In Vitro Cancer Models: A Closer Look at Limitations on Translation
Abstract
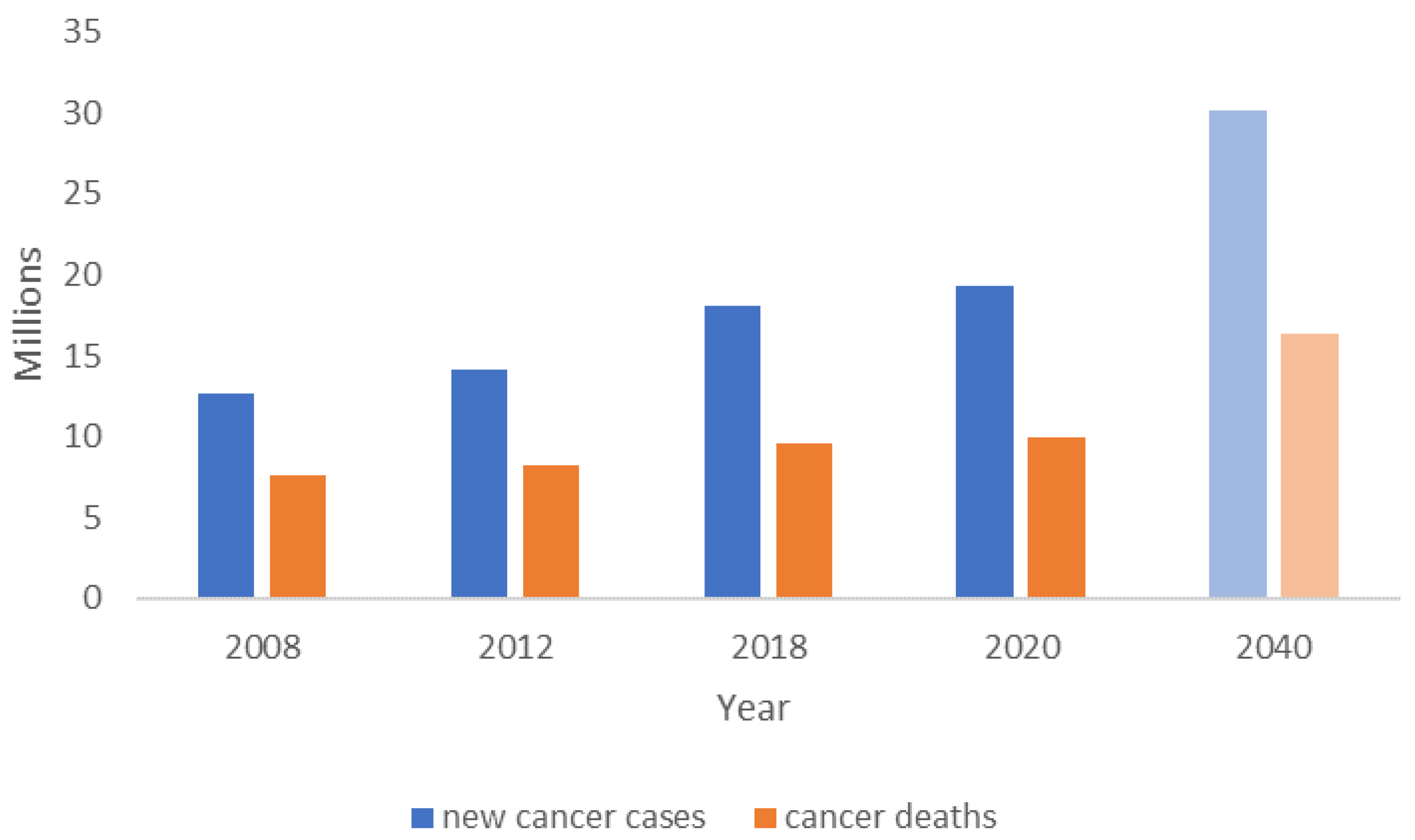
1. Introduction
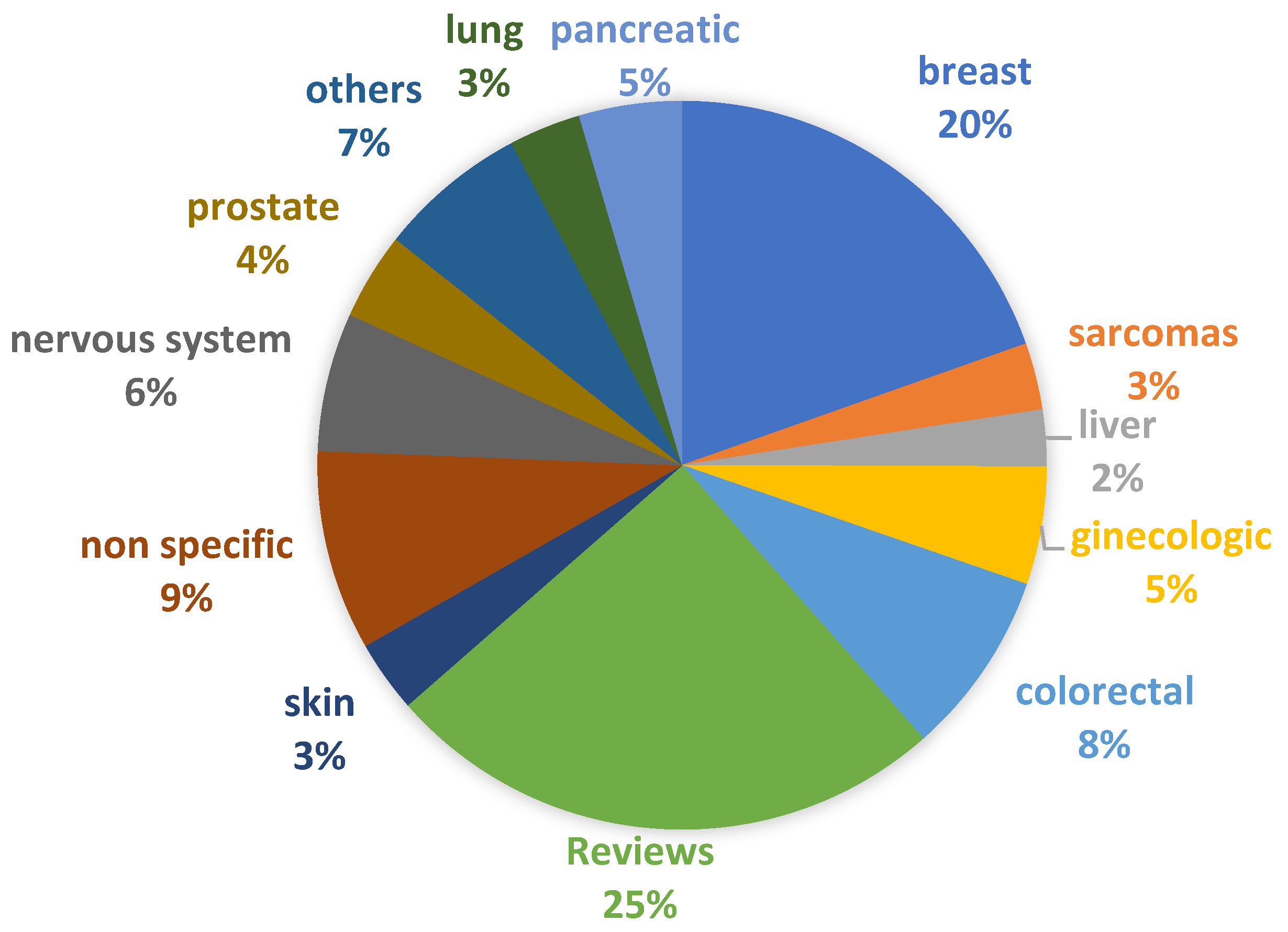
2. 3D Cancer Models: Product Segments, Commercial Tools, Prototypes, and Patents
2.1. Surfaces and 3D Culture Plates
2.2. Scaffolds/Matrices
2.3. Patient-Derived and Cell Line-Based Assays/Services, Prototypes
2.4. Microfluidic Platforms
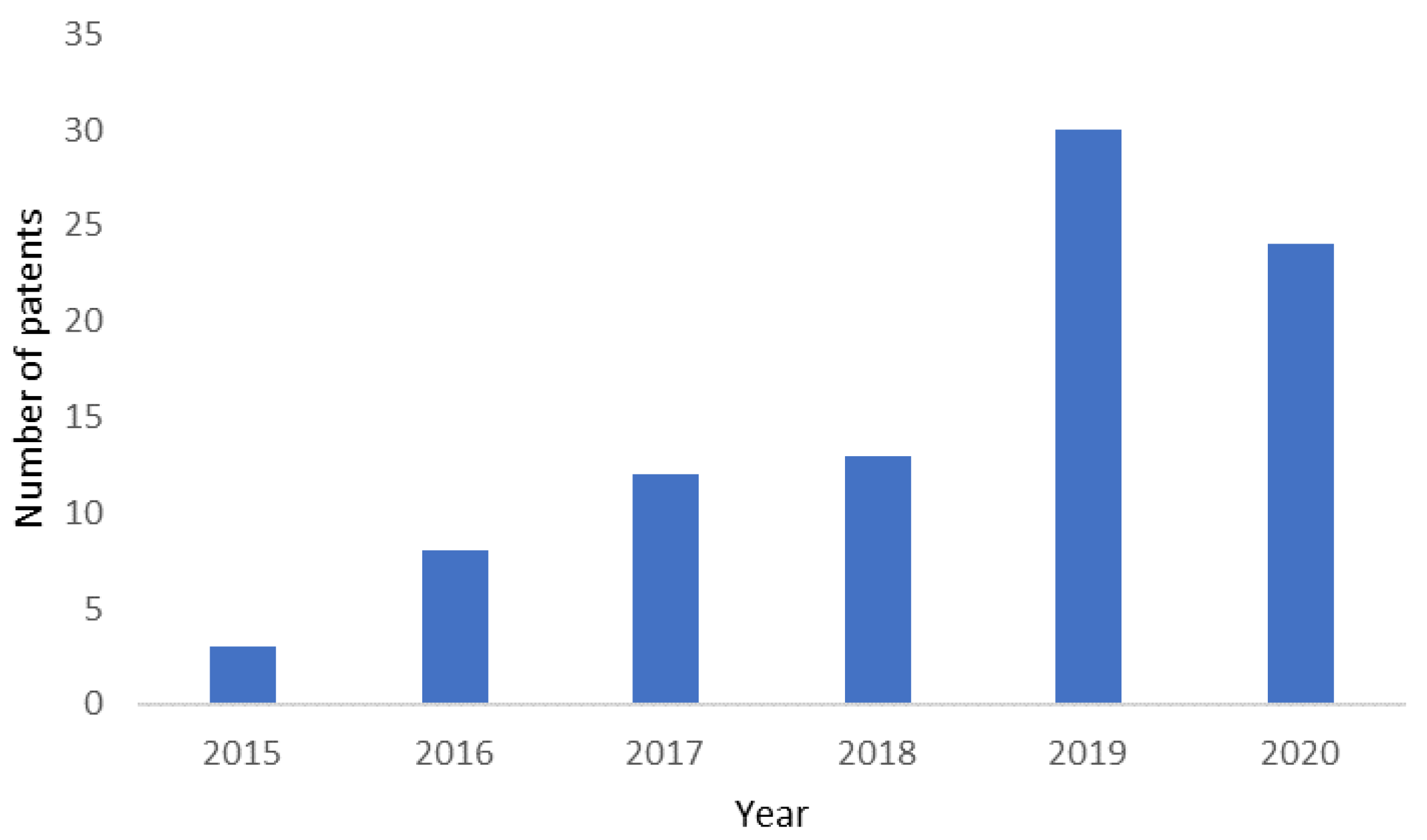
2.5. In Vitro Cancer Models: Patents
3. Gap Analysis: Limitations and Challenges of Existing Models
- The hierarchical heterogeneous structure of cancer results in phenotypic and genotypic diversities among the subpopulations of cancer cells. They are not possible to recapitulate in clinical models to date. The reductionist approaches to cancer modelling and the anti-systematic method of therapeutic screening are potent clinical failure recipes [93].
- There are differences between the biology of the model system and the context of the human body. For instance, tumors generally grow faster in laboratory animals or in vitro models than in humans [94].
- The discrepancy between site and stage of the disease in the preclinical model; for example, the subcutaneous tumor xenografts do not mimic the location and setting of the patient’s tumor. Therefore, the experimental therapeutic molecule fails to elicit the desired response at the pre-validated dose concentration [95].
- The inherited constraint of mimicking the advanced disease stage using commonly available cell lines, using more aggressive metastatic variants, such as MDA-MB 231/LM2-4 (triple-negative breast cancer cell line of human into immunodeficient mice (SCID)), to screen the FDA approved anticancer therapeutic Sunitinib, as the therapeutic for advanced metastatic breast cancer, also fails to elicit any response in mono or combination therapy [96].
- The introduction of immune therapy offers a logical approach to overcome the limitations mentioned above and exhibits promising results in treating breast, melanoma, urogenital or non-small cell lung cancers [97]. For instance, Keytruda is a humanized antibody that has received FDA approval as an immune therapeutic agent in the treatment of melanoma, head and neck cancer, and lung cancer patients [98]. However, in these success stories, little consideration is paid to the systematic or local compensatory immune–non-immune response mechanism, the cellular immune composition of site-directed tissues, the oxidation-reduction profile against checkpoint inhibitions, host immune–non-immune response, and adverse side-effects [99,100,101,102,103,104,105]. The systematic insight investigation of the mechanisms of these interdependent pathways and acute inflammatory and effective immune responses must be considered for more effective cancer immune therapy.
- A closer examination of detailed data spanning several decades reveals that persistent injuries, chronic infections, or inflammations cause genetic changes at site-specific tissues, increasing the risk of cancer, particularly in the elderly [102].

4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melo, F.D.S.E.; Vermeulen, L.; Fessler, E.; Medema, J.P. Cancer heterogeneity—A multifaceted view. EMBO Rep. 2013, 14, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?types=0&single_unit=500000 (accessed on 11 May 2021).
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Jackson, S.J.; Thomas, G.J. Human tissue models in cancer research: Looking beyond the mouse. Dis. Model. Mech. 2017, 10, 939–942. [Google Scholar] [CrossRef]
- Rodrigues, T.; Kundu, B.; Silva-Correia, J.; Kundu, S.C.; Oliveira, J.M.; Reis, R.L.; Correlo, V.M. Emerging tumor spheroids technologies for 3D in vitro cancer modeling. Pharmacol. Ther. 2018, 184, 201–211. [Google Scholar] [CrossRef]
- Paget, S. The Distribution of Secondary Growths in Cancer of The Breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- About|Predect. Available online: http://www.predect.eu/about/ (accessed on 28 October 2020).
- Rothbauer, M.; Rosser, J.M.; Zirath, H.; Ertl, P. Tomorrow today: Organ-on-a-chip advances towards clinically relevant pharmaceutical and medical in vitro models. Curr. Opin. Biotechnol. 2019, 55, 81–86. [Google Scholar] [CrossRef] [PubMed]
- FDA D.I.S.C.O. Burst Edition: FDA Approvals of Brukinsa (Zanubrutinib), for Adult Patients with Relapsed or Refractory Marginal Zone Lymphoma, and Exkivity (Mobocertinib) for Adult Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor Exon 20 Insertion Mutations|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approvals-brukinsa-zanubrutinib-adult-patients-relapsed-or-refractory (accessed on 13 February 2022).
- Liu, Z.; Liu, J.; Zhang, T.; Li, L.; Zhang, S.; Jia, H.; Xia, Y.; Shi, M.; Zhang, J.; Yue, S.; et al. Distinct BTK inhibitors differentially induce apoptosis but similarly suppress chemotaxis and lipid accumulation in mantle cell lymphoma. BMC Cancer 2021, 21, 732. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Vega, V.; Hou, S.; Plenker, D.; Tiriac, H.; Baillargeon, P.; Shumate, J.; Scampavia, L.; Seldin, J.; Souza, G.R.; Tuveson, D.A.; et al. Lead Identification using 3D Models of Pancreatic Cancer. SLAS Discov. 2022, 27, 159–166. [Google Scholar] [CrossRef]
- Human Cancer Models Initiative. Available online: https://www.lgcstandards-atcc.org/Products/CellsandMicroorganisms/HCMI.aspx?geo_country=pt (accessed on 20 May 2019).
- Ehrlich, P.; Apolant, H. Beobachtungen über Maligne Mäusetumoren; Antiquariat für Medizin—Fritz-Dieter Söhn: Berlin, Germany, 1905. [Google Scholar]
- 3D Cancer Spheroids|STEMCELL Technologies. Available online: https://www.stemcell.com/cancer-spheroids (accessed on 20 May 2019).
- 3D Cell Culture|3D Cell Culture Models|Corning. Available online: https://www.corning.com/cala/pt/products/life-sciences/applications/cell-culture/3D-cell-culture.html (accessed on 20 May 2019).
- 3D Cell Culture from Greiner Bio-One. Available online: https://3dcellculture.gbo.com/products/ (accessed on 20 May 2019).
- Lipidure-COAT Plates|Amsbio. Available online: http://www.amsbio.com/Lipidure-Coat.aspx (accessed on 20 May 2019).
- Alvetex 3D Cell Culture|Amsbio. Available online: http://www.amsbio.com/alvetex-3D-cell-culture.aspx (accessed on 20 May 2019).
- Biogelx|3D In-Vitro Tumor Models Are Changing Cancer Research. Available online: https://www.biogelx.com/3d-in-vitro-tumor-models-are-changing-cancer-research/ (accessed on 20 May 2019).
- Advanced BioMatrix—Advanced BioMatrix Manufacturers and Distributes High Quality Collagen Including PureCol® for Cell Culture, Gels, Coatings and Other Research Uses. Available online: https://www.advancedbiomatrix.com/ (accessed on 20 May 2019).
- HyStemTM Hyaluronic Acid Based Hydrogels for 3D Cell Culture Applications. Available online: https://www.sigmaaldrich.com/PT/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/3d-cell-culture/hystem-3d-hydrogels (accessed on 13 February 2022).
- MaxGelTM ECM Mixture, Liquid|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/catalog/product/sigma/e0282?lang=pt®ion=PT (accessed on 2 December 2020).
- TrueGel3DTM Hydrogel for 3D Cell Culture|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biology/truegel3d.html (accessed on 2 December 2020).
- MillicoatTM Human Collagen Type I Coated Strips (96-Wells)|ECM104. Available online: http://www.merckmillipore.com/PT/en/product/Millicoat-Human-Collagen-Type-I-Coated-Strips-96-Wells,MM_NF-ECM104?ReferrerURL=https%3A%2F%2Fwww.google.com%2F (accessed on 20 May 2019).
- MAPTrix HyGelTM Line of Products|Kollodis. Available online: https://www.kollodis.com/down/MAPTrixHyGelFlyer.pdf (accessed on 20 May 2019).
- PD3D® Models. Available online: https://www.cellphenomics.com/scientific-background/pd3dr-models/ (accessed on 20 May 2019).
- Products|InSphero. Available online: https://insphero.com/products/ (accessed on 20 May 2019).
- 3D Cell Culture with the OncoPanelTM Cell-Based Profiling Service. Available online: https://www.eurofinsdiscoveryservices.com/cms/cms-content/services/phenotypic-assays/oncology/oncopanel/oncopanel-3d/ (accessed on 20 May 2019).
- 3D Cancer ORGANDOT Model. Available online: https://www.bioivt.com/3d-cancer-organdot-model/ (accessed on 20 May 2019).
- KIYATEC. Available online: http://kiyatec.com/ (accessed on 20 May 2019).
- Patient-Derived Xenograft—PDX Models—CrownBio. Available online: https://www.crownbio.com/oncology/in-vivo-services/patient-derived-xenograft-pdx-tumor-models (accessed on 28 October 2020).
- Charles River Oncology. Available online: https://www.charlesriveroncology.com/ (accessed on 28 October 2020).
- Pharmalegacy-Pharmalegacy. Available online: http://www.pharmalegacy.com/index.html (accessed on 28 October 2020).
- Indivumed: Indivumed. Available online: https://www.indivumed.com/ (accessed on 28 October 2020).
- Repositive—Accelerating Preclinical Cancer Research. Available online: https://repositive.io/ (accessed on 28 October 2020).
- Halmai, N.B.; Carvajal-Carmona, L.G. Diversifying preclinical research tools: Expanding patient-derived models to address cancer health disparities. Trends Cancer 2022, 8, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Reis, R.L.; Kundu, S.C. Metastasis in 3D biomaterials. In Biomaterials for 3D Tumor Modelling; Kundu, S.C., Reis, R.L., Eds.; Elsevier Publications: London, UK, 2020; pp. 191–210. [Google Scholar]
- SynTumor 3D Cancer Model|SynVivo. Available online: https://www.synvivobio.com/syntumor/ (accessed on 20 May 2019).
- 2D Versus 3D Cell Cultures|Mimetas. Available online: https://mimetas.com/article/2d-versus-3d-cell-cultures (accessed on 20 May 2019).
- CellASIC® ONIX Microfluidic Platform—CellASIC ONIX Microfluidic System and Accessories. Available online: http://www.merckmillipore.com/PT/en/product/CellASIC-ONIX-Microfluidic-Platform,MM_NF-C117908?ReferrerURL=https%3A%2F%2Fwww.google.com%2F (accessed on 20 May 2019).
- Udrea, A. Market analysis Market Analysis Report. In Proceedings of the 2nd International Conference on Cancer Science and Cancer Therapy, Zurich, Switzerland, 28–29 September 2020; Volume 2, pp. 9–10. [Google Scholar]
- 3D Cell Culture Market Size and Share|Industry Growth, 2027. Available online: https://www.alliedmarketresearch.com/3d-cell-cultures-market (accessed on 16 September 2021).
- Bar-Shalom, A.; Cook-Deegan, R. Patents and Innovation in Cancer Therapeutics: Lessons from CellPro. Milbank Q. 2002, 80, 637–676. [Google Scholar] [CrossRef]
- Eisenberg, R.S. Public research and private development: Patents and technology transfer in government-sponsored research. Va. Law Rev. 1996, 82, 1663. [Google Scholar] [CrossRef]
- Kovarik, J.E. Cancer Moonshot: Patents for Patients. Trends Cancer 2018, 4, 515–516. [Google Scholar] [CrossRef]
- WIPO—Search International and National Patent Collections. Available online: https://patentscope.wipo.int/search/en/result.jsf (accessed on 13 February 2022).
- Chen, S.; Boda, S.K.; Batra, S.K.; Li, X.; Xie, J. Emerging Roles of Electrospun Nanofibers in Cancer Research. Adv. Healthc. Mater. 2018, 7, e1701024. [Google Scholar] [CrossRef]
- Mohammad-Hadi, L.; MacRobert, A.J.; Loizidou, M.; Yaghini, E. Photodynamic therapy in 3D cancer models and the utilisation of nanodelivery systems. Nanoscale 2018, 10, 1570–1581. [Google Scholar] [CrossRef]
- Holt, S.E.; Ward, E.S.; Ober, R.J.; Alge, D.L. Shooting for the moon: Using tissue-mimetic hydrogels to gain new insight on cancer biology and screen therapeutics. MRS Commun. 2017, 7, 427–441. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Park, K.Y.; Virumbrales-Muñoz, M.; Beebe, D.J. Toward improved in vitro models of human cancer. APL Bioeng. 2021, 5, 10902. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.; Heinrich, M.A.; Teixeira, L.M.; Prakash, J. 3D In Vitro Model (R)evolution: Unveiling Tumor–Stroma Interactions. Trends Cancer 2021, 7, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted cancer models: Revolutionizing personalized cancer therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fang, J.; Huang, S.; Wu, X.; Xie, X.; Wang, J.; Liu, F.; Zhang, M.; Peng, Z.; Hu, N. Tumor-on-a-chip: From bioinspired design to biomedical application. Microsyst. Nanoeng. 2021, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Sant, S.; Johnston, P.A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov. Today Technol. 2017, 23, 27–36. [Google Scholar] [CrossRef]
- Wong, J.K.; Seifalian, A.; Mohseni, R.; Hamidieh, A.A.; MacLaren, R.E.; Habib, N.; Seifalian, A.M. Emerging In Vitro 3D Tumour Models in Nanoparticle-Based Gene and Drug Therapy. Trends Biotechnol. 2018, 36, 477–480. [Google Scholar] [CrossRef]
- Van Oppen, L.M.P.E.; Pille, J.; Stuut, C.; van Stevendaal, M.; van der Vorm, L.N.; Smeitink, J.A.M.; Koopman, W.J.H.; Willems, P.H.G.M.; van Hest, J.C.M.; Brock, R. Octa-arginine boosts the penetration of elastin-like polypeptide nanoparticles in 3D cancer models. Eur. J. Pharm. Biopharm. 2019, 137, 175–184. [Google Scholar] [CrossRef]
- Ullah, S.; Seidel, K.; Türkkan, S.; Warwas, D.P.; Dubich, T.; Rohde, M.; Hauser, H.; Behrens, P.; Kirschning, A.; Köster, M.; et al. Macrophage entrapped silica coated superparamagnetic iron oxide particles for controlled drug release in a 3D cancer model. J. Control. Release 2019, 294, 327–336. [Google Scholar] [CrossRef]
- Srinivasa Reddy, T.; Privér, S.H.; Rao, V.V.; Mirzadeh, N.; Bhargava, S.K. Gold(i) and gold(iii) phosphine complexes: Synthesis, anticancer activities towards 2D and 3D cancer models, and apoptosis inducing properties. Dalton Trans. 2018, 47, 15312–15323. [Google Scholar] [CrossRef]
- Scolamiero, G.; Pazzini, C.; Bonafè, F.; Guarnieri, C.; Muscari, C. Effects of α-mangostin on viability, growth and cohesion of multicellular spheroids derived from human breast cancer cell lines. Int. J. Med. Sci. 2018, 15, 23–30. [Google Scholar] [CrossRef]
- Mármol, I.; Virumbrales-Muñoz, M.; Quero, J.; Sánchez-de-Diego, C.; Fernández, L.; Ochoa, I.; Cerrada, E.; Yoldi, M.J.R. Alkynyl gold(I) complex triggers necroptosis via ROS generation in colorectal carcinoma cells. J. Inorg. Biochem. 2017, 176, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Tada, A.; Isoyama, J.; Nagayama, S.; Yao, R.; Adachi, J.; Tomonaga, T. Improved phosphoproteomic analysis for phosphosignaling and active-kinome profiling in Matrigel-embedded spheroids and patient-derived organoids. Sci. Rep. 2018, 8, 11401. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, M.; D’Angelo, E.; Crotti, S.; Sensi, F.; Urbani, L.; Maghin, E.; Burns, A.; De Coppi, P.; Fassan, M.; Rugge, M.; et al. Decellularized colorectal cancer matrix as bioactive microenvironment for in vitro 3D cancer research. J. Cell. Physiol. 2018, 233, 5937–5948. [Google Scholar] [CrossRef] [PubMed]
- Brancato, V.; Gioiella, F.; Imparato, G.; Guarnieri, D.; Urciuolo, F.; Netti, P.A. 3D breast cancer microtissue reveals the role of tumor microenvironment on the transport and efficacy of free-doxorubicin in vitro. Acta Biomater. 2018, 75, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Nocelo, M.; Raimondo, T.M.; Vining, K.H.; López-López, R.; de la Fuente, M.; Mooney, D.J. Matrix stiffness and tumor-associated macrophages modulate epithelial to mesenchymal transition of human adenocarcinoma cells. Biofabrication 2018, 10, 035004. [Google Scholar] [CrossRef]
- Le, B.; Kang, D.; Yun, S.; Jeong, Y.; Kwak, J.-Y.; Yoon, S.; Jin, S. Three-Dimensional Hepatocellular Carcinoma/Fibroblast Model on a Nanofibrous Membrane Mimics Tumor Cell Phenotypic Changes and Anticancer Drug Resistance. Nanomaterials 2018, 8, 64. [Google Scholar] [CrossRef]
- Close, D.A.; Camarco, D.P.; Shan, F.; Kochanek, S.J.; Johnston, P.A. The Generation of Three-Dimensional Head and Neck Cancer Models for Drug Discovery in 384-Well Ultra-Low Attachment Microplates. In High Content Screening; Humana Press: New York, NY, USA, 2018; pp. 355–369. [Google Scholar]
- Meinert, C.; Theodoropoulos, C.; Klein, T.J.; Hutmacher, D.W.; Loessner, D. A Method for Prostate and Breast Cancer Cell Spheroid Cultures Using Gelatin Methacryloyl-Based Hydrogels. In Prostate Cancer; Humana Press: New York, NY, USA, 2018; pp. 175–194. [Google Scholar]
- Ahonen, I.; Åkerfelt, M.; Toriseva, M.; Oswald, E.; Schüler, J.; Nees, M. A high-content image analysis approach for quantitative measurements of chemosensitivity in patient-derived tumor microtissues. Sci. Rep. 2017, 7, 6600. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, B.; Dong, Y.; Wang, W.; Zheng, X.; Zhou, W.; Zhang, K.; Du, Z. Three-dimensional prostate tumor model based on a hyaluronic acid-alginate hydrogel for evaluation of anti-cancer drug efficacy. J. Biomater. Sci. Polym. Ed. 2017, 28, 1603–1616. [Google Scholar] [CrossRef]
- Brancato, V.; Gioiella, F.; Profeta, M.; Imparato, G.; Guarnieri, D.; Urciuolo, F.; Melone, P.; Netti, P.A. 3D tumor microtissues as an in vitro testing platform for microenvironmentally-triggered drug delivery systems. Acta Biomater. 2017, 57, 47–58. [Google Scholar] [CrossRef]
- Brancato, V.; Comunanza, V.; Imparato, G.; Corà, D.; Urciuolo, F.; Noghero, A.; Bussolino, F.; Netti, P.A. Bioengineered tumoral microtissues recapitulate desmoplastic reaction of pancreatic cancer. Acta Biomater. 2017, 49, 152–166. [Google Scholar] [CrossRef]
- Pradhan, S.; Hassani, I.; Seeto, W.J.; Lipke, E.A. PEG-fibrinogen hydrogels for three-dimensional breast cancer cell culture. J. Biomed. Mater. Res. Part A 2017, 105, 236–252. [Google Scholar] [CrossRef]
- Brancato, V.; Garziano, A.; Gioiella, F.; Urciuolo, F.; Imparato, G.; Panzetta, V.; Fusco, S.; Netti, P.A. 3D is not enough: Building up a cell instructive microenvironment for tumoral stroma microtissues. Acta Biomater. 2017, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Bastos, A.R.F.; Brancato, V.; Cerqueira, M.T.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Mechanical Property of Hydrogels and the Presence of Adipose Stem Cells in Tumor Stroma Affect Spheroid Formation in the 3D Osteosarcoma Model. ACS Appl. Mater. Interfaces 2019, 11, 14548–14559. [Google Scholar] [CrossRef] [PubMed]
- Scannell, J.W.; Blanckley, A.; Boldon, H.; Warrington, B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat. Rev. Drug Discov. 2012, 11, 191–200. [Google Scholar] [CrossRef]
- Cook, D.; Brown, D.; Alexander, R.; March, R.; Morgan, P.; Satterthwaite, G.; Pangalos, M.N. Lessons learned from the fate of AstraZeneca’s drug pipeline: A five-dimensional framework. Nat. Rev. Drug Discov. 2014, 13, 419–431. [Google Scholar] [CrossRef]
- Scannell, J.W.; Bosley, J. When quality beats quantity: Decision theory, drug discovery, and the reproducibility crisis. PLoS ONE 2016, 11, e0147215. [Google Scholar] [CrossRef]
- Pammolli, F.; Magazzini, L.; Riccaboni, M. The productivity crisis in pharmaceutical R&D. Nat. Rev. Drug Discov. 2011, 10, 428–438. [Google Scholar] [CrossRef]
- Chong, L.; Ray, L.B. Whole-istic biology. Science 2002, 295, 1661. [Google Scholar] [CrossRef][Green Version]
- Ekert, J.E.; Deakyne, J.; Pribul-Allen, P.; Terry, R.; Schofield, C.; Jeong, C.G.; Storey, J.; Mohamet, L.; Francis, J.; Naidoo, A.; et al. Recommended Guidelines for Developing, Qualifying, and Implementing Complex In Vitro Models (CIVMs) for Drug Discovery. SLAS Discov. 2020, 25, 1174–1190. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Fontana, F.; Figueiredo, P.; Martins, J.P.; Santos, H.A. Requirements for Animal Experiments: Problems and Challenges. Small 2021, 17, e2004182. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative human three-dimensional tissue-engineered models as an alternative to animal testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Frequently Asked Questions|Transgenic, Knockout, and Tumor Model Center|Stanford Medicine. Available online: https://med.stanford.edu/tktc/faq.html (accessed on 13 February 2022).
- In Vitro Differentiation of Human iPS Cells into Colon Organoids in Serum-Free Cell Culture Conditions. Available online: https://www.sigmaaldrich.com/PT/en/technical-documents/protocol/cell-culture-and-cell-culture-analysis/3d-cell-culture/human-colon-organoids (accessed on 13 February 2022).
- Morgan, P.; Van Der Graaf, P.H.; Arrowsmith, J.; Feltner, D.E.; Drummond, K.S.; Wegner, C.D.; Street, S.D.A. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov. Today 2012, 17, 419–424. [Google Scholar] [CrossRef] [PubMed]
- About Project Data Sphere|Project Data Sphere. Available online: https://www.projectdatasphere.org/about (accessed on 30 October 2020).
- Hickman, J.A.; Graeser, R.; de Hoogt, R.; Vidic, S.; Brito, C.; Gutekunst, M.; van der Kuip, H. Imi Predect consortium Three-dimensional models of cancer for pharmacology and cancer cell biology: Capturing tumor complexity in vitro/ex vivo. Biotechnol. J. 2014, 9, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Lemos, R.; Powis, G. The promise of patient-derived xenografts: The best laid plans of mice and men. Clin. Cancer Res. 2012, 18, 5160–5162. [Google Scholar] [CrossRef] [PubMed]
- Francia, G.; Cruz-Munoz, W.; Man, S.; Xu, P.; Kerbel, R.S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer 2011, 11, 135–141. [Google Scholar] [CrossRef]
- Guerin, E.; Man, S.; Xu, P.; Kerbel, R.S. A model of postsurgical advanced metastatic breast cancer more accurately replicates the clinical efficacy of antiangiogenic drugs. Cancer Res. 2013, 73, 2743–2748. [Google Scholar] [CrossRef]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef]
- FDA Approves Merck’s KEYTRUDA® (pembrolizumab) for Patients With MSI-H/dMMR Advanced Endometrial Carcinoma, Who Have Disease Progression Following Prior Systemic Therapy in Any Setting and Are Not Candidates for Curative Surgery or Radiation—Merck.com. Available online: https://www.merck.com/news/fda-approves-mercks-keytruda-pembrolizumab-for-patients-with-msi-h-dmmr-advanced-endometrial-carcinoma-who-have-disease-progression-following-prior-systemic-therapy-in-any-se/ (accessed on 13 February 2022).
- Ikeda, M.; Ioka, T.; Fukutomi, A.; Morizane, C.; Kasuga, A.; Takahashi, H.; Todaka, A.; Okusaka, T.; Creasy, C.L.; Gorman, S.; et al. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci. 2018, 109, 215–224. [Google Scholar] [CrossRef]
- Khatami, M. Inflammation, Aging and Cancer; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-66473-6. [Google Scholar]
- Zavala, V.A.; Kalergis, A.M. New clinical advances in immunotherapy for the treatment of solid tumours. Immunology 2015, 145, 182–201. [Google Scholar] [CrossRef]
- Khatami, M. Safety concerns and hidden agenda behind HPV vaccines: Another generation of drug-dependent society? Clin. Transl. Med. 2016, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M. Inflammation, aging, and cancer: Tumoricidal versus tumorigenesis of immunity. Cell Biochem. Biophys. 2009, 55, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M. Is cancer a severe delayed hypersensitivity reaction and histamine a blueprint? Clin. Transl. Med. 2016, 5, e35. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef]
- Cancer Burden Statistics and Trends Across Europe|ECIS. Available online: https://ecis.jrc.ec.europa.eu/?Cancer=0 (accessed on 30 October 2020).
- Milat, A.J.; Bauman, A.; Redman, S. Narrative review of models and success factors for scaling up public health interventions. Implement. Sci. 2015, 10, 113. [Google Scholar] [CrossRef]
- Proctor, W.R.; Foster, A.J.; Vogt, J.; Summers, C.; Middleton, B.; Pilling, M.A.; Shienson, D.; Kijanska, M.; Ströbel, S.; Kelm, J.M.; et al. Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury. Arch. Toxicol. 2017, 91, 2849–2863. [Google Scholar] [CrossRef]
- Breitenbach, M.; Hoffmann, J. Editorial: Cancer models. Front. Oncol. 2018, 8, 401. [Google Scholar] [CrossRef]
- Fetah, K.L.; DiPardo, B.J.; Kongadzem, E.M.; Tomlinson, J.S.; Elzagheid, A.; Elmusrati, M.; Khademhosseini, A.; Ashammakhi, N. Cancer Modeling-on-a-Chip with Future Artificial Intelligence Integration. Small 2019, 15, e1901985. [Google Scholar] [CrossRef]
- Innovative Medicines Initiative. New Models for Preclinical Evaluation of Drug Efficacy in Common Solid Tumours. In IMI1 Final Project Report Public Summary (PREDECT); Innovative Medicines Initiative: Brussels, Belgium, 2016. [Google Scholar]

| Commercial Products | Marketed by | Features | Limitations | References |
|---|---|---|---|---|
| AggreWell™ | STEMCELL™ Technologies |
|
| [19] |
| Corning® Spheroid Microplates | Corning® |
| [20] | |
| CELLSTAR® Cell-Repellent Surface | Greiner Bio-One |
| [21] | |
| NanoShuttle™-PL |
| |||
| Lipidure®-COAT plates | AMS Biotechnology |
| [22] |
| Commercial Products | Marketed by | Features | References |
|---|---|---|---|
| Alvetex® | AMS Biotechnology |
| [23] |
| Biogelx™-S | BIOGELX™ |
| [24] |
| BiogelxTM-RGD, BiogelxTM-IKVAV, BiogelxTM-YIGSR and BiogelxTM-GFOGER |
| ||
| Matrigel® and PuraMatrix™ | Corning® |
| [20] |
| CytoSoft® Rigidity plates | Advanced BioMatrix |
| [25] |
| HyStem® | Sigma-Aldrich® |
| [26] |
| MaxGel™ |
| [27] | |
| TrueGel3D™ |
| [28] | |
| Millicoat™ |
| [29] | |
| MAPTrix™ | Kollodis BioSciences, Inc. |
| [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, N.; Kundu, B.; Kundu, S.C.; Reis, R.L.; Correlo, V. In Vitro Cancer Models: A Closer Look at Limitations on Translation. Bioengineering 2022, 9, 166. https://doi.org/10.3390/bioengineering9040166
Antunes N, Kundu B, Kundu SC, Reis RL, Correlo V. In Vitro Cancer Models: A Closer Look at Limitations on Translation. Bioengineering. 2022; 9(4):166. https://doi.org/10.3390/bioengineering9040166
Chicago/Turabian StyleAntunes, Nina, Banani Kundu, Subhas C. Kundu, Rui L. Reis, and Vítor Correlo. 2022. "In Vitro Cancer Models: A Closer Look at Limitations on Translation" Bioengineering 9, no. 4: 166. https://doi.org/10.3390/bioengineering9040166
APA StyleAntunes, N., Kundu, B., Kundu, S. C., Reis, R. L., & Correlo, V. (2022). In Vitro Cancer Models: A Closer Look at Limitations on Translation. Bioengineering, 9(4), 166. https://doi.org/10.3390/bioengineering9040166









