Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro
Abstract
1. Introduction
2. The Wound Healing Process
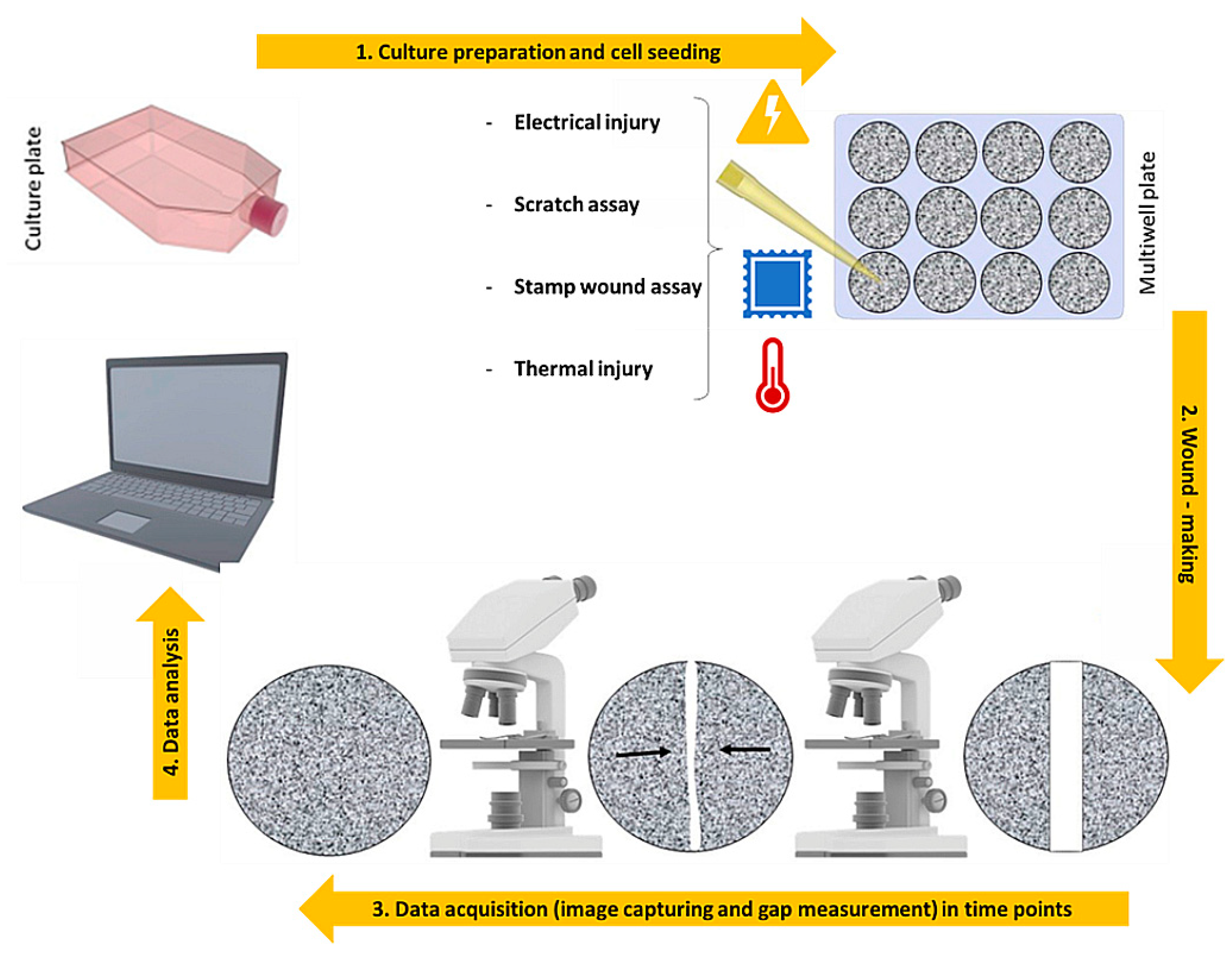
3. 2D Models: An over Simplified View of the Repair Dynamics
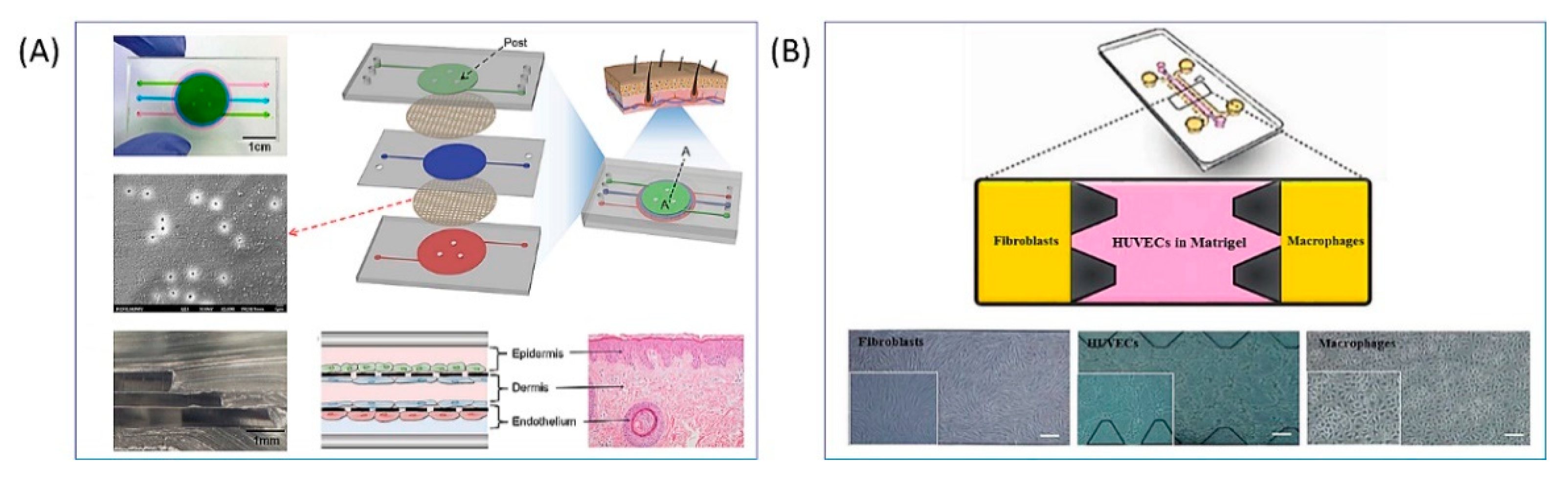
4. Full-Thickness Models: Toward the Replication of Deep Wounds Repair
4.1. Deep Wound Models Based on Exogenous Dermis Equivalents
4.2. Deep Wound Models Based on Endogenous Dermis Equivalents
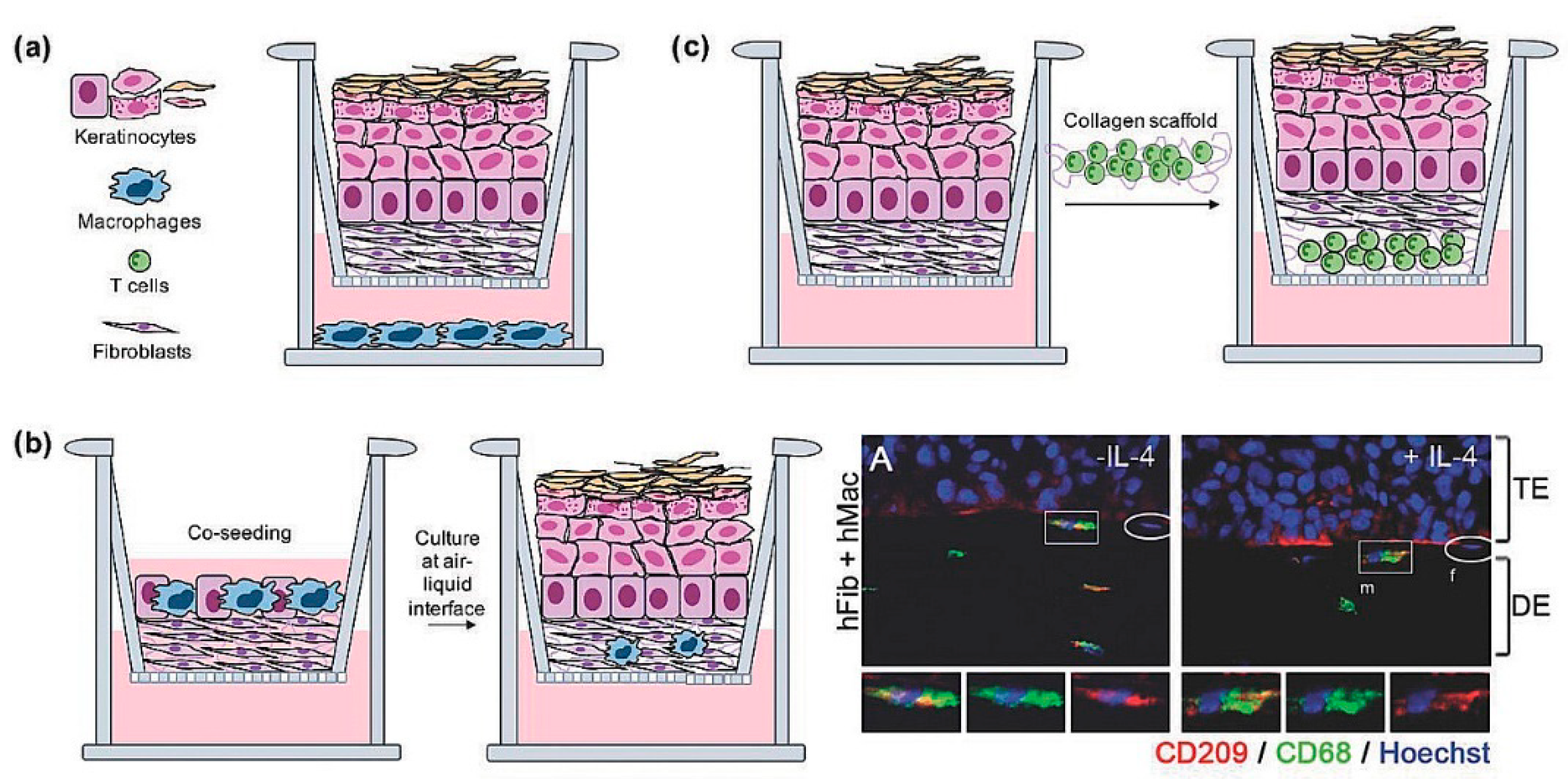
5. The Need of Immune Response In Vitro
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Eckes, B.; Nischt, R.; Krieg, T. Cell-matrix interactions in dermal repair and scarring. Fibrogenes. Tissue Repair 2010, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; Somenek, M. Scar revision review. Arch. Facial Plast. Surg. 2012, 14, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, A.S. Wound healing and scarring. Adv. Exp. Surg. 2018, 2, 255–267. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in wound healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Scholle, R.H. Learning by the rules. CDS Rev. 1995, 88, 72. [Google Scholar] [CrossRef]
- Fernández, J.M.A.; Pablo, C.L. Body temperature and heating temperature in major burns patients care. Enferm. Glob. 2021, 20, 466–488. [Google Scholar] [CrossRef]
- Escámez, M.J.; García, M.; Larcher, F.; Meana, A.; Muñoz, E.; Jorcano, J.L.; Del Río, M. An in vivo model of wound healing in genetically modified skin-humanized mice. J. Investig. Dermatol. 2004, 123, 1182–1191. [Google Scholar] [CrossRef]
- Polo, M.; Kim, Y.J.; Kucukcelebi, A.; Hayward, P.G.; Ko, F.; Robson, M.C. An in vivo model of human proliferative scar. J. Surg. Res. 1998, 74, 187–195. [Google Scholar] [CrossRef]
- Coolen, N.A.; Schouten, K.C.W.M.; Boekema, B.K.H.L.; Middelkoop, E.; Ulrich, M.M.W. Wound healing in a fetal, adult, and scar tissue model: A comparative study. Wound Repair Regen. 2010, 18, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kloeters, O.; Tandara, A.; Mustoe, T.A. Hypertrophic scar model in the rabbit ear: A reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2007, 15, 40–45. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, L.J.; Limandjaja, G.C.; Niessen, F.B.; Gibbs, S. Human hypertrophic and keloid scar models: Principles, limitations and future challenges from a tissue engineering perspective. Exp. Dermatol. 2014, 23, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Sami, D.G.; Heiba, H.H.; Abdellatif, A. Wound healing models: A systematic review of animal and non-animal models. Wound Med. 2019, 24, 8–17. [Google Scholar] [CrossRef]
- Urciuolo, F.; Casale, C.; Imparato, G.; Netti, P.A. Bioengineered skin substitutes: The role of extracellular matrix and vascularization in the healing of deep wounds. J. Clin. Med. 2019, 8, 2083. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Behm, B.; Babilas, P.; Landthaler, M.; Schreml, S. Cytokines, chemokines and growth factors in wound healing. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 812–820. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Departments of Biochemistry, Anatomy, Emergency Medicine and Virginia Commonwealth University, Richmond Virginia. Front. Biosci. 2004, 283–289. [Google Scholar] [CrossRef]
- Sorg, J.M.R.H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. General principles of wound healing. Surg. Clin. N. Am. 1997, 77, 509–528. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Orgill, D.P.; Murphy, G.F. The pathophysiologic basis for wound healing and cutaneous regeneration. In Biomaterials for Treating Skin Loss; A Volume in Woodhead Publishing Series in Biomaterials; Orgill, D., Blanco, C., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 25–57. [Google Scholar] [CrossRef]
- Delavary, B.M.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H.J. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M.M. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.W.; Muir, I.F.K. The role of lymphocytes in wound healing. Br. J. Plast. Surg. 1990, 43, 655–662. [Google Scholar] [CrossRef]
- Boothby, I.C.; Cohen, J.N.; Rosenblum, M.D. Regulatory T cells in Skin Injury: At the Crossroads of Tolerance and Tissue Repair. Sci. Immunol. 2020, 5, eaaz9631. [Google Scholar] [CrossRef]
- Short, W.D.; Wang, X.; Keswani, S.G. The Role of T Lymphocytes in Cutaneous Scarring. Adv. Wound Care 2022, 11, 121–131. [Google Scholar] [CrossRef]
- Grieb, G.; Steffens, G.; Pallua, N.; Bernhagen, J.; Bucala, R. Circulating Fibrocytes-Biology and Mechanisms in Wound Healing and Scar Formation. Int. Rev. Cell Mol. Biol. 2011, 291, 1–19. [Google Scholar] [CrossRef]
- Senger, D.R.; Davis, G.E. Angiogenesis. Cold Spring Harb. Perspect. Biol. 2011, 3, a005090. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Larson, B.J.; Nauta, A.; Kawai, K.; Longaker, M.T.; Lorenz, H.P. Scarring and scarless wound healing. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 77–111. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Schrementi, M. Oral Mucosal Healing. In Wound Healing: Stem Cells Repair and Restorations, Basic and Clinical Aspects; Turksen, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 125–132. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Wu, X.; Guan, J.L. Wound-healing assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Jonkman, J.E.N.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. Cell Adhesion & Migration An introduction to the wound healing assay using livecell microscopy An introduction to the wound healing assay using livecell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Doyle, W.; Shide, E.; Thapa, S.; Chandrasekaran, V. The effects of energy beverages on cultured cells. Food Chem. Toxicol. 2012, 50, 3759–3768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhai, W.; Xie, Y.; Chen, Q.; Zhu, W.; Sun, X. Mesenchymal stem cells derived from breast cancer tissue promote the proliferation and migration of the MCF-7 cell line in vitro. Oncol. Lett. 2013, 6, 1577–1582. [Google Scholar] [CrossRef] [PubMed]
- Klettner, A.; Tahmaz, N.; Dithmer, M.; Richert, E.; Roider, J. Effects of aflibercept on primary RPE cells: Toxicity, wound healing, uptake and phagocytosis. Br. J. Ophthalmol. 2014, 98, 1448–1452. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Geng, H.; Hwang, Y.; Mishra, P.; Skloss, W.L.; Sprague, E.A.; Saikumar, P.; Venkatachalam, M. A novel wounding device suitable for quantitative biochemical analysis of wound healing and regeneration of cultured epithelium. Wound Repair Regen. 2010, 18, 159–167. [Google Scholar] [CrossRef]
- Lee, J.; Wang, Y.L.; Ren, F.; Lele, T.P. Stamp wound assay for studying coupled cell migration and cell debris clearance. Langmuir 2010, 26, 16672–16676. [Google Scholar] [CrossRef]
- Goetsch, K.P.; Niesler, C.U. Optimization of the scratch assay for in vitro skeletal muscle wound healing analysis. Anal. Biochem. 2011, 411, 158–160. [Google Scholar] [CrossRef]
- Ashby, W.J.; Zijlstra, A. Established and novel methods of interrogating two-dimensional cell migration. Integr. Biol. United Kingd. 2012, 4, 1338–1350. [Google Scholar] [CrossRef]
- Liu, M.; Saeki, K.; Matsunobu, T.; Okuno, T.; Koga, T.; Sugimoto, Y.; Yokoyama, C.; Nakamizo, S.; Kabashima, K.; Narumiya, S.; et al. 12-hydroxyheptadecatrienoic acid promotes epidermal wound healing by accelerating keratinocyte migration via the BLT2 receptor. J. Exp. Med. 2014, 211, 1063–1078. [Google Scholar] [CrossRef]
- Brönneke, S.; Brückner, B.; Söhle, J.; Siegner, R.; Smuda, C.; Stäb, F.; Wenck, H.; Kolbe, L.; Grönniger, E.; Winnefeld, M. Genome-wide expression analysis of wounded skin reveals novel genes involved in angiogenesis. Angiogenesis 2015, 18, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.Y.K.; Leung, E.P.Y.; Mak, N.K.; Wong, R.N.S. A Simplified Method for Quantifying Cell Migration/Wound Healing in 96-Well Plates. J. Biomol. Screen. 2010, 15, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Hettler, A.; Werner, S.; Eick, S.; Laufer, S.; Weise, F. A new in vitro model to study cellular responses after thermomechanical damage in monolayer cultures. PLoS ONE 2013, 8, e82635. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef] [PubMed]
- Keese, C.R.; Wegener, J.; Walker, S.R.; Giaever, I. Electrical wound-healing assay for cells in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Szulcek, R.; Bogaard, H.J.; van Nieuw Amerongen, G.P. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. J. Vis. Exp. 2014, 85, e51300. [Google Scholar] [CrossRef]
- Zordan, M.D.; Mill, C.P.; Riese, D.J.; Leary, J.F. A high throughput, interactive imaging, bright-field wound healing assay. Cytom. Part A 2011, 79A, 227–232. [Google Scholar] [CrossRef]
- Chim, S.M.; Qin, A.; Tickner, J.; Pavlos, N.; Davey, T.; Wang, H.; Guo, Y.; Zheng, M.H.; Xu, J. EGFL6 promotes endothelial cell migration and angiogenesis through the activation of extracellular signal-regulated kinase. J. Biol. Chem. 2011, 286, 22035–22046. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Razzell, W.; Martin, P. “White wave” analysis of epithelial scratch wound healing reveals how cells mobilise back from the leading edge in a myosin-II-dependent fashion. J. Cell Sci. 2011, 124, 1017–1021. [Google Scholar] [CrossRef]
- Bellas, E.; Seiberg, M.; Garlick, J.; Kaplan, D.L. In vitro 3D Full-Thickness Skin-Equivalent Tissue Model Using Silk and Collagen Biomaterials. Macromol. Biosci. 2012, 12, 1627–1636. [Google Scholar] [CrossRef]
- Das, D.; Noh, I. Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Springer: Singapore, 2018; ISBN 9789811304446. [Google Scholar]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Cao, Y.; Li, M.; Yan, Y.; Cheng, R.; Zhao, Y.; Shao, Q.; Wang, J.; Sang, S. Construction of tissue-engineered skin with rete ridges using co-network hydrogels of gelatin methacrylated and poly(ethylene glycol) diacrylate. Mater. Sci. Eng. C 2021, 129, 112360. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, M.; Pajorova, J.; Broz, A.; Hadraba, D.; Lopot, F.; Zavadakova, A.; Vistejnova, L.; Kostic, I.; Jencova, V.; Bacakova, L. Erratum to a Two-layer skin construct consisting of a collagen hydrogel reinforced by a fibrin-coated polylactide nanofibrous membrane. Int. J. Nanomed. 2019, 14, 7215–7216. [Google Scholar] [CrossRef] [PubMed]
- Casale, C.; Imparato, G.; Urciuolo, F.; Netti, P.A. Endogenous human skin equivalent promotes in vitro morphogenesis of follicle-like structures. Biomaterials 2016, 101, 86–95. [Google Scholar] [CrossRef]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef]
- Larouche, D.; Cantin-Warren, L.; Desgagné, M.; Guignard, R.; Martel, I.; Ayoub, A.; Lavoie, A.; Gauvin, R.; Auger, F.A.; Moulin, V.J.; et al. Improved Methods to Produce Tissue-Engineered Skin Substitutes Suitable for the Permanent Closure of Full-Thickness Skin Injuries. Biores. Open Access 2016, 5, 320–329. [Google Scholar] [CrossRef]
- Imparato, G.; Urciuolo, F.; Casale, C.; Netti, P.A. The role of microscaffold properties in controlling the collagen assembly in 3D dermis equivalent using modular tissue engineering. Biomaterials 2013, 34, 7851–7861. [Google Scholar] [CrossRef]
- Urciuolo, F.; Garziano, A.; Imparato, G.; Panzetta, V.; Fusco, S.; Casale, C.; Netti, P.A. Biophysical properties of dermal building-blocks affect extra cellular matrix assembly in 3D endogenous macrotissue. Biofabrication 2016, 8, 015010. [Google Scholar] [CrossRef]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef]
- Vaughan, M.B.; Ramirez, R.D.; Brown, S.A.; Yang, J.C.; Wright, W.E.; Shay, J.W. A reproducible laser-wounded skin equivalent model to study the effects of aging in vitro. Rejuvenat. Res. 2004, 7, 99–110. [Google Scholar] [CrossRef]
- Oberringer, M.; Meins, C.; Bubel, M.; Pohlemann, T. A new in vitro wound model based on the co-culture of human dermal microvascular endothelial cells and human dermal fibroblasts. Biol. Cell 2007, 99, 197–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iljas, J.D.; Röhl, J.; McGovern, J.A.; Moromizato, K.H.; Parker, T.J.; Cuttle, L. A human skin equivalent burn model to study the effect of a nanocrystalline silver dressing on wound healing. Burns 2021, 47, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isaacs, C.; Paquette, D.; Downing, G.; Kouttab, N.; Butmarc, J.; Badiavas, E.; Hardin-Young, J. Wounding of bioengineered skin: Cellular and molecular aspects after injury. J. Investig. Dermatol. 2002, 119, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Geer, D.J.; Swartz, D.D.; Andreadis, S.T. Fibrin promotes migration in a three-dimensional in vitro model of wound regeneration. Tissue Eng. 2002, 8, 787–798. [Google Scholar] [CrossRef]
- Xie, Y.; Rizzi, S.C.; Dawson, R.; Lynam, E.; Richards, S.; Leavesley, D.I.; Upton, Z. Development of a three-dimensional human skin equivalent wound model for investigating novel wound healing therapies. Tissue Eng. Part C Methods 2010, 16, 1111–1123. [Google Scholar] [CrossRef]
- Biglari, S.; Le, T.Y.L.; Tan, R.P.; Wise, S.G.; Zambon, A.; Codolo, G.; De Bernard, M.; Warkiani, M.; Schindeler, A.; Naficy, S.; et al. Simulating Inflammation in a Wound Microenvironment Using a Dermal Wound-on-a-Chip Model. Adv. Healthc. Mater. 2019, 8, 1801307. [Google Scholar] [CrossRef]
- Tefft, J.B.; Chen, C.S.; Eyckmans, J. Reconstituting the dynamics of endothelial cells and fibroblasts in wound closure. APL Bioeng. 2021, 5, 016102. [Google Scholar] [CrossRef]
- Shabestani Monfared, G.; Ertl, P.; Rothbauer, M. An on-chip wound healing assay fabricated by xurography for evaluation of dermal fibroblast cell migration and wound closure. Sci. Rep. 2020, 10, 16192. [Google Scholar] [CrossRef]
- Sakar, M.S.; Eyckmans, J.; Pieters, R.; Eberli, D.; Nelson, B.J.; Chen, C.S. Cellular forces and matrix assembly coordinate fibrous tissue repair. Nat. Commun. 2016, 7, 11036. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.H.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S.H. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef]
- Imparato, G.; Casale, C.; Scamardella, S.; Urciuolo, F.; Bimonte, M.; Apone, F.; Colucci, G.; Netti, P.A. A novel engineered dermis for in vitro photodamage research. J. Tissue Eng. Regen. Med. 2017, 11, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; Pardo, A. Fibroageing: An ageing pathological feature driven by dysregulated extracellular matrix-cell mechanobiology. Ageing Res. Rev. 2021, 70, 101393. [Google Scholar] [CrossRef] [PubMed]
- Gioiella, F.; Urciuolo, F.; Imparato, G.; Brancato, V.; Netti, P.A. An Engineered Breast Cancer Model on a Chip to Replicate ECM-Activation In Vitro during Tumor Progression. Adv. Healthc. Mater. 2016, 5, 3074–3084. [Google Scholar] [CrossRef] [PubMed]
- Casale, C.; Imparato, G.; Urciuolo, F.; Rescigno, F.; Scamardella, S.; Escolino, M.; Netti, P.A. Engineering a human skin equivalent to study dermis remodelling and epidermis senescence in vitro after UVA exposure. J. Tissue Eng. Regen. Med. 2018, 12, 1658–1669. [Google Scholar] [CrossRef]
- Lombardi, B.; Casale, C.; Imparato, G.; Urciuolo, F.; Netti, P.A. Spatiotemporal Evolution of the Wound Repairing Process in a 3D Human Dermis Equivalent. Adv. Healthc. Mater. 2017, 6, 1601422. [Google Scholar] [CrossRef]
- Yousuf, Y.; Amini-Nik, S.; Jeschke, M.G. Overall Perspective on the Clinical Importance of Skin Models; Elsevier, Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128110003. [Google Scholar]
- Pastar, I.; Liang, L.; Sawaya, A.P.; Wikramanayake, T.C.; Glinos, G.D.; Drakulich, S.; Chen, V.; Stojadinovic, O.; Davis, S.C.; Tomic-Canic, M. Preclinical Models for Wound-Healing Studies; Elsevier, Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128110003. [Google Scholar]
- Maestri, E. The 3Rs Principle in Animal Experimentation: A Legal Review of the State of the Art in Europe and the Case in Italy. BioTech 2021, 10, 9. [Google Scholar] [CrossRef]
- Pupovac, A.; Senturk, B.; Griffoni, C.; Maniura-Weber, K.; Rottmar, M.; McArthur, S.L. Toward Immunocompetent 3D Skin Models. Adv. Healthc. Mater. 2018, 7, 1701405. [Google Scholar] [CrossRef]
- Linde, N.; Gutschalk, C.M.; Hoffmann, C.; Yilmaz, D.; Mueller, M.M. Integrating macrophages into organotypic co-cultures: A 3D in vitro model to study tumor-associated macrophages. PLoS ONE 2012, 7, e40058. [Google Scholar] [CrossRef]
- Todd, C.; Hewitt, S.D.; Kempenaar, J.; Noz, K.; Thody, A.J.; Ponec, M. Co-culture of human melanocytes and keratinocytes in a skin equivalent model: Effect of ultraviolet radiation. Arch. Dermatol. Res. 1993, 285, 455–459. [Google Scholar] [CrossRef]
- Li, L.; Fukunaga-Kalabis, M.; Herlyn, M. The Three-Dimensional Human Skin Reconstruct Model: A Tool to Study Normal Skin and Melanoma Progression. J. Vis. Exp. 2011, 54, e2937. [Google Scholar] [CrossRef]
- Kosten, I.J.; Spiekstra, S.W.; de Gruijl, T.D.; Gibbs, S. MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Toxicol. Appl. Pharmacol. 2015, 287, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, K.; Scheper, R.J.; De Gruijl, T.D.; Gibbs, S. Epidermis-to-dermis migration of immature Langerhans cells upon topical irritant exposure is dependent on CCL2 and CCL5. Eur. J. Immunol. 2010, 40, 2026–2034. [Google Scholar] [CrossRef] [PubMed]
- Chau, D.Y.S.; Johnson, C.; Macneil, S.; Haycock, J.W.; Ghaemmaghami, A.M. The development of a 3D immunocompetent model of human skin. Biofabrication 2013, 5, 035011. [Google Scholar] [CrossRef] [PubMed]
- Kühbacher, A.; Henkel, H.; Stevens, P.; Grumaz, C.; Finkelmeier, D.; Burger-Kentischer, A.; Sohn, K.; Rupp, S. Central role for dermal fibroblasts in skin model protection against candida albicans. J. Infect. Dis. 2017, 215, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Lorthois, I.; Simard, M.; Morin, S.; Pouliot, R. Infiltration of T cells into a three-dimensional psoriatic skin model mimics pathological key features. Int. J. Mol. Sci. 2019, 20, 1670. [Google Scholar] [CrossRef]
- Shin, J.U.; Abaci, H.E.; Herron, L.; Guo, Z.; Sallee, B.; Pappalardo, A.; Jackow, J.; Wang, E.H.C.; Doucet, Y.; Christiano, A.M. Recapitulating T cell infiltration in 3D psoriatic skin models for patient-specific drug testing. Sci. Rep. 2020, 10, 4123. [Google Scholar] [CrossRef]
- Bechetoille, N.; Vachon, H.; Gaydon, A.; Boher, A.; Fontaine, T.; Schaeffer, E.; Decossas, M.; André-Frei, V.; Mueller, C.G. A new organotypic model containing dermal-type macrophages. Exp. Dermatol. 2011, 20, 1035–1037. [Google Scholar] [CrossRef]
- Wang, X.F.; Fang, Q.Q.; Jia, B.; Hu, Y.Y.; Wang, Z.C.; Yan, K.P.; Yin, S.Y.; Liu, Z.; Tan, W.Q. Potential effect of non-thermal plasma for the inhibition of scar formation: A preliminary report. Sci. Rep. 2020, 10, 1064. [Google Scholar] [CrossRef]
- Kubinova, S.; Zaviskova, K.; Uherkova, L.; Zablotskii, V.; Churpita, O.; Lunov, O.; Dejneka, A. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci. Rep. 2017, 7, 45183. [Google Scholar] [CrossRef]
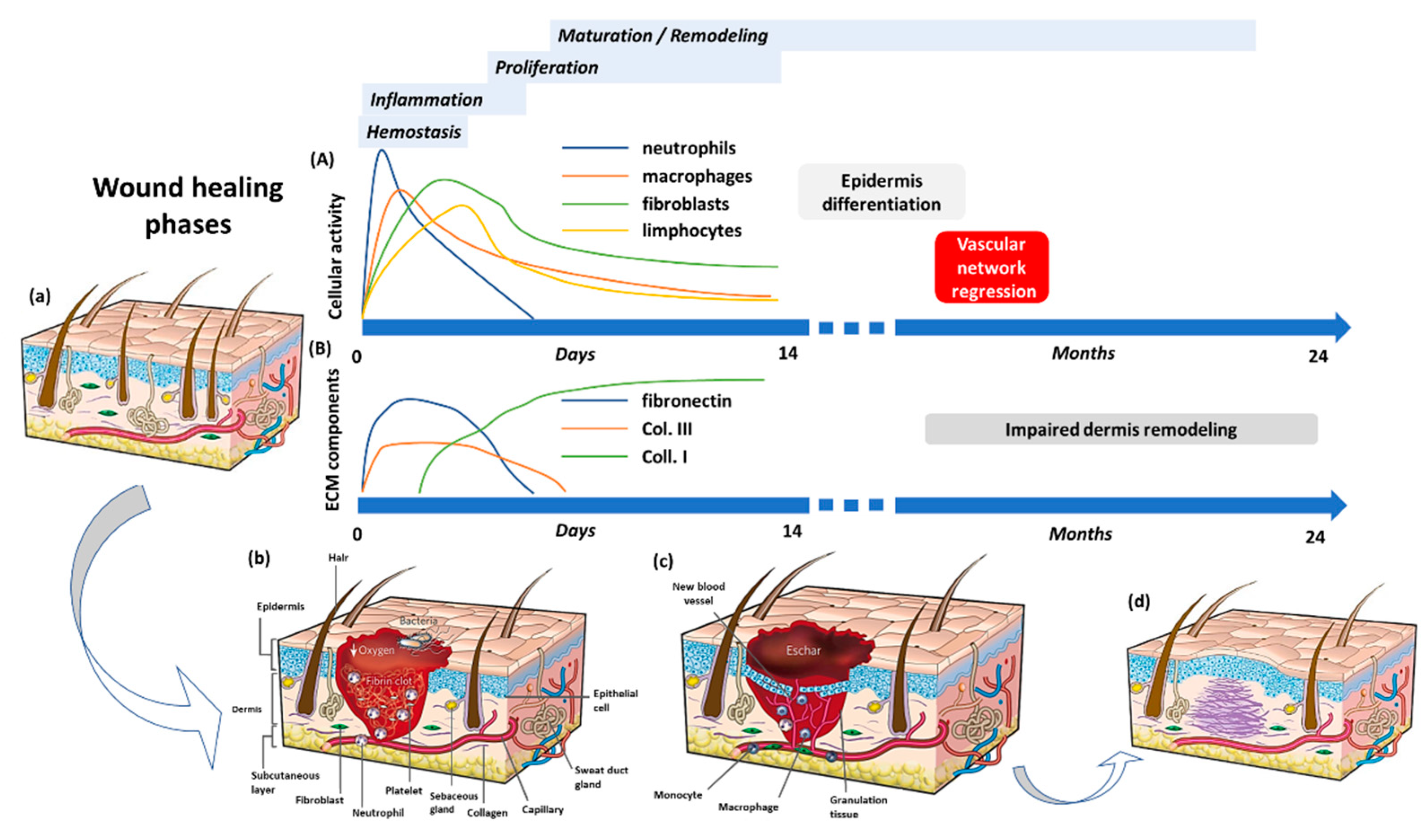


| Cell Type | Activated by | Molecules Released | Effects |
|---|---|---|---|
| PLATELETS [21] | Exposure to the underlying collagen and vWF after blood vessel rupture | PDGF, TGF-β, bFGF, KGF, EGF, IGF | Fibrin clot (scab) formation; enhance neutrophils, macrophages, fibroblasts, and endothelial cells chemotaxis and infiltration |
| ENDOTHELIAL CELLS (HEMOSTASIS PHASE) [21] | Blood vessel injury | Prostaglandins, Leukotrienes | Vasodilation and platelets disassembly; increase in vascular permeability and in leukocytes chemotaxis and adhesion |
| ENDOTHELIAL CELLS (PROLIFERATION PHASE) [21] | Tissue hypoxia, bFGF, KGF, VEGF, TNF-α, TGF-β, thrombin | Proteolytic enzymes, matrix MMP | Angiogenesis |
| DERMAL MAST CELLS [21] | Complement system (C3a and C5a), physical stimuli (heat or mechanical injury) | Histamine, TNF-α, IL-1, TGF-β, PDGF, serine protease, chymase, tryptase, Prostaglandins, Leukotrienes | More leaky and permeable blood vessels; breakdown of the ECM to pave the way for fibroblasts and endothelial cells proliferation |
| NEUTROPHILS [23] | Factor released by platelets, by-products of bacterial degradation | ROS, NO, antimicrobial peptides, antimicrobial proteases, IL-17, VEGF | Phagocytosis; antimicrobial function; wound debridement |
| MACROPHAGES (M1 PHENOTYPE—PRO- INFLAMMATORY) [23] | Derived from chemotaxis of migrating monocytes activated by bacterial products, complement degradation products (C5a), and factor released by platelets and neutrophils | Proteinases, antimicrobial peptides and proteases, TNF-α, TGF-β, IL-1, IL-8 | Phagocytosis; antimicrobial function; wound debridement |
| MACROPHAGES (M2 PHENOTYPE—ANTI- INFLAMMATORY) [23] | Proteinases, TGF-β, EGF, PDGF, TNF-α, IL-1, IFN-γ, IGF, IL-6, Fibronectin, bFGF, VEGF | Matrix synthesis regulation; cell recruitment and activation; angiogenesis | |
| T LYMPHOCYTES [26] | IFN-γ released by macrophages | IL-2, IFN-γ, IL-4, IL-10, TGF-β, TNF-α, FGF | Macrophages production and differentiation regulation; synthesis and proliferation of fibroblast |
| EPITHELIAL CELLS/ KERATINOCYTES [21] | Mainly, EGF, secreted by platelets, and TGF-α produced by macrophages, platelets, and keratinocytes | bFGF, VEGF, TNF-α | Re-epithelization |
| FIBROBLASTS/ MYOFIBROBLASTS [21] | PDGF, TGF-β, FGF, EGF, and IGF released by platelets and macrophages | Collagen type I and III, elastin, GAGs, adhesive glycoproteins | Matrix components synthesis; wound contraction |
| 2D Models | Aim | Ref. |
|---|---|---|
| Keratinocytes monolayers | Study of the migration behavior to reproduce re-epithelialization process | [33,38,39,42,44] |
| Fibroblasts monolayers | Evaluation of the migratory potential to study their speed, persistence, and polarity during granulation tissue formation | [31,32,35,46] |
| Endothelial cells | How endothelial cells migrate and grow towards an angiogenic stimulus to form sprouts | [43,45,48,50] |
| 2D wound assay | Description | Ref. |
| Scratch Assay | Scratch ofa confluent monolayer of cells with pipette tip, cell scrapers, toothpicks or metallic micro indenters | [30,31,32,33,35,36,37,41,42,43,44,50,51] |
| Stamp Wound Assay | Determine a lesion on the cell culture with a high-pressure force | [38,39] |
| Thermal injury Assay | Injury a specific zone of the cell monolayer with very high or low temperatures | [45] |
| Electrical Injury Assay | Destruction of a cell portion by applying an electric current | [46,47,48] |
| Optical Injury Assay | Formation of a wounded area by means of a laser beam | [49] |
| 3D Models | Aim | Ref. |
|---|---|---|
| Exogenous skin wound models | ||
| Fibroblasts-populated Rat tail Collagen I + keratinocytes | Besides new tissue formation, these in vitro platforms enable us to screen molecules able to speed up the re-epithelization step | [62,65] |
| Fibroblasts in DED + keratinocytes | [64,66,67] | |
| Exogenous dermal wound models | ||
| Fibroblasts-populated Rat tail Collagen I | Reproduction of the complex processes concerning ECM remodelling and cell-ECM crosstalk | [71] |
| Fibroblast and endothelial cells embedded in Rat tail Collagen I and fibrinogen | [69] | |
| Endogenous dermal wound model | ||
| Fibroblasts embedded in their own ECM | Able to replicate in vitro morphogenesis, neo-synthesis, assembly, ECM turnover, and modification of ECM composition/architecture during a pathological state. | [75] |
| 3D wound assay | Description | Ref. |
| Mechanical Injury | Creation of a wound through a scalpel, a biopsy punch, or a rotating drill | [66] |
| Laser wound | Tissue ablation in a specific area by a laser beam | [62] |
| Thermal injury Assay | Deep burn wounds generated through the contact with a stainless-steel rod connected to a soldering iron | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urciuolo, F.; Passariello, R.; Imparato, G.; Casale, C.; Netti, P.A. Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro. Bioengineering 2022, 9, 233. https://doi.org/10.3390/bioengineering9060233
Urciuolo F, Passariello R, Imparato G, Casale C, Netti PA. Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro. Bioengineering. 2022; 9(6):233. https://doi.org/10.3390/bioengineering9060233
Chicago/Turabian StyleUrciuolo, Francesco, Roberta Passariello, Giorgia Imparato, Costantino Casale, and Paolo Antonio Netti. 2022. "Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro" Bioengineering 9, no. 6: 233. https://doi.org/10.3390/bioengineering9060233
APA StyleUrciuolo, F., Passariello, R., Imparato, G., Casale, C., & Netti, P. A. (2022). Bioengineered Wound Healing Skin Models: The Role of Immune Response and Endogenous ECM to Fully Replicate the Dynamic of Scar Tissue Formation In Vitro. Bioengineering, 9(6), 233. https://doi.org/10.3390/bioengineering9060233






