Modelling Mixed Microbial Culture Polyhydroxyalkanoate Accumulation Bioprocess towards Novel Methods for Polymer Production Using Dilute Volatile Fatty Acid Rich Feedstocks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass from a Lab-Scale SBR
2.2. Biomass from a Pilot-Scale SBR
2.3. Pulse-Stimulation Respiration Experiments
2.4. Analytical Methods
2.5. Dissolved Oxygen and Chemical Oxygen Demand Mass Balance
2.6. Monte Carlo Accumulation Process Simulation
3. Results and Discussion
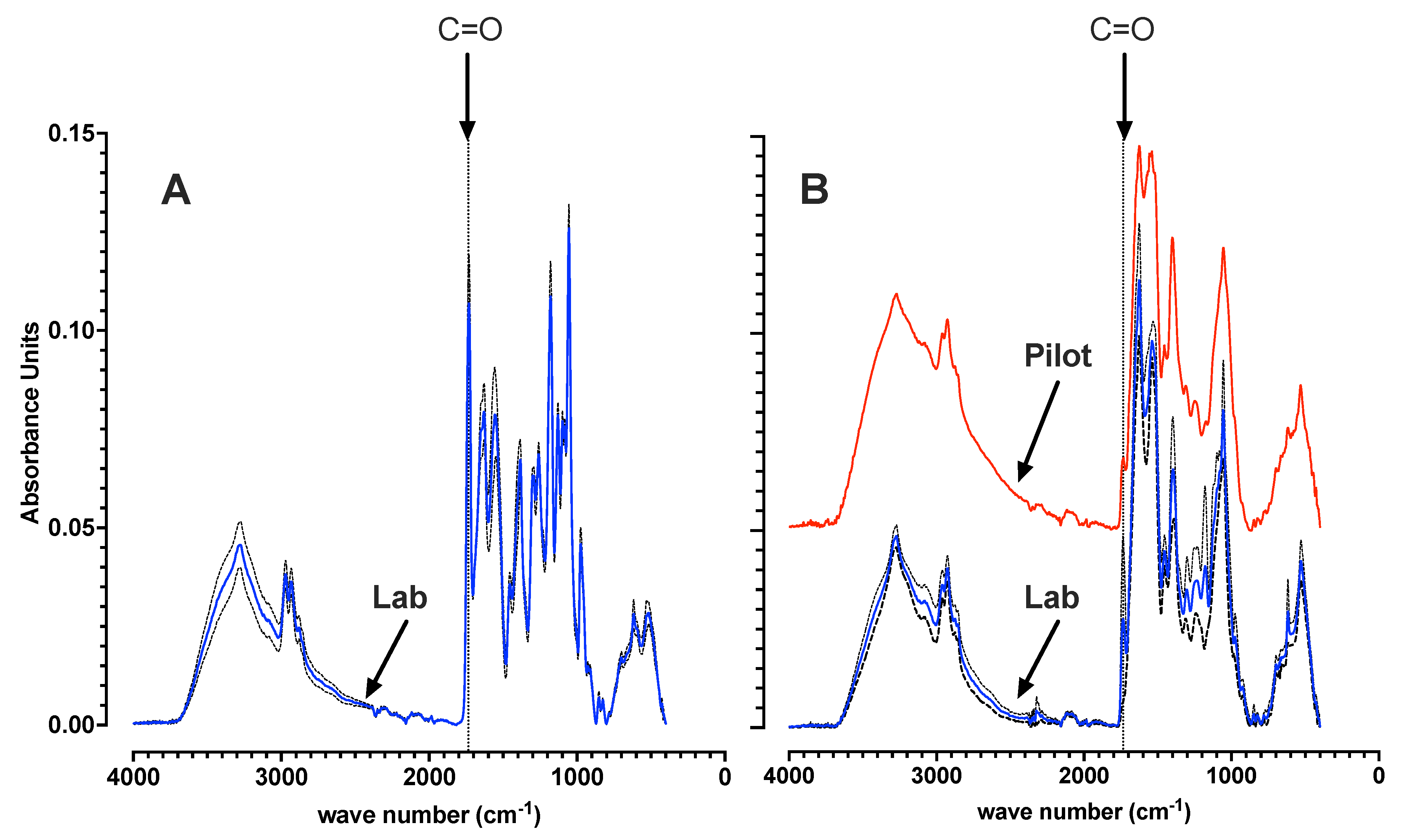
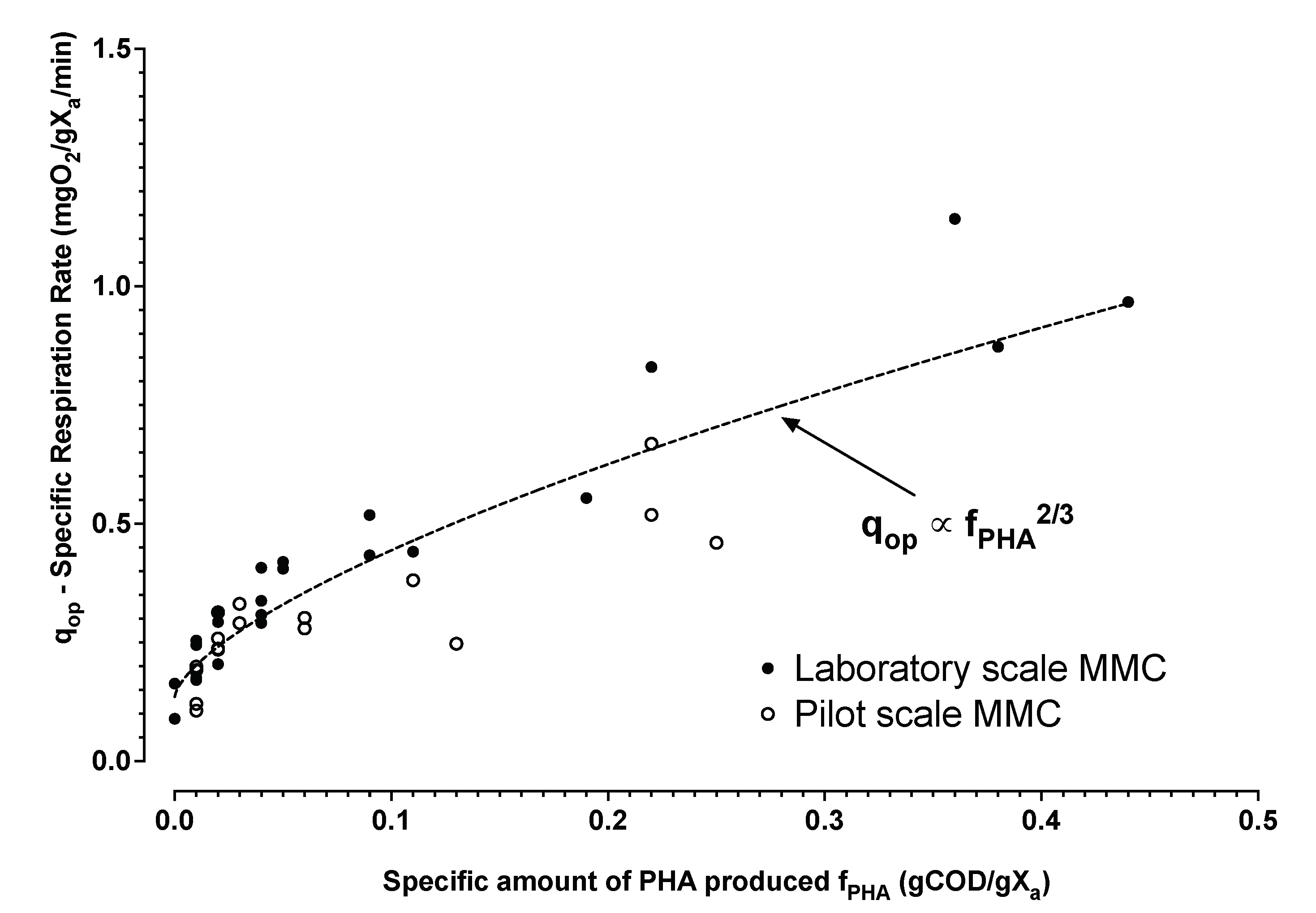
3.1. Laboratory and Pilot MMC Enrichment Biomass Characterization
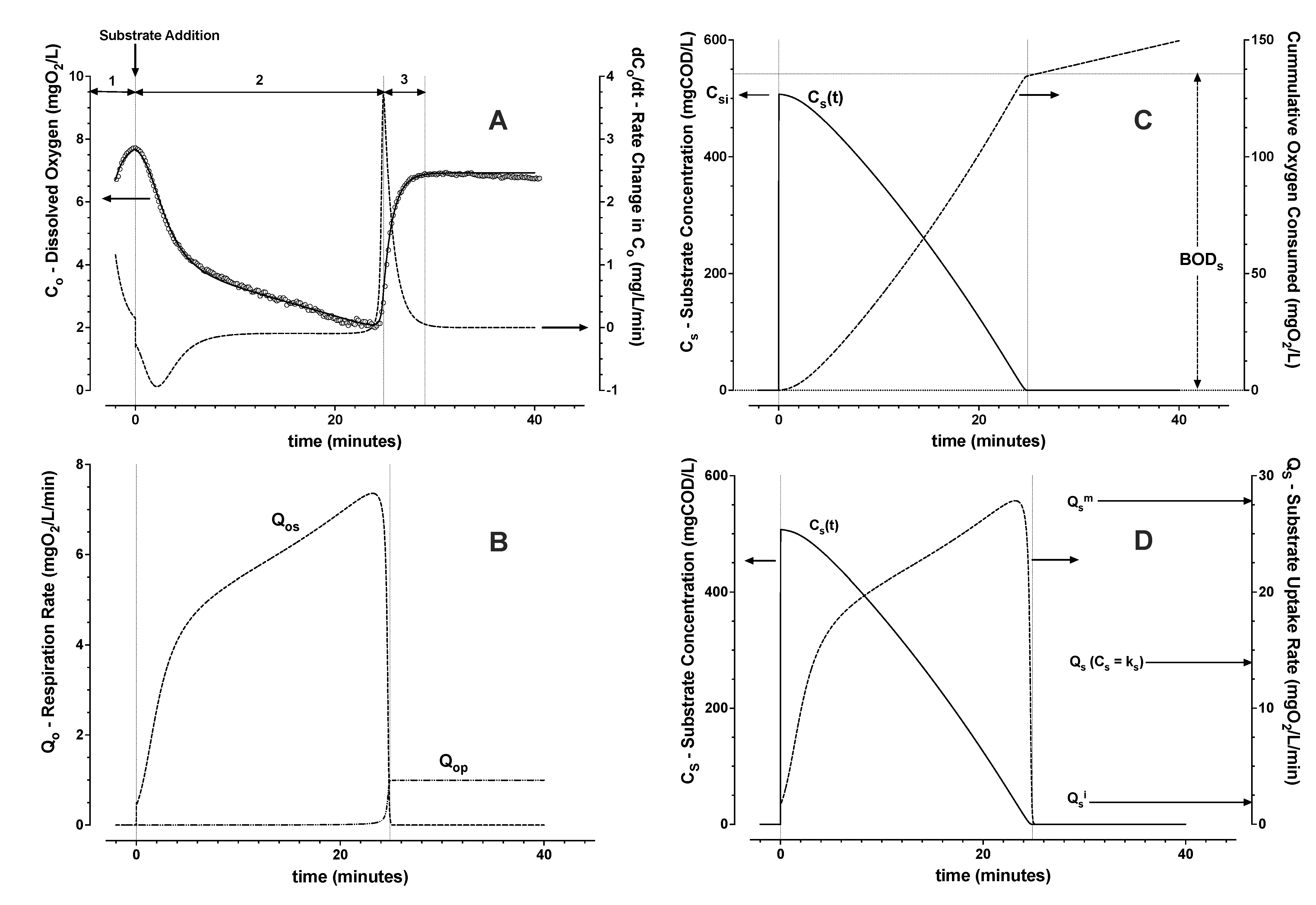
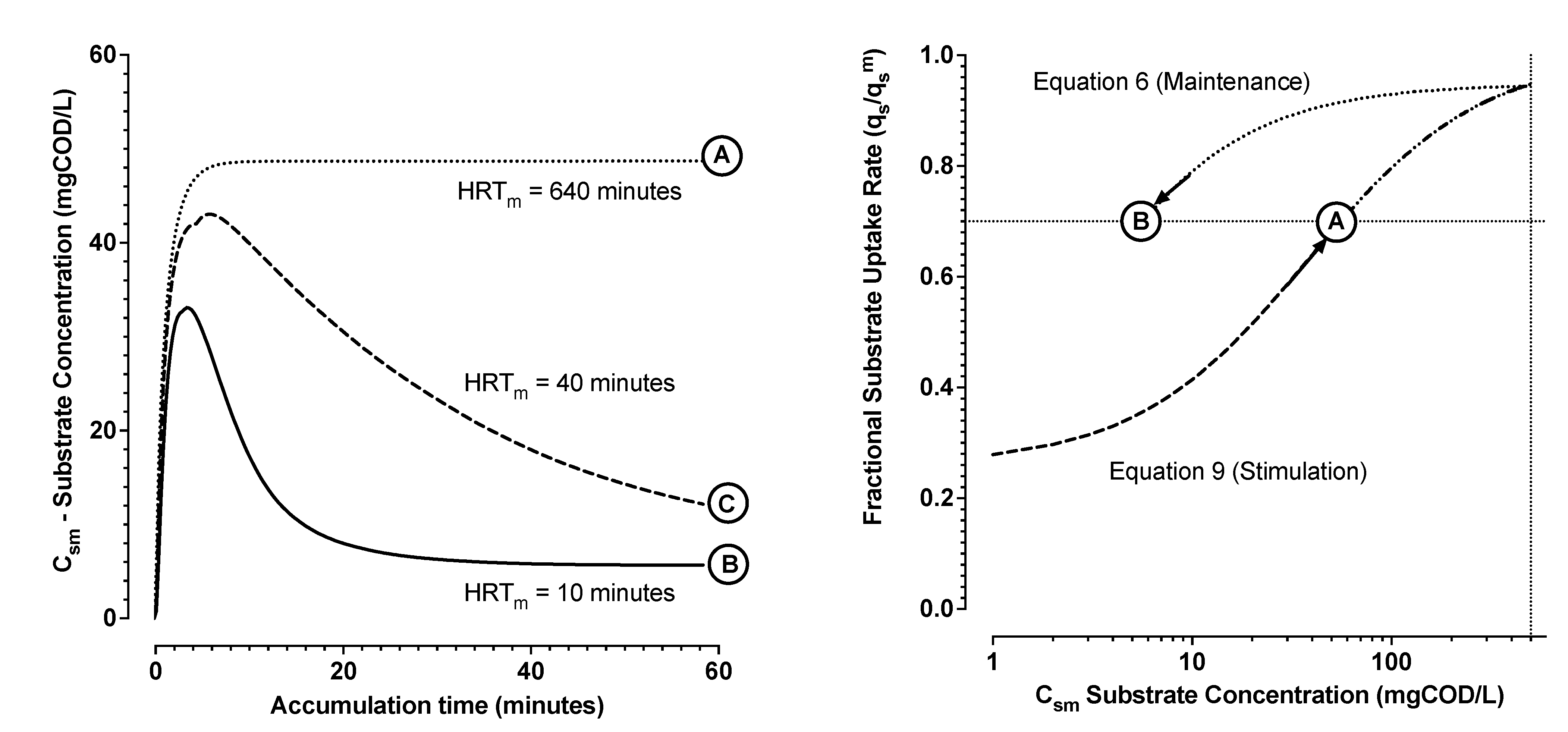
3.1.1. Model Evaluation with No Active Aeration
3.1.2. Model Evaluation with Active Aeration
- From Zone 2, the integral of Qos, the cumulative biochemical oxygen demand (BODs) due to substrate removal, was estimated. Then the average yield Yos and the trend of substrate concentration were calculated (Equation (2));
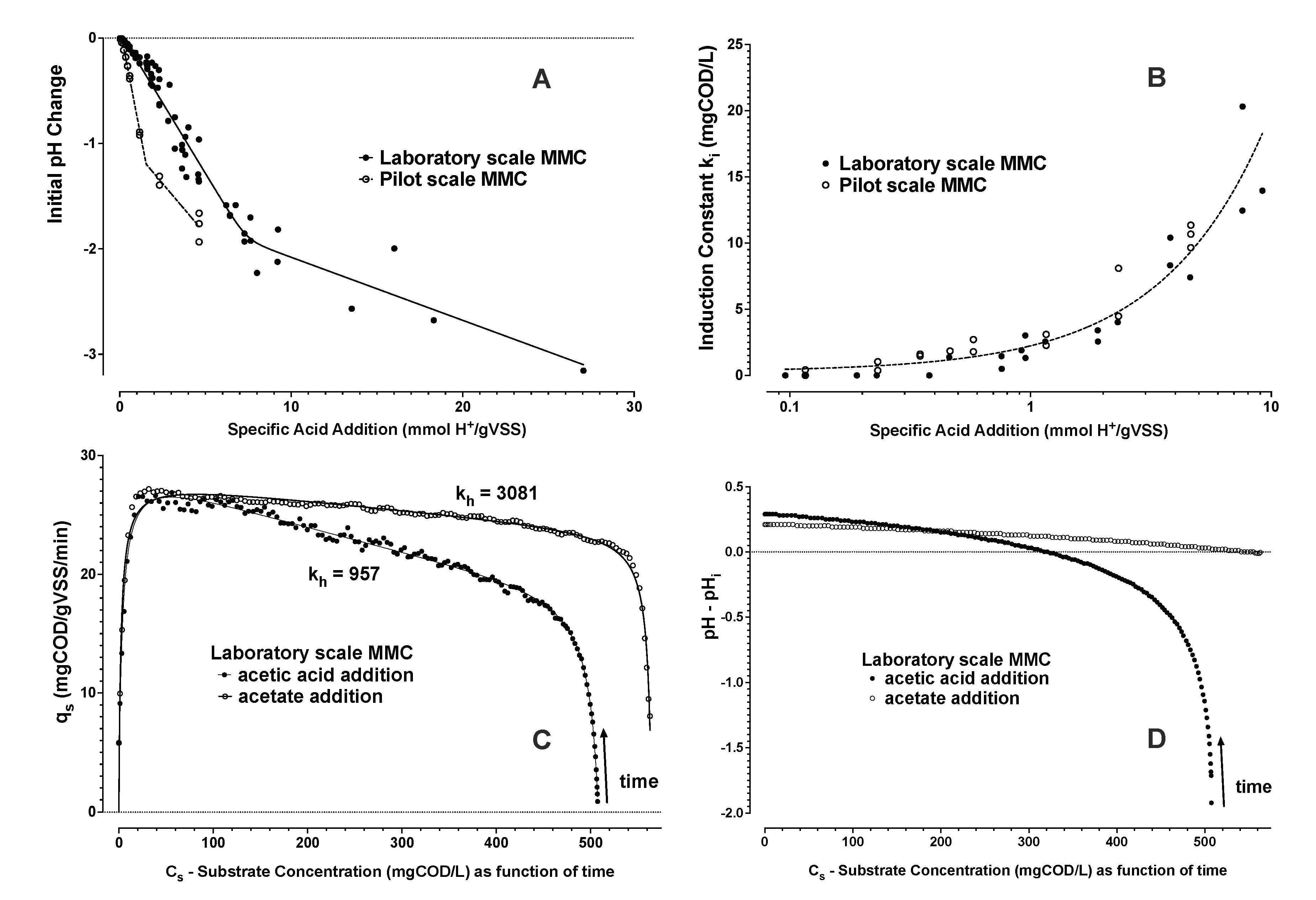
- From Zone 2, the trend of substrate uptake rate as a function of estimated substrate concentration was then used to determine remaining parameters (Equations (6)–(8)). The induction and downshift substrate affinity constants (ki and ks, respectively) were interpolated from the derived trend of substrate uptake rate as a function of interpreted substrate concentration. Remaining parameters were estimated by nonlinear least squares regression analysis (see Figure 4 and Figure 5).
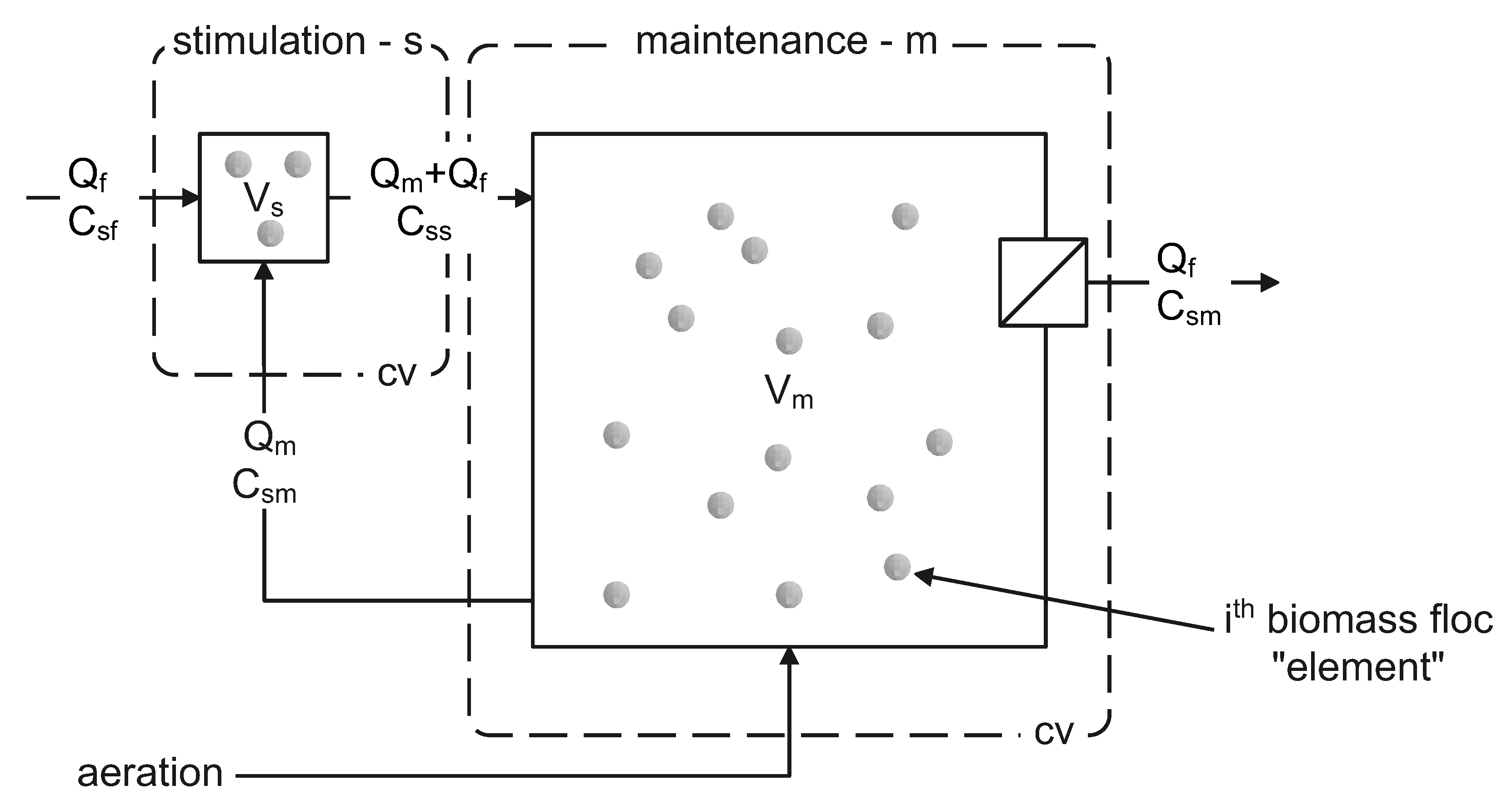
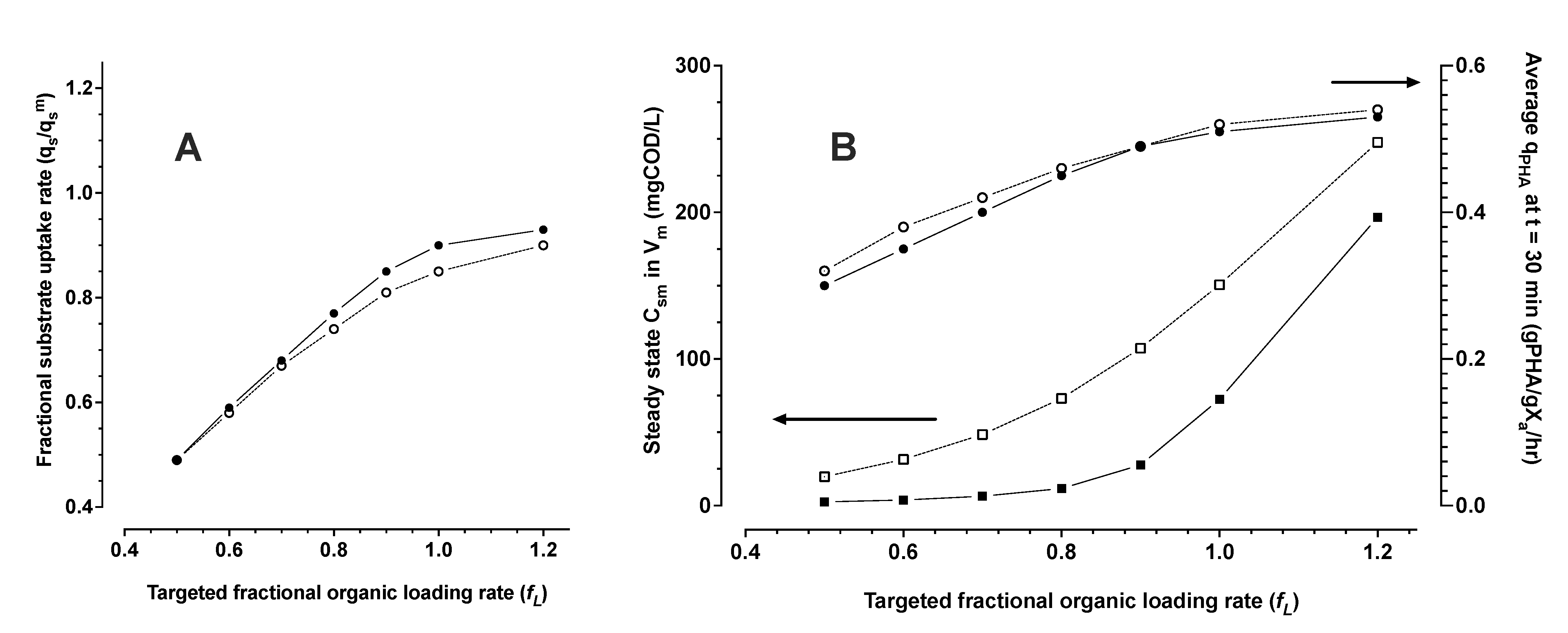
3.1.3. Monte Carlo Simulation of PHA Accumulation in MMC Enrichment Biomass
3.1.4. Future Research Perspectives and Challenges
4. Conclusions
- A property of hysteresis in the dynamic response of MMCs storing PHA could be demonstrated for two distinct enrichment cultures using dissolved oxygen and chemical oxygen demand mass balance experiments.
- This hysteresis could be modelled with readily identifiable parameters using Monod equations describing the distinct upshift and downshift dynamics in substrate uptake rates as a function of substrate concentrations and as a function of time. It was found that the substrate concentration, required to stimulate a substrate uptake rate, was higher than the substrate concentration required to maintain an attained substrate uptake rate.
- The system of equations in numerical simulations suggest for an opportunity to exploit this property of hysteresis in industrial scale bioprocesses for PHA production. MMC PHA production processes can be operated with continuous feeding strategies, even with low concentration feedstocks. The model simulations found that engineered stimulation zones can be applied in continuous flow PHA production bioprocesses as a strategy to reach maximum possible performance in volumetric productivity without sacrificing performance in substrate utilization efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR-FTIR | attenuated total reflection-FTIR |
| BOD | biochemical oxygen demand |
| COD | chemical oxygen demand |
| DO | dissolved oxygen |
| FTIR | Fourier transform infrared spectroscopy |
| HRT | hydraulic retention time |
| MMC | mixed microbial culture |
| PHA | polyhydroxyalkanoate |
| PHB | polyhydroxybutyrate |
| SBR | sequencing batch reactor |
| SRT | solids retention time |
| VFA | volatile fatty acid |
| VSS | volatile suspended solids |
| WWTP | wastewater treatment plant |
| PHA accumulation inhibition exponent | |
| DO concentration (mgO2/L) | |
| apparent maximum DO (saturation) concentration (mgO2/L) | |
| exogenous dissolved substrate concentration (mgCOD/L) | |
| initial or peak upshift (mgCOD/L) | |
| / | |
| aeration oxygen mass transfer coefficient (1/min) | |
| Haldane substrate inhibition constant (mgCOD/L) | |
| substrate induction constant (mgCOD/L) | |
| Monod apparent affinity constant on DO concentration (mgO2/L) | |
| Monod apparent downshift affinity constant on substrate concentration (mgCOD/L) | |
| Monod apparent upshift affinity constant on peak substrate concentration (mgCOD/L) | |
| DO supply rate due to aeration (mgO2/L/min) | |
| DO consumption rate due to endogenous respiration (mgO2/L/min) | |
| DO consumption rate due to stored PHA (mgO2/L/min) | |
| DO consumption reate due to substrate consumption (mgO2/L/min) | |
| specific (mgO2/gVSS/min) | |
| specific (mgO2/gVSS/min) | |
| biomass resting specific substrate uptake rate (mgCOD/gVSS/min) | |
| specific substrate uptake rate (mgCOD/gVSS/min) | |
| maximum extant specific substrate uptake rate (mgCOD/gVSS/min) | |
| initial maximum extant specific substrate uptake rate (mgCOD/gVSS/min) | |
| maximum specific substrate uptake rate (mgCOD/gVSS/min) | |
| t | time (minutes) |
| biomass maximum upshift in specific substrate uptake rate (mgCOD/gVSS/min) | |
| active biomass concentration (gVSS/L) | |
| PHA concentration (gCOD/L) | |
| yield of oxygen consumed with substrate (mgO2/mgCOD) | |
| influent feedstock substrate concentration (mgCOD/L) | |
| maintenance volume substrate concentration (mgCOD/L) | |
| stimulation volume substrate concentration (mgCOD/L) | |
| applied fraction of maximum possible organic loading rate | |
| ith-active biomass element specific PHA level (/) | |
| active biomass maximum PHA level for / | |
| HRT in due to (min) | |
| HRT in due to (min) | |
| number of biomass elements in | |
| substrate feed (and effluent) flow rate (L/min) | |
| recirculation flow rate (L/min) | |
| ith-biomass element maximum specific substrate uptake rate (mgCOD/gVSS/min) | |
| maintenance volume (L) | |
| stimulation volume (L) | |
| element specific active biomass (gVSS/element) | |
| element specific PHA content (gPHA/element) |
References
- Anderson, A.J.; Dawes, E.A. Occurrence, Metabolism, Metabolic Role, and Industrial Uses of Bacterial Polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef] [PubMed]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2014, 39, 397–442. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef]
- Vandi, L.J.; Chan, C.M.; Werker, A.; Richardson, D.; Laycock, B.; Pratt, S. Wood-PHA Composites: Mapping Opportunities. Polymers 2018, 10, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikodinovic-Runic, J.; Guzik, M.; Kenny, S.T.; Babu, R.; Werker, A.; O’Connor, K.E. Carbon-rich wastes as feedstocks for biodegradable polymer (polyhydroxyalkanoate) production using bacteria. Adv. Appl. Microbiol. 2013, 84, 139–200. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Bharagava, R.N.; Kumar, G.; Kim, D.S.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Marang, L.; van Loosdrecht, M.C.; Kleerebezem, R. Enrichment of PHA-producing bacteria under continuous substrate supply. New Biotechnol. 2018, 41, 55–61. [Google Scholar] [CrossRef]
- Stouten, G.R.; Hogendoorn, C.; Douwenga, S.; Kilias, E.S.; Muyzer, G.; Kleerebezem, R. Temperature as competitive strategy determining factor in pulse-fed aerobic bioreactors. ISME J. 2019, 13, 3112–3125. [Google Scholar] [CrossRef]
- Anterrieu, S.; Quadri, L.; Geurkink, B.; Dinkla, I.; Bengtsson, S.; Arcos-Hernandez, M.; Alexandersson, T.; Morgan-Sagastume, F.; Karlsson, A.; Hjort, M.; et al. Integration of biopolymer production with process water treatment at a sugar factory. New Biotechnol. 2014, 31, 308–323. [Google Scholar] [CrossRef]
- Bengtsson, S.; Karlsson, A.; Alexandersson, T.; Quadri, L.; Hjort, M.; Johansson, P.; Morgan-Sagastume, F.; Anterrieu, S.; Arcos-Hernandez, M.; Karabegovic, L.; et al. A process for polyhydroxyalkanoate (PHA) production from municipal wastewater treatment with biological carbon and nitrogen removal demonstrated at pilot-scale. New Biotechnol. 2017, 35, 42–53. [Google Scholar] [CrossRef]
- Estevez-Alonso, A.; Pei, R.; Loosdrecht, M.C.M.V.; Kleerebezem, R.; Werker, A. Scaling-up microbial community-based polyhydroxyalkanoate production: Status and challenges. Bioresour. Technol. 2021, 327, 124790. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon recovery from wastewater through bioconversion into biodegradable polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos, P.C.; Serafim, L.S.; Reis, M.A.M. Synthesis of polyhydroxyalkanoates from different short-chain fatty acids by mixed cultures submitted to aerobic dynamic feeding. J. Biotechnol. 2006, 122, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, S.; Pisco, A.R.; Johansson, P.; Lemos, P.C.; Reis, M.A. Molecular weight and thermal properties of polyhydroxyalkanoates produced from fermented sugar molasses by open mixed cultures. J. Biotechnol. 2010, 147, 172–179. [Google Scholar] [CrossRef]
- Mel, B.; Torres-giner, S.; Reis, M.A.M.; Silva, F.; Matos, M.; Cabedo, L. Blends of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) with Fruit Pulp Biowaste Derived Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate-co-3-Hydroxyhexanoate) for Organic Recycling Food Packaging. Polymers 2021, 13, 1155. [Google Scholar]
- Silva, F.; Matos, M.; Pereira, B.; Ralo, C.; Pequito, D.; Marques, N.; Carvalho, G.; Reis, M.A. An integrated process for mixed culture production of 3-hydroxyhexanoate-rich polyhydroxyalkanoates from fruit waste. Chem. Eng. J. 2022, 427, 131908. [Google Scholar] [CrossRef]
- Werker, A.; Bengtsson, S.; Johansson, P.; Magnusson, P.; Gustafsson, E.; Hjort, M.; Anterrieu, S.; Karabegovic, L.; Karlsson, A.; Morgan-Sagastume, F.; et al. Production Quality Control of Mixed Culture Poly(3-hydroxbutyrate-co-3-hydroxyvalerate) blends using full-scale municipal activated sludge and non-chlorinated solvent extraction. In The Handbook of Polyhydroxyalkanoates: Kinetics, Bioengineering, and Industrial Aspects, 1st ed.; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 329–386. [Google Scholar]
- Morgan-Sagastume, F.; Valentino, F.; Hjort, M.; Zanaroli, G.; Majone, M.; Werker, A. Acclimation Process for Enhancing Polyhydroxyalkanoate Accumulation in Activated-Sludge Biomass. Waste Biomass Valorization 2019, 10, 1065–1082. [Google Scholar] [CrossRef]
- Estévez-Alonso, Á.; van Loosdrecht, M.C.; Kleerebezem, R.; Werker, A. Simultaneous nitrification and denitrification in microbial community-based polyhydroxyalkanoate production. Bioresour. Technol. 2021, 337, 125420. [Google Scholar] [CrossRef]
- Grazia, G.D.; Quadri, L.; Majone, M.; Morgan-Sagastume, F.; Werker, A. Influence of temperature on mixed microbial culture polyhydroxyalkanoate production while treating a starch industry wastewater. J. Environ. Chem. Eng. 2017, 5, 5067–5075. [Google Scholar] [CrossRef] [Green Version]
- Koller, M. A review on established and emerging fermentation schemes for microbial production of polyhydroxyalkanoate (PHA) biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Koller, M.; Braunegg, G. Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering 2015, 2, 94–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.C.; Serafim, L.S.; Nadais, H.; Arroja, L.; Capela, I. Acidogenic Fermentation towards Valorisation of Organic Waste Streams into Volatile Fatty Acids. Chem. Biochem. Eng. Q. 2013, 27, 467–476. [Google Scholar]
- Lagoa-Costa, B.; Kennes, C.; Veiga, M.C. Cheese whey fermentation into volatile fatty acids in an anaerobic sequencing batch reactor. Bioresour. Technol. 2020, 308, 123226. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Karabegovic, L.; Majone, M.; Morgan-Sagastume, F.; Werker, A. Polyhydroxyalkanoate (PHA) storage within a mixed-culture biomass with simultaneous growth as a function of accumulation substrate nitrogen and phosphorus levels. Water Res. 2015, 77, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Penabad, N.; Kennes, C.; Veiga, M.C. Anaerobic digestion of tuna waste for the production of volatile fatty acids. Waste Manag. 2017, 68, 96–102. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Fernández-Feito, R.; Massanet-Nicolau, J.; Dinsdale, R.; Guwy, A. Continuous recovery and enhanced yields of volatile fatty acids from a continually-fed 100 L food waste bioreactor by filtration and electrodialysis. Waste Manag. 2021, 122, 81–88. [Google Scholar] [CrossRef]
- Burniol-Figols, A.; Pinelo, M.; Skiadas, I.V.; Gavala, H.N. Enhancing polyhydroxyalkanoate productivity with cell-retention membrane bioreactors. Biochem. Eng. J. 2020, 161, 107687. [Google Scholar] [CrossRef]
- Tamis, J.; Marang, L.; Jiang, Y.; van Loosdrecht, M.C.M.; Kleerebezem, R. Modeling PHA-producing microbial enrichment cultures-towards a generalized model with predictive power. New Biotechnol. 2014, 31, 324–334. [Google Scholar] [CrossRef]
- Werker, A.; Bengtsson, S.; Karlsson, A. Method for Accumulation of Polyhydroxyalkanoates in Biomass with On-Line Monitoring for Feed Rate Control and Process Termination. U.S. Patent US8748138B2, 10 June 2014. [Google Scholar]
- Lorini, L.; di Re, F.; Majone, M.; Valentino, F. High rate selection of PHA accumulating mixed cultures in sequencing batch reactors with uncoupled carbon and nitrogen feeding. New Biotechnol. 2020, 56, 140–148. [Google Scholar] [CrossRef]
- Valentino, F.; Moretto, G.; Lorini, L.; Bolzonella, D.; Pavan, P.; Majone, M. Pilot-Scale Polyhydroxyalkanoate Production from Combined Treatment of Organic Fraction of Municipal Solid Waste and Sewage Sludge. Ind. Eng. Chem. Res. 2019, 58, 12149–12158. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Chan, C.M.; Johansson, P.; Magnusson, P.; Vandi, L.J.L.J.; Arcos-Hernandez, M.; Halley, P.; Laycock, B.; Pratt, S.; Werker, A. Mixed culture polyhydroxyalkanoate-rich biomass assessment and quality control using thermogravimetric measurement methods. Polym. Degrad. Stab. 2017, 144, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Savitzky, A.; Golay, M.J. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Spanjers, H.; Olsson, G. Modelling of the dissolved oxygen probe response in the improvement of the performance of a continuous respiration meter. Water Res. 1992, 26, 945–954. [Google Scholar] [CrossRef]
- Krishna, C.; Loosdrecht, M.C.V. Substrate flux into storage and growth in relation to activated sludge modeling. Water Res. 1999, 33, 3149–3161. [Google Scholar] [CrossRef]
- Panikov, N.S. Microbial Growth Kinetics; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Johnson, K.; Kleerebezem, R.; van Loosdrecht, M.C. Model-based data evaluation of polyhydroxybutyrate producing mixed microbial cultures in aerobic sequencing batch and fed-batch reactors. Biotechnol. Bioeng. 2009, 104, 50–67. [Google Scholar] [CrossRef]
- Arcos-Hernandez, M.V.; Gurieff, N.; Pratt, S.; Magnusson, P.; Werker, A.; Vargas, A.; Lant, P. Rapid quantification of intracellular PHA using infrared spectroscopy: An application in mixed cultures. J. Biotechnol. 2010, 150, 372–379. [Google Scholar] [CrossRef]
- Beun, J.J.; Dircks, K.; Loosdrecht, M.C.V.; Heijnen, J.J. Poly-beta-hydroxybutyrate metabolism in dynamically fed mixed microbial cultures. Water Res. 2002, 36, 1167–1180. [Google Scholar] [CrossRef]
- van Aalast-van Leeuwen, M.A.; Pot, M.A.; van Loosdrecht, M.C.; Heijnen, J.J. Kinetic Modeling of Poly (beta-hydroxybutyrate) Production and Consumption by Paracoccus pantotrophus under Dynamic Substrate Supply. Biotechnol. Bioeng. 1997, 55, 773–782. [Google Scholar] [CrossRef]
- Jolkver, E.; Emer, D.; Ballan, S.; Krämer, R.; Eikmanns, B.J.; Marin, K. Identification and characterization of a bacterial transport system for the uptake of pyruvate, propionate, and acetate in Corynebacterium glutamicum. J. Bacteriol. 2009, 191, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Gurieff, N. Production of Biodegradable Polyhydroxyalkanoate Polymers Using Advanced Biological Wastewater Treatment Process Technology; The University of Queensland: Brisbane, Australia, 2007. [Google Scholar]
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.; Reis, M.A. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Russo, I.; Bolzonella, D.; Pavan, P.; Majone, M.; Valentino, F. An urban biorefinery for food waste and biological sludge conversion into polyhydroxyalkanoates and biogas. Water Res. 2020, 170, 115371. [Google Scholar] [CrossRef] [PubMed]
- Tamis, J.; Lužkov, K.; Jiang, Y.; van Loosdrecht, M.C.M.; Kleerebezem, R. Enrichment of Plasticicumulans acidivorans at pilot-scale for PHA production on industrial wastewater. J. Biotechnol. 2014, 192, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Sagastume, F.; Bengtsson, S.; Grazia, G.D.; Alexandersson, T.; Quadri, L.; Johansson, P.; Magnusson, P.; Werker, A. Mixed-culture polyhydroxyalkanoate (PHA) production integrated into a food-industry effluent biological treatment: A pilot-scale evaluation. J. Environ. Chem. Eng. 2020, 8, 104469. [Google Scholar] [CrossRef]
- Albuquerque, M.G.; Concas, S.; Bengtsson, S.; Reis, M.A. Mixed culture polyhydroxyalkanoates production from sugar molasses: The use of a 2-stage CSTR system for culture selection. Bioresour. Technol. 2010, 101, 7112–7122. [Google Scholar] [CrossRef]
- Marang, L.; van Loosdrecht, M.C.M.; Kleerebezem, R. Modeling the competition between PHA-producing and non-PHA-producing bacteria in feast-famine SBR and staged CSTR systems. Biotechnol. Bioeng. 2015, 112, 2475–2484. [Google Scholar] [CrossRef]
- Mulders, M.; Tamis, J.; Stouten, G.R.; Kleerebezem, R. Simultaneous growth and poly(3-hydroxybutyrate) (PHB) accumulation in a Plasticicumulans acidivorans dominated enrichment culture. J. Biotechnol. 2020, 8, 100027. [Google Scholar] [CrossRef]
- Haimi, H.; Mulas, M.; Corona, F.; Vahala, R. Data-derived soft-sensors for biological wastewater treatment plants: An overview. Environ. Model. Softw. 2013, 47, 88–107. [Google Scholar] [CrossRef]


| Parameter | Units | Laboratory Scale | Pilot Scale |
|---|---|---|---|
| Temperature | °C | 24.6 ± 0.3 (24) | 23.8 ± 0.6 (17) |
| pH (t = 0) | - | 9.2 ± 0.1 (24) | 9.0 ± 0.1 (17) |
| Xa | gVSS/L | 1.0 ± 0.1 (11) | 1.71 ± 0.04 (2) |
| ka | 1/min | 1.11 ± 0.14 (24) | 0.60 ± 0.15 (17) |
| qoe | mgO2/gVSS/min | 0.64 ± 0.12 (24) | 0.25 ± 0.07 (17) |
| Yos | gO2/gCOD | 0.26 ± 0.02 (24) | 0.23 ± 0.05 (17) |
| ks | mgCOD/L | 2.0 ± 0.7 (24) | 1.8 ± 0.4 (17) |
| ku | mgCOD/L | 38 ± 7 (24) | 20 ± 2 (17) |
| mgCOD/gVSS/min | 7.4 ± 0.1 (24) | 2.5 ± 0.2 (17) | |
| mgCOD/gVSS/min | 21.1 ± 1.1 (24) | 6.8 ± 0.2 (17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werker, A.; Lorini, L.; Villano, M.; Valentino, F.; Majone, M. Modelling Mixed Microbial Culture Polyhydroxyalkanoate Accumulation Bioprocess towards Novel Methods for Polymer Production Using Dilute Volatile Fatty Acid Rich Feedstocks. Bioengineering 2022, 9, 125. https://doi.org/10.3390/bioengineering9030125
Werker A, Lorini L, Villano M, Valentino F, Majone M. Modelling Mixed Microbial Culture Polyhydroxyalkanoate Accumulation Bioprocess towards Novel Methods for Polymer Production Using Dilute Volatile Fatty Acid Rich Feedstocks. Bioengineering. 2022; 9(3):125. https://doi.org/10.3390/bioengineering9030125
Chicago/Turabian StyleWerker, Alan, Laura Lorini, Marianna Villano, Francesco Valentino, and Mauro Majone. 2022. "Modelling Mixed Microbial Culture Polyhydroxyalkanoate Accumulation Bioprocess towards Novel Methods for Polymer Production Using Dilute Volatile Fatty Acid Rich Feedstocks" Bioengineering 9, no. 3: 125. https://doi.org/10.3390/bioengineering9030125
APA StyleWerker, A., Lorini, L., Villano, M., Valentino, F., & Majone, M. (2022). Modelling Mixed Microbial Culture Polyhydroxyalkanoate Accumulation Bioprocess towards Novel Methods for Polymer Production Using Dilute Volatile Fatty Acid Rich Feedstocks. Bioengineering, 9(3), 125. https://doi.org/10.3390/bioengineering9030125









