Turabian StyleMarin, Adriana A., Richard E. Decker, Shreya Kumar, Zachary Lamantia, Hiroki Yokota, Todd Emrick, and Marxa L. Figueiredo. 2022. "Enhancing Prednisone-Based Arthritis Therapy with Targeted IL-27 Gene Delivery" Bioengineering 9, no. 6: 248. https://doi.org/10.3390/bioengineering9060248
APA StyleMarin, A. A., Decker, R. E., Kumar, S., Lamantia, Z., Yokota, H., Emrick, T., & Figueiredo, M. L. (2022). Enhancing Prednisone-Based Arthritis Therapy with Targeted IL-27 Gene Delivery. Bioengineering, 9(6), 248. https://doi.org/10.3390/bioengineering9060248





