Abstract
Fractures of the femur are a frequent problem in elderly people, and it has been demonstrated that treating them with a diagnostic–therapeutic–assistance path within 48 h of admission to the hospital reduces complications and shortens the length of the hospital stay (LOS). In this paper, the preoperative data of 1082 patients were used to further extend the previous research and to generate several models that are capable of predicting the overall LOS: First, the LOS, measured in days, was predicted through a regression analysis; then, it was grouped by weeks and was predicted with a classification analysis. The KNIME analytics platform was applied to divide the dataset for a hold-out cross-validation, perform a multiple linear regression and implement machine learning algorithms. The best coefficient of determination (R2) was achieved by the support vector machine (R2 = 0.617), while the mean absolute error was similar for all the algorithms, ranging between 2.00 and 2.11 days. With regard to the classification analysis, all the algorithms surpassed 80% accuracy, and the most accurate algorithm was the radial basis function network, at 83.5%. The use of these techniques could be a valuable support tool for doctors to better manage orthopaedic departments and all their resources, which would reduce both waste and costs in the context of healthcare.
1. Introduction
Fractures of the neck of the femur are very frequent in elderly people (over 65 years of age); the risk for this type of fracture seems to be higher for women than for men [1]. The main causes are linked to falls, or even to apparently insignificant traumas and chronic bone diseases, such as osteoporosis [2]. The international guidelines state that the best treatment for fracture of the neck of the femur is surgery [3]. In particular, the scientific evidence shows that surgery within 48 h of admission is an effective treatment that can significantly reduce complications in the short, medium and long terms [4,5]. For this reason, the A. Cardarelli Hospital has introduced the diagnostic–therapeutic–assistance path (DTAP), which is a clinical pathway that involves a complex intervention for the organization of care processes for a well-defined group of patients over a well-defined period. It is an organizational model that is characterized by the appropriateness of the interventions, by the integration of the skills of the various professional figures and by the creation of the continuity of care between the hospital and the territory [6]. DTAPs support hospitals in the care quality management, thereby promoting organized and efficient patient care [7], and reducing complications, the length of the hospital stay (LOS) and the medical costs [8]. Previous studies have also proven that their implementation, combined with management approaches, such as Lean and Six Sigma, reduces the variability in clinical practice and improves outcomes [9,10,11,12]. In the specific case of the A. Cardarelli Hospital, the DTAP describes the approaches, organizational procedures and processes that are mandatory for all hospital workers, with the aim of speeding up the surgical process for patients with femoral neck fractures. This clinical pathway includes three phases: (1) Initial patient care after arrival in the emergency room; (2) Perioperative management; and (3) The predischarge period. The implementation of the DTAP, in this case, significantly improved the management of patients with femoral neck fractures; indeed, Six Sigma studies by Improta et al. and Ricciardi et al. show that the implementation of a DTAP led to a significant reduction in the preoperative LOS and in the overall LOS, respectively, at the A. Cardarelli Hospital [13,14].
Mathematical modelling has been used in the healthcare sector for various purposes: Tesfahun et al. developed a model that can predict the rate of the production of medical waste to optimize the management processes of this waste [15] and to predict the spread of viruses and bacteria [16,17]. In this situation, multiple linear regression was used to assess which factors influence the LOS in patients with dengue haemorrhagic fever [18]. Similarly, Liu et al. used regression to evaluate the clinical factors that most influence the LOS in adult patients with peritonsillar abscesses [19]. Furthermore, Trunfio et al. used regression to predict the LOS of patients with femoral neck fractures at the University Hospital of Salerno [20].
Compared to other approaches for the analysis, simulation and modelling of biomedical data [21,22,23,24,25,26,27,28,29,30,31,32,33], machine learning (ML) techniques are useful in this context to the creation of a model that can help clinicians to manage patients; indeed, ML has been used in the literature for many aims. Regarding the diagnosis, it has been employed for Parkinson’s disease in neurology, in cardiology for the detection of coronary artery disease and in oncology for the classification of the tumour grade and the nodal status in oropharyngeal and oral cavity squamous-cell carcinoma [34]. Regarding the prognostic use of ML, it has allowed for the assessment of the risk of cardiac death or cardiovascular risks in several studies [35,36,37,38,39,40,41,42,43].
While in the abovementioned fields of medicine, ML has been widely applied for solving biomedical problems, and even outperforming human specialists in some cases, the review of Cabitza et al. outlines a different scenario in the orthopaedic field, where ML is still in a preliminary phase [44]. The authors compare ML to a health technology by stating that it needs assessments and evaluations in the real world setting in order to go from a Phase 2 trial, where it is now in orthopaedics, to a Phase 3 trial. In another study, Ramkumar et al. created an ML laboratory that was focused exclusively on orthopaedic surgery, with a two-fold aim: patient-specific value-based care, and human movement [45]. Moreover, interest in the use of ML to develop a predictive model of the hospital LOS has grown in recent years [46,47]. Researchers used a database composed of more than 120,000 patients to predict the LOS, measured in days, and the costs for patients who underwent a total hip and knee arthroplasty [48,49]. Similarly, Karnuta et al. developed a naïve Bayes ML algorithm and artificial neural networks to predict the LOS and costs for patients with fractures of the hip that used 103,592 patients [48,50,51]. More recently, Dogu et al. [47,52,53] integrated statistically based fuzzy cognitive maps and artificial neural networks to build an LOS prediction model for patients with an acute exacerbation of chronic obstructive pulmonary disease.
The present study aims to propose a multiparametric approach that is based on both ML and multiple linear regression for LOS prediction. In this work, the preoperative data of elderly patients who were undergoing femur fracture surgery at the A. Cardarelli Hospital of Naples were collected, before and after the implementation of a DTAP, which was specifically dedicated to the management of elderly patients who had been diagnosed with fractures of the neck of the femur. All the gathered data were then used to model and predict the overall hospital LOS by following a three-way approach (see Figure 1): (i) A traditional multiple linear regression analysis; (ii) ML algorithms, which were trained and tested; and the obtained results were compared with (iii) An ML classification analysis, which was performed to predict the LOS grouped into weeks. The final aim of the study was not only to test and compare different prediction models that could support the estimation of the LOS starting from preoperative information, as already proposed in previous studies [48,49,50], but also to offer a general approach to assessing the impact of a newly implemented DTAP on the patients’ overall LOS.
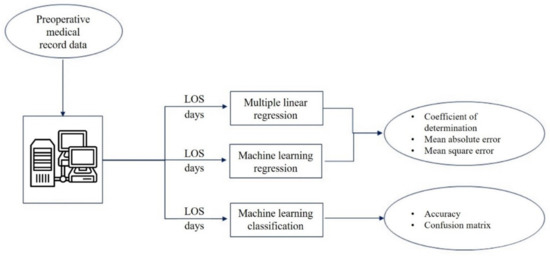
Figure 1.
Workflow of the study.
2. Materials and Methods
2.1. Dataset
Data related to 1082 patients who underwent surgery due to a femur fracture were collected. They were extrapolated from the hospital′s information system (QUANI, which is owned by BIM Italia) and included patients who underwent surgery 14 months before and 14 months after the introduction of the DTAP, as also described in [54,55].
The following inclusion criteria were applied:
- Over 65 years of age;
- Primary or secondary discharge diagnosis: femur fracture.
The exclusion criteria were as follows:
- Polytrauma;
- Cancer as a primary or secondary diagnosis;
- Voluntary discharge;
- Death.
These criteria were taken from both the Essential Level of Assistance grid, released by the Italian Ministry of Health, and the hospital operative protocol.
The following variables were extracted and considered for subsequent analyses:
- Demographic information:
- ∘
- Age.
- Timing information:
- ∘
- Date and time of admission;
- ∘
- Date and time of surgery;
- ∘
- Date and time of discharge.
- Admission modality:
- ∘
- Standard hospitalization;
- ∘
- Hospitalization through the dedicated DTAP for femur fractures.
- Comorbid conditions:
- ∘
- None;
- ∘
- Allergies;
- ∘
- Diabetes;
- ∘
- Cardiovascular disease.
- Risk variables:
- ∘
- American Society of Anesthesiologists (ASA) score.
In order to understand the influence of the variables considered for the LOS, a univariate statistical analysis was performed. First, a Kolmogorov–Smirnov test was performed on the data; then, since the p-values of the tests were lower than 0.05, the data were considered nonnormally distributed and a Mann–Whitney test was used to compare the mean LOS of the independent dichotomous variables (allergies, cardiovascular disease, diabetes, ASA score and DTAP), while a Kruskal–Wallis test was used to compare the mean LOS of the non-dichotomous variable (age). A significance level of 0.05 was adopted for the statistical analysis.
The group of researchers who collected and analysed the data was composed of the following:
- Biomedical engineers;
- An expert clinician in health management;
- The directors of the two departments of orthopaedics at the A. Cardarelli Hospital;
- The former director of the Complex Operative Unity of Health Planning and Programming at the A. Cardarelli Hospital;
- The Chief Medical Officer of the A. Cardarelli Hospital.
Table 1 shows the descriptive statistics of the dataset.

Table 1.
Descriptive statistics of the dataset.
Table 1 shows that the most influent variables are the presence of the DTAP, cardiovascular diseases and a high ASA score, thus confirming the results obtained by previous studies in the literature [54,55]: its presence definitely reduces the preoperative LOS.
2.2. The Diagnostic–Therapeutic–Assistance Path
International guidelines state that surgery within 48 h of admission is an effective treatment for significantly reducing short-, medium- and long-term complications [56]. In the A. Cardarelli Hospital, there was a nine-day average preoperative hospitalization, while the national average was four days. The proportion of hospitalizations for the fracture of the neck of the femur with surgery within 48 h in patients over 65 years of age was approximately 4%, versus 33% of the national average [57,58].
A. Cardarelli is not a single building, but a set of “pavilions”. Specifically, it consists of 21 pavilions, of which 14 are used for diagnosis and treatment, and the remaining 7 are used for technical and administrative services. Therefore, it was necessary to transport the patients among all the pavilions by ambulances in order to carry out the instrumental exams and visits. This was one of the major problems that arose during preoperative hospitalization and it suggests the need for a DTAP.
A DTAP was implemented to improve this situation at the A. Cardarelli Hospital; the plan consisted of 3 phases:
- The early hospital assistance phase, which includes all preoperative exams and transfer to the orthopaedic pavilion;
- The phase of perioperative management, which includes the anaesthesiologist evaluation, antibiotic prophylaxis and the acquisition of informed consent to be ready for surgery;
- The postoperative phase and the predischarge period, which includes a rehabilitative treatment conducted by a multidisciplinary team (surgeon, physiotherapist, nurses and social worker).
Patients are discharged only after the Individual Rehabilitative Project has been sent to the directors of the districts where the patients live in order to guarantee that the necessary activities for the continuity of care are conducted according to their individual needs.
2.3. Multiple Linear Regression Model
Multiple linear regression (MLR) is a statistical technique that is used to investigate the relationship between more than two variables [20,59]. In particular, it is very useful in predicting the best relationship between a dependent variable and several independent variables [60]. This is the reason why, in this study, multiple linear regression was implemented with consideration to 7 independent variables, and the LOS as a dependent variable, and the following equation was obtained:
where y represents the LOS; is the considered variables; is the intercept; and is the regression coefficients. This equation enables the prediction of the LOS from the patient′s characteristics and further makes it possible to determine which of these characteristics most influences the output.
In order to use this model, however, the following assumptions must be verified:
- That the relationship between the independent and dependent variables is linear;
- That there is no multicollinearity in the data;
- That the values of the residuals are independent;
- That the variance in the residuals is constant;
- That the values of the residuals are normally distributed;
- That there are no influential cases biasing the model.
The analyses that were carried out with SPSS (Statistical Package for the Social Sciences) statistics software indicate that, in this case, the assumptions are verified, and the regression analysis could then be carried out. A significance level of 0.05 was adopted for the statistical and regression analysis. For more details on all the tests performed, please refer to the additional material that is provided with this paper (see Supplementary Materials from Tables S1–S8 and Figures S1–S17).
2.4. Machine Learning Analysis
In order to perform the ML analyses, the KNIME analytics platform was used, which is a business intelligence, or predictive analytical, tool that has already been employed in previous biomedical studies [61]. Both the regression and the classification analyses were performed with random forests (RFs), radial basis function (RBF) networks, multilayer perceptrons (MLPs) and support vector machines (SVMs). The first is an extension of the decision tree methodology through ensemble learning techniques, which are, namely, bagging and randomization: many trees are learned in parallel on a random subset of features, and the final prediction is produced by majority voting [62]. RBF and MLP are two examples of artificial neural network approaches [63,64], and they are thus characterized by an input layer, an output layer and hidden layers. Their differences are present in the literature: the basic difference is that the parameters of MLP are nonlinear, while those of RBF are linear. Finally, the SVM is an instance-based algorithm that assigns the class to the test data on the basis of their distance from similar training data. SVM is capable of addressing problems such as overfitting, small datasets and nonlinear and/or high-dimensional data; it can be used for both classification and regression [65,66,67].
These four ML algorithms were chosen because they are based on different operating principles: RF employs ensemble learning techniques on one of the most famous and applied ML algorithms (decision tree); SVM is clearly different from the previous algorithm since it is an instance-based algorithm, similar to the k nearest neighbours; while RBF and MLP are types of neural networks and are, thus, completely different from RF and SVM. Covering a different range of operating principles for the algorithms ensures an investigation of the data without bias caused by a single chosen algorithm. However, all the algorithms have been used in the literature for several biomedical studies, which shows their feasibility [68,69,70,71].
Since there were enough records in the dataset, the data were divided into training and test sets for the hold-out cross-validation, and the evaluation metrics were computed for both the regression and classification analyses. The coefficient of determination (R2), the mean absolute error and the root mean square error are used to evaluate the regression analysis, while, because of the high number of classes, the classification analysis is assessed with accuracy and presents the full confusion matrix.
3. Results
The dataset was divided into a training set and a test set (70% and 30% of the total, respectively) for the hold-out cross-validation. Then, a multiple linear regression analysis, RF, MLP, an RBF network and SVM were performed. The evaluation metrics on the test set are reported in Table 2 (multiple R2 are reported for all the tested models).

Table 2.
Evaluation metrics for the regression analysis of LOS, measured in days.
The regression coefficients and the t-test for the multiple linear regression are shown in Table 3.

Table 3.
Coefficients of the multiple linear regression model.
The obtained predictive model can be considered valid, although the coefficient of determination (R2 = 0.610) is not very high. The ML algorithms obtained sufficient but not excellent results with regard to the coefficient of determination. The best one was obtained through the implementation of SVM (R2 = 0.617), but the RBF network obtained similar scoring (R2 = 0.616). The mean absolute error was similar for all the algorithms: it ranged between 2.000 (obtained by SVM) and 2.109 (obtained by MLP) days.
A second ML analysis was conducted with the same algorithms, but by performing a classification of the LOS, measured in weeks. The dataset was divided again into training and test sets with the same proportions previously mentioned, and the results are shown in Table 4.

Table 4.
The accuracies and the best confusion matrix for the classification analysis of LOS, measured in weeks.
The numbers reported in the confusion matrix represent the numbers of weeks. This confusion matrix is presented in order to show how the patient LOS, measured in weeks, is predicted by the best algorithm, and what the wrong predictions are (for example, 12 times, a patient with a one-week LOS received the prediction of a two-week LOS).
All the algorithms exceeded an 80% accuracy, and the most accurate was the RBF network, at 83.5%. The confusion matrix shows that the model was able to correctly identify the number of weeks for the LOS.
Figure 2 shows the feature importance of the classification analysis computed on the RF that is based on the ratio between the number of splits performed through the considered feature, and the number of candidates for each level.
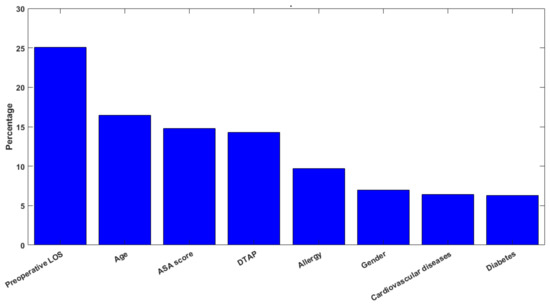
Figure 2.
Histogram describing the feature importance.
The most important feature was, of course, the preoperative LOS (25.1%), followed by the age (16.4%), the ASA score (14.8%) and the DTAP (14.3%). A comparison between the significance of the regression coefficients and the feature-importance ranking obtained by applying the machine learning models reveals that the most influencing factors, according to the RF algorithm, are the preoperative LOS and the age, which only partially overlaps the significance of the regression coefficients. Indeed, the preoperative LOS showed itself to be a significant predictor in both the multiple regression and machine learning models, while cardiovascular diseases assumed a higher significance as a predictor of the LOS in the regression analysis, rather than in the machine learning models. Such results suggest that the interpretation of the predictive models of the healthcare process should be carried out cautiously and in view of the value and effect of the chosen predictors used in the models. Indeed, the comparison of the predictors’ relevance in the examined regression and classification models is an essential part in the assessment of the validity of the findings, and it should be the guidance to achieving reasonable and interpretable results when dealing with predictive algorithms in the healthcare context.
4. Discussion and Conclusions
In this paper, multiple linear regression and several ML algorithms were used to provide a model that is able to predict the LOS (measured in days) of patients hospitalized for femur fracture at the A. Cardarelli Hospital of Naples. Moreover, ML algorithms were applied to classify the LOS, grouped by weeks. In addition, the analyses have provided indications of the variables that most influence the LOS, and they agree with the results that have been obtained in the literature with regard to the application of DTAPs [54,55]. The feature importance, which is represented in Figure 2, also confirms what the literature has expressed over the years. First, reducing the preoperative LOS has a preeminent role in the overall hospital stay because, as proven by previous studies [4,5], patients undergoing surgery within 48 h from admission to the hospital experience lower complications and, consequently, a reduced LOS. Furthermore, despite being in fourth place in feature importance, the presence of the DTAP on patients who are affected by a fracture of the femur provides the classification of the LOS with almost the same contribution from the age and the ASA score. Indeed, this is another confirmation of the literature because Improta et al. and Ricciardi et al. show that the use of a DTAP was useful to decrease the preoperative and overall LOS [54,55].
The errors of the classification ML analysis were mostly just one week. The misclassified records were those patients with the highest number of weeks for the LOS because the number of cases in the dataset was not large enough to train and test the models.
Although this paper focuses only on the LOS, it holds technical value as it applies a wide range of ML algorithms that have not been previously investigated, such as RF, the RBF network, MLP and SVM, since previous researchers have employed only a naïve Bayes model [48,49,50]. The study achieved good results, with accuracies beyond 80% in the classification of the LOS. The aim of this research was to improve the models and to change the perspective. In the first analysis, although it was a hard task, the regression performed punctual predictions since the LOS was measured in days, with discrete results (the R2 was greater than 0.60). In the second analysis, the LOS was grouped into four classes by week, and a harder prediction had to be performed (in previous research, it was grouped into “1–2 days”, “3–5 days” and “6+ days” [48,49]), but the results were good again.
A limitation of this work could be the presence of a clinical pathway (DTAP) that was specific to the hospital of Naples, which would not allow the models to be used in many other hospitals. Indeed, regarding the possibility of importing this path into other facilities, it must be mentioned that the distributed pavilion-based structure of the facility is an intrinsic characteristic of A. Cardarelli that cannot be found in all hospitals; therefore, the DTAP could be useful for hospitals that are organized and structured in similar ways. An example of an analogous pathway is reported in [72], where the authors discuss the implementation of a DTAP for patients with femur fracture in the Italian hospital, “San Giovanni di Dio e Ruggi d’Aragona”, of Salerno. Some similarities can be found between the two DTAPs, such as the presence of a protocol for the rapid transfer of the patient from the emergency department to the orthopaedic ward, the timely multiprofessional (orthopaedic, internal medicine, anaesthesiology and nursing) assessment and, finally, the early rehabilitation care.
Nevertheless, this clinical pathway has earned trust from health policy and it has also been discussed in other Italian regions. The collection of more variables, rather than more patients or the testing of different algorithms, is fundamental in order to obtain further improvements to these models. Although the models that were obtained are not perfectly accurate predictors of the LOS, the obtained results are promising, and since the selected predictors are readily available in the patients’ clinical records, and since the adopted algorithms do not require time-consuming procedures or dedicated hardware, the proposed methodology may be applicable in other settings, and may potentially represent a support tool for the management of department resources and workflows.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bioengineering9040172/s1: Tables S1–S8 and Figures S1–S17, which report the methodological details of the multiple linear regression model, are available as Supplementary Materials.
Author Contributions
Conceptualization, A.B., M.M., G.R. (Gaetano Romano), G.R. (Giuseppe Russo), M.T. and G.I.; data curation, A.B., M.M., G.R. (Gaetano Romano) and G.R. (Giuseppe Russo); formal analysis, C.R., A.M.P. and A.S.; investigation, C.R., A.M.P. and G.I.; methodology, C.R., A.M.P. and A.S.; resources, A.B., M.M., G.R. (Gaetano Romano) and G.R. (Giuseppe Russo); supervision, M.T. and G.I.; validation, M.T. and G.I.; visualization, C.R., A.M.P. and A.S.; writing–original draft, C.R., A.M.P. and A.S.; writing–review and editing, C.R., A.M.P., A.S., A.B., M.M., G.R. (Gaetano Romano), G.R. (Giuseppe Russo), M.T. and G.I. The authors, C.R. and A.M.P., contributed equally to the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board
In view of the retrospective nature of the study, since all the procedures that were performed were part of routine care, all the collected data were anonymized and no information was linked or is linkable to a specific person, no ethical approval was asked of the Ethics Committee.
Informed Consent Statement
No applicable.
Data Availability Statement
The data cannot be made available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rossini, M.; Piscitelli, P.; Fitto, F.; Camboa, P.; Angeli, A.; Guida, G.; Adami, S. Incidence and Socioeconomic Burden of Hip Fractures in Italy. Reumatismo 2005, 57, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Yeom, J.-W.; Song, H.K.; Hwang, K.-T.; Hwang, J.-H.; Yoo, J.-H. Lateral Locked Plating for Distal Femur Fractures by Low-Energy Trauma: What Makes a Difference in Healing? Int. Orthop. 2018, 42, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Hip Fracture: Evidence Update March 2013: A Summary of Selected New Evidence Relevant to NICE Clinical Guideline 124 ‘The Management of Hip Fracture in Adults’ (2011); National Institute for Health and Clinical Excellence (UK): London, UK, 2013.
- Colais, P.; Di Martino, M.; Fusco, D.; Perucci, C.A.; Davoli, M. The Effect of Early Surgery after Hip Fracture on 1-Year Mortality. BMC Geriatr. 2015, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, A.M.; Gromov, K.; Palm, H.; Brix, M.; Kallemose, T.; Troelsen, A. Danish Fracture Database Collaborators Time to Surgery Is Associated with Thirty-Day and Ninety-Day Mortality After Proximal Femoral Fracture: A Retrospective Observational Study on Prospectively Collected Data from the Danish Fracture Database Collaborators. J. Bone Joint Surg. Am. 2015, 97, 1333–1339. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Scotti, E. Internal Medicine Network: A New Way of Thinking Hospital-Territory Integration and Public-Private Partnership. Ital. J. Med. 2017, 11, 85–94. [Google Scholar] [CrossRef]
- Schrijvers, G.; van Hoorn, A.; Huiskes, N. The Care Pathway: Concepts and Theories: An Introduction. Int. J. Integr. Care 2012, 12, e192. [Google Scholar] [CrossRef]
- Zhang, Y.; Padman, R.; Patel, N. Paving the COWpath: Learning and Visualizing Clinical Pathways from Electronic Health Record Data. J. Biomed. Inform. 2015, 58, 186–197. [Google Scholar] [CrossRef]
- Carradori, T.; Bravi, F.; Butera, D.S.; Iannazzo, E.; Valpiani, G.; Wienand, U. Continuity of care in oncology. Quantitative analysis of data from patients treated in two different settings in Emilia—Romagna. Recenti Prog. Med. 2017, 108, 288–293. [Google Scholar] [CrossRef]
- Antony, J.; Forthun, S.C.; Trakulsunti, Y.; Farrington, T.; McFarlane, J.; Brennan, A.; Dempsey, M. An Exploratory Study into the Use of Lean Six Sigma to Reduce Medication Errors in the Norwegian Public Healthcare Context. Leadersh. Health Serv. Bradf. Engl. 2019, 32, 509–524. [Google Scholar] [CrossRef]
- Akifuddin, S.; Khatoon, F. Reduction of Complications of Local Anaesthesia in Dental Healthcare Setups by Application of the Six Sigma Methodology: A Statistical Quality Improvement Technique. J. Clin. Diagn. Res. JCDR 2015, 9, ZC34–ZC38. [Google Scholar] [CrossRef]
- Arafeh, M.; Barghash, M.A.; Haddad, N.; Musharbash, N.; Nashawati, D.; Al-Bashir, A.; Assaf, F. Using Six Sigma DMAIC Methodology and Discrete Event Simulation to Reduce Patient Discharge Time in King Hussein Cancer Center. J. Healthc. Eng. 2018, 2018, 3832151. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, C.; Sorrentino, A.; Improta, G.; Abbate, V.; Latessa, I.; Perrone, A.; Triassi, M.; Dell’aversana Orabona, G. A Health Technology Assessment between Two Pharmacological Therapies through Six Sigma: The Case Study of Bone Cancer. TQM J. 2020, 32. ahead-of-print. [Google Scholar] [CrossRef]
- Ponsiglione, A.M.; Ricciardi, C.; Improta, G.; Orabona, G.D.; Sorrentino, A.; Amato, F.; Romano, M. A Six Sigma DMAIC Methodology as a Support Tool for Health Technology Assessment of Two Antibiotics. Math. Biosci. Eng. 2021, 18, 3469–3490. [Google Scholar] [CrossRef] [PubMed]
- Tesfahun, E.; Kumie, A.; Beyene, A. Developing Models for the Prediction of Hospital Healthcare Waste Generation Rate. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2016, 34, 75–80. [Google Scholar] [CrossRef]
- Chatterjee, K.; Chatterjee, K.; Kumar, A.; Shankar, S. Healthcare Impact of COVID-19 Epidemic in India: A Stochastic Mathematical Model. Med. J. Armed Forces India 2020, 76, 147–155. [Google Scholar] [CrossRef]
- Gingras, G.; Guertin, M.-H.; Laprise, J.-F.; Drolet, M.; Brisson, M. Mathematical Modeling of the Transmission Dynamics of Clostridium Difficile Infection and Colonization in Healthcare Settings: A Systematic Review. PLoS ONE 2016, 11, e0163880. [Google Scholar] [CrossRef]
- Arianti, M.D.; Prijambodo, J.; Wujoso, H. Relationships between Age, Sex, Laboratory Parameter, and Length of Stay in Patients with Dengue Hemorrhagic Fever. J. Epidemiol. Public Health 2019, 4, 307–313. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Su, H.-H.; Tsai, Y.-W.; Hou, Y.-Y.; Chang, K.-P.; Chi, C.-C.; Lin, M.-Y.; Wu, P.-H. Initial Factors Influencing Duration of Hospital Stay in Adult Patients with Peritonsillar Abscess. Clin. Exp. Otorhinolaryngol. 2017, 10, 115–120. [Google Scholar] [CrossRef][Green Version]
- Trunfio, T.A.; Scala, A.; Vecchia, A.D.; Marra, A.; Borrelli, A. Multiple Regression Model to Predict Length of Hospital Stay for Patients Undergoing Femur Fracture Surgery at “San Giovanni Di Dio e Ruggi d’Aragona” University Hospital. In Proceedings of the 8th European Medical and Biological Engineering Conference, Portorož, Slovenia, 29 November–3 December 2020; Jarm, T., Cvetkoska, A., Mahnič-Kalamiza, S., Miklavcic, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 840–847. [Google Scholar]
- Bhardwaj, R.; Nambiar, A.R.; Dutta, D. A Study of Machine Learning in Healthcare. In Proceedings of the 2017 IEEE 41st Annual Computer Software and Applications Conference (COMPSAC), Turin, Italy, 4–8 July 2017; Volume 2, pp. 236–241. [Google Scholar]
- Improta, G.; Ponsiglione, A.M.; Parente, G.; Romano, M.; Cesarelli, G.; Rea, T.; Russo, M.; Triassi, M. Evaluation of Medical Training Courses Satisfaction: Qualitative Analysis and Analytic Hierarchy Process. In Proceedings of the 8th European Medical and Biological Engineering Conference, Portorož, Slovenia, 29 November–3 December 2020; Jarm, T., Cvetkoska, A., Mahnič-Kalamiza, S., Miklavcic, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 518–526. [Google Scholar]
- Wiens, J.; Shenoy, E.S. Machine Learning for Healthcare: On the Verge of a Major Shift in Healthcare Epidemiology. Clin. Infect. Dis. 2018, 66, 149–153. [Google Scholar] [CrossRef]
- Donisi, L.; Cesarelli, G.; Balbi, P.; Provitera, V.; Lanzillo, B.; Coccia, A.; D’Addio, G. Positive Impact of Short-Term Gait Rehabilitation in Parkinson Patients: A Combined Approach Based on Statistics and Machine Learning. Math. Biosci. Eng. MBE 2021, 18, 6995–7009. [Google Scholar] [CrossRef]
- Chowriappa, P.; Dua, S.; Todorov, Y. Introduction to Machine Learning in Healthcare Informatics. In Machine Learning in Healthcare Informatics; Dua, S., Acharya, U.R., Dua, P., Eds.; Intelligent Systems Reference Library; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–23. ISBN 978-3-642-40017-9. [Google Scholar]
- Ricciardi, C.; Ponsiglione, A.M.; Converso, G.; Santalucia, I.; Triassi, M.; Improta, G. Implementation and Validation of a New Method to Model Voluntary Departures from Emergency Departments. Math. Biosci. Eng. 2021, 18, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, C.; Jónsson, H.; Jacob, D.; Improta, G.; Recenti, M.; Gíslason, M.K.; Cesarelli, G.; Esposito, L.; Minutolo, V.; Bifulco, P.; et al. Improving Prosthetic Selection and Predicting BMD from Biometric Measurements in Patients Receiving Total Hip Arthroplasty. Diagnostics 2020, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Scrutinio, D.; Ricciardi, C.; Donisi, L.; Losavio, E.; Battista, P.; Guida, P.; Cesarelli, M.; Pagano, G.; D’Addio, G. Machine Learning to Predict Mortality after Rehabilitation among Patients with Severe Stroke. Sci. Rep. 2020, 10, 20127. [Google Scholar] [CrossRef]
- Ricciardi, C.; Valente, A.S.; Edmund, K.; Cantoni, V.; Green, R.; Fiorillo, A.; Picone, I.; Santini, S.; Cesarelli, M. Linear Discriminant Analysis and Principal Component Analysis to Predict Coronary Artery Disease. Health Inform. J. 2020, 26, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Ponsiglione, A.M.; Cosentino, C.; Cesarelli, G.; Amato, F.; Romano, M. A Comprehensive Review of Techniques for Processing and Analyzing Fetal Heart Rate Signals. Sensors 2021, 21, 6136. [Google Scholar] [CrossRef]
- Fiscon, G.; Weitschek, E.; Cialini, A.; Felici, G.; Bertolazzi, P.; De Salvo, S.; Bramanti, A.; Bramanti, P.; De Cola, M.C. Combining EEG Signal Processing with Supervised Methods for Alzheimer’s Patients Classification. BMC Med. Inform. Decis. Mak. 2018, 18, 35. [Google Scholar] [CrossRef]
- Fiscon, G.; Weitschek, E.; Felici, G.; Bertolazzi, P.; De Salvo, S.; Bramanti, P.; De Cola, M.C. Alzheimer’s Disease Patients Classification through EEG Signals Processing. In Proceedings of the 2014 IEEE Symposium on Computational Intelligence and Data Mining (CIDM), Orlando, FL, USA, 9–12 December 2014; pp. 105–112. [Google Scholar]
- Ponsiglione, A.M.; Romano, M.; Amato, F. A Finite-State Machine Approach to Study Patients Dropout from Medical Examinations. In Proceedings of the 2021 IEEE 6th International Forum on Research and Technology for Society and Industry (RTSI), Naples, Italy, 6–9 September 2021; pp. 289–294. [Google Scholar]
- Ricciardi, C.; Cuocolo, R.; Cesarelli, G.; Ugga, L.; Improta, G.; Solari, D.; Romeo, V.; Guadagno, E.; Maria, C.L.; Cesarelli, M. Distinguishing Functional from Non-Functional Pituitary Macroadenomas with a Machine Learning Analysis. In XV Mediterranean Conference on Medical and Biological Engineering and Computing—MEDICON 2019, Proceedings of MEDICON 2019, Coimbra, Portugal, 26–28 September 2019; Henriques, J., de Carvalho, P., Neves, N., Eds.; Springer: Cham, Switzerland, 2020; Volume 76, pp. 1822–1829. [Google Scholar]
- Ricciardi, C.; Cantoni, V.; Green, R.; Improta, G.; Cesarelli, M. Is It Possible to Predict Cardiac Death? Springer: Cham, Switzerland, 2020; Volume 76, ISBN 978-3-030-31635-8. [Google Scholar]
- Ricciardi, C.; Improta, G.; Amato, F.; Cesarelli, G.; Romano, M. Classifying the Type of Delivery from Cardiotocographic Signals: A Machine Learning Approach. Comput. Methods Programs Biomed. 2020, 196, 105712. [Google Scholar] [CrossRef]
- Ponsiglione, A.M.; Amato, F.; Romano, M. Multiparametric Investigation of Dynamics in Fetal Heart Rate Signals. Bioengineering 2022, 9, 8. [Google Scholar] [CrossRef]
- Mena, L.J.; Orozco, E.E.; Felix, V.G.; Ostos, R.; Melgarejo, J.; Maestre, G.E. Machine Learning Approach to Extract Diagnostic and Prognostic Thresholds: Application in Prognosis of Cardiovascular Mortality. Comput. Math. Methods Med. 2012, 2012, e750151. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Bonaccio, M.; Costanzo, S.; Gialluisi, A.; Antinori, A.; Berselli, N.; Blandi, L.; Bruno, R.; Cauda, R.; Guaraldi, G.; et al. Common Cardiovascular Risk Factors and In-Hospital Mortality in 3894 Patients with COVID-19: Survival Analysis and Machine Learning-Based Findings from the Multicentre Italian CORIST Study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1899–1913. [Google Scholar] [CrossRef]
- Rajliwall, N.S.; Davey, R.; Chetty, G. Machine Learning Based Models for Cardiovascular Risk Prediction. In Proceedings of the 2018 International Conference on Machine Learning and Data Engineering (iCMLDE), Sydney, NSW, Australia, 3–7 December 2018; pp. 142–148. [Google Scholar]
- Sánchez-Cabo, F.; Rossello, X.; Fuster, V.; Benito, F.; Manzano, J.P.; Silla, J.C.; Fernández-Alvira, J.M.; Oliva, B.; Fernández-Friera, L.; López-Melgar, B.; et al. Machine Learning Improves Cardiovascular Risk Definition for Young, Asymptomatic Individuals. J. Am. Coll. Cardiol. 2020, 76, 1674–1685. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Li, S.; Zhang, J.; Jiang, D.; Li, X.; Li, Y.; Du, J. A Machine Learning-Based Prediction Model for Cardiovascular Risk in Women with Preeclampsia. Front. Cardiovasc. Med. 2021, 8, 736491. [Google Scholar] [CrossRef] [PubMed]
- Ponsiglione, A.M.; Cesarelli, G.; Amato, F.; Romano, M. Optimization of an Artificial Neural Network to Study Accelerations of Foetal Heart Rhythm. In Proceedings of the 2021 IEEE 6th International Forum on Research and Technology for Society and Industry (RTSI), Naples, Italy, 6–9 September 2021; pp. 159–164. [Google Scholar]
- Cabitza, F.; Locoro, A.; Banfi, G. Machine Learning in Orthopedics: A Literature Review. Front. Bioeng. Biotechnol. 2018, 6, 75. [Google Scholar] [CrossRef]
- Ramkumar, P.N.; Haeberle, H.S.; Bloomfield, M.R.; Schaffer, J.L.; Kamath, A.F.; Patterson, B.M.; Krebs, V.E. Artificial Intelligence and Arthroplasty at a Single Institution: Real-World Applications of Machine Learning to Big Data, Value-Based Care, Mobile Health, and Remote Patient Monitoring. J. Arthroplasty 2019, 34, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.M.; Hua, N.; Zhang, E.; Robinson, R.; Spyker, A.; Armstrong, D.; Whittaker, R.; Robinson, T.; Ullah, E. A Machine Learning Model for Predicting Risk of Hospital Readmission within 30 Days of Discharge: Validated with LACE Index and Patient at Risk of Hospital Readmission (PARR) Model. Med. Biol. Eng. Comput. 2020, 58, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Dogu, E.; Albayrak, Y.E.; Tuncay, E. Length of Hospital Stay Prediction with an Integrated Approach of Statistical-Based Fuzzy Cognitive Maps and Artificial Neural Networks. Med. Biol. Eng. Comput. 2021, 59, 483–496. [Google Scholar] [CrossRef]
- Ramkumar, P.N.; Navarro, S.M.; Haeberle, H.S.; Karnuta, J.M.; Mont, M.A.; Iannotti, J.P.; Patterson, B.M.; Krebs, V.E. Development and Validation of a Machine Learning Algorithm After Primary Total Hip Arthroplasty: Applications to Length of Stay and Payment Models. J. Arthroplasty 2019, 34, 632–637. [Google Scholar] [CrossRef]
- Navarro, S.M.; Wang, E.Y.; Haeberle, H.S.; Mont, M.A.; Krebs, V.E.; Patterson, B.M.; Ramkumar, P.N. Machine Learning and Primary Total Knee Arthroplasty: Patient Forecasting for a Patient-Specific Payment Model. J. Arthroplasty 2018, 33, 3617–3623. [Google Scholar] [CrossRef]
- Karnuta, J.M.; Navarro, S.M.; Haeberle, H.S.; Billow, D.G.; Krebs, V.E.; Ramkumar, P.N. Bundled Care for Hip Fractures: A Machine-Learning Approach to an Untenable Patient-Specific Payment Model. J. Orthop. Trauma 2019, 33, 324–330. [Google Scholar] [CrossRef]
- Ramkumar, P.N.; Karnuta, J.M.; Navarro, S.M.; Haeberle, H.S.; Scuderi, G.R.; Mont, M.A.; Krebs, V.E.; Patterson, B.M. Deep Learning Preoperatively Predicts Value Metrics for Primary Total Knee Arthroplasty: Development and Validation of an Artificial Neural Network Model. J. Arthroplast. 2019, 34, 2220–2227.e1. [Google Scholar] [CrossRef]
- Dogu, E.; Albayrak, Y.E. Criteria Evaluation for Pricing Decisions in Strategic Marketing Management Using an Intuitionistic Cognitive Map Approach. Soft Comput. 2018, 22, 4989–5005. [Google Scholar] [CrossRef]
- Dogu, E.; Albayrak, Y.E.; Tuncay, E. An Integrated Decision Support System for Hospital Management: Statistical-Based Fuzzy Cognitive Maps. J. Mult. Valued Log Soft Comput 2020, 34, 527–552. [Google Scholar]
- Ricciardi, C.; Fiorillo, A.; Valente, A.S.; Borrelli, A.; Verdoliva, C.; Triassi, M.; Improta, G. Lean Six Sigma Approach to Reduce LOS through a Diagnostic-Therapeutic-Assistance Path at A.O.R.N. A. Cardarelli. TQM J. 2019, 31, 657–672. [Google Scholar] [CrossRef]
- Improta, G.; Ricciardi, C.; Borrelli, A.; D’Alessandro, A.; Verdoliva, C.; Cesarelli, M. The Application of Six Sigma to Reduce the Pre-Operative Length of Hospital Stay at the Hospital Antonio Cardarelli. Int. J. Lean Six Sigma Print 2019, 11, 555–576. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE) Hip Fracture: Management. Clinical Guideline CG124; National Institute for Health and Care Excellence: London, UK, 2011. [Google Scholar]
- Azienda Ospedaliera di Rilievo Nazionale Antonio Cardarelli di Napoli Percorso Assistenziale per Fratture Di Femore Nel Paziente Anziano. Available online: http://www.ospedalecardarelli.it/doc/1092/137431/DOCUMENT_FILE_137431.pdf (accessed on 12 January 2021).
- Frattura Del Collo Del Femore: Proporzione Di Interventi Chirurgici Entro 2 Giorni. Available online: https://www.sanita24.ilsole24ore.com/pdf2010/Editrice/ILSOLE24ORE/QUOTIDIANO_SANITA/Online/_Oggetti_Correlati/Documenti/2015/11/18/femore.pdf?uuid=ACmVZobB (accessed on 12 January 2021).
- Tiryaki, S.; Aydın, A. An Artificial Neural Network Model for Predicting Compression Strength of Heat-Treated Woods and Comparison with a Multiple Linear Regression Model. Constr. Build. Mater. 2014, 62, 102–108. [Google Scholar] [CrossRef]
- Williams, M.; Grajales, C.; Kurkiewicz, D. Assumptions of Multiple Regression: Correcting Two Misconceptions. Pract. Assess. Res. Eval. 2019, 18, 11. [Google Scholar] [CrossRef]
- Recenti, M.; Ricciardi, C.; Gìslason, M.; Edmunds, K.; Carraro, U.; Gargiulo, P. Machine Learning Algorithms Predict Body Mass Index Using Nonlinear Trimodal Regression Analysis from Computed Tomography Scans. In Proceedings of the XV Mediterranean Conference on Medical and Biological Engineering and Computing—MEDICON, Coimbra, Portugal, 26–28 September 2019; Henriques, J., Neves, N., de Carvalho, P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 839–846. [Google Scholar]
- Liaw, A.; Wiener, M.C. Classification and Regression by Random Forest. R news. 2002, 2, 18–22. [Google Scholar]
- Broomhead, D.S.; Lowe, D. Radial Basis Functions, Multi-Variable Functional Interpolation and Adaptive Networks; Royal Signals and Radar Establishment Malvern: Malvern, UK, 2018. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, Second Edition; Springer Series in Statistics; Springer: New York, NY, USA, 2009; ISBN 978-0-387-84857-0. [Google Scholar]
- Suykens, J.A.K.; Vandewalle, J. Least Squares Support Vector Machine Classifiers. Neural Process. Lett. 1999, 9, 293–300. [Google Scholar] [CrossRef]
- Anguita, D.; Ghio, A.; Greco, N.; Oneto, L.; Ridella, S. Model Selection for Support Vector Machines: Advantages and Disadvantages of the Machine Learning Theory. In Proceedings of the 2010 International Joint Conference on Neural Networks (IJCNN), Barcelona, Spain, 18–23 July 2010; pp. 1–8. [Google Scholar]
- Mierswa, I. Controlling Overfitting with Multi-Objective Support Vector Machines. In Proceedings of the 9th Annual Conference on Genetic and Evolutionary Computation, London, UK, 7–11 July 2007; Association for Computing Machinery: New York, NY, USA, 2007; pp. 1830–1837. [Google Scholar]
- D’Addio, G.; Ricciardi, C.; Improta, G.; Bifulco, P.; Cesarelli, M. Feasibility of Machine Learning in Predicting Features Related to Congenital Nystagmus; Springer: Cham, Switzerland, 2020; Volume 76, ISBN 978-3-030-31635-8. [Google Scholar]
- Romeo, V.; Cuocolo, R.; Ricciardi, C.; Ugga, L.; Cocozza, S.; Verde, F.; Stanzione, A.; Napolitano, V.; Russo, D.; Improta, G.; et al. Prediction of Tumor Grade and Nodal Status in Oropharyngeal and Oral Cavity Squamous-Cell Carcinoma Using a Radiomic Approach. ANTICANCER Res. 2020, 40, 271–280. [Google Scholar] [CrossRef]
- Nayyar, A.; Gadhavi, L.; Zaman, N. Chapter 2—Machine Learning in Healthcare: Review, Opportunities and Challenges. In Machine Learning and the Internet of Medical Things in Healthcare; Singh, K.K., Elhoseny, M., Singh, A., Elngar, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 23–45. ISBN 978-0-12-821229-5. [Google Scholar]
- Shailaja, K.; Seetharamulu, B.; Jabbar, M.A. Machine Learning in Healthcare: A Review. In Proceedings of the 2018 Second International Conference on Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 29–31 March 2018; pp. 910–914. [Google Scholar]
- Scala, A.; Ponsiglione, A.M.; Loperto, I.; Della Vecchia, A.; Borrelli, A.; Russo, G.; Triassi, M.; Improta, G. Lean Six Sigma Approach for Reducing Length of Hospital Stay for Patients with Femur Fracture in a University Hospital. Int. J. Environ. Res. Public. Health 2021, 18, 2843. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).