Abstract
Type 1 diabetes is a chronic autoimmune disease affecting nearly 35 million people. This disease develops as T-cells continually attack the β-cells of the islets of Langerhans in the pancreas, which leads to β-cell death, and steadily decreasing secretion of insulin. Lowered levels of insulin minimize the uptake of glucose into cells, thus putting the body in a hyperglycemic state. Despite significant progress in the understanding of the pathophysiology of this disease, there is a need for novel developments in the diagnostics and management of type 1 diabetes. Extracellular vesicles (EVs) are lipid-bound nanoparticles that contain diverse content from their cell of origin and can be used as a biomarker for both the onset of diabetes and transplantation rejection. Furthermore, vesicles can be loaded with therapeutic cargo and delivered in conjunction with a transplant to increase cell survival and long-term outcomes. Crucially, several studies have linked EVs and their cargos to the progression of type 1 diabetes. As a result, gaining a better understanding of EVs would help researchers better comprehend the utility of EVs in regulating and understanding type 1 diabetes. EVs are a composition of biologically active components such as nucleic acids, proteins, metabolites, and lipids that can be transported to particular cells/tissues through the blood system. Through their varied content, EVs can serve as a flexible aid in the diagnosis and management of type 1 diabetes. In this review, we provide an overview of existing knowledge about EVs. We also cover the role of EVs in the pathogenesis, detection, and treatment of type 1 diabetes and the function of EVs in pancreas and islet β-cell transplantation.
1. Introduction
Diabetes mellitus is a lifelong, incurable disease in which the production or response to insulin is dysregulated. Diabetes diagnoses are divided into separate categories based on their mechanism, with the two most commonly discussed groups being type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). Approximately 90% of diabetes cases can be considered T2DM, which occurs when cells do not respond appropriately to circulating insulin (called insulin resistance) and thus need higher insulin levels to regulate sugar processing. Doctors often recommend an initial intervention of modified diet and exercise to increase insulin sensitivity. As needed, T2DM patients are given external insulin to decrease blood sugar levels.
T1DM accounts for the remaining 10% of diabetes cases and is caused by the immune-mediated T-cell attack on insulin-producing β-cells in the pancreatic islets. This produces a deficiency in insulin, hyperglycemia (high glucose levels), and changes to the metabolism of proteins and lipids [1]. In total, 34.2 million patients are currently living with T1DM globally, a number that has tripled in the last decade. An estimated 500,000 of these patients are less than 15 years of age, giving T1DM its common moniker of “juvenile diabetes” [2,3,4]. Treatment of T1DM is more complex than T2DM, as it cannot be regulated solely by diet and exercise, is regularly accompanied by severe complications, and has extreme variability due to the diverse ages of diagnosis. Primary treatment options for T1DM include continuous exogenous insulin and immunosuppressive drugs, which ameliorate the primary pathology of T1DM. Globally, one in every two patients are unable to afford or lack access to the T1DM therapies they need [5]. The number of diabetes diagnoses is steadily rising, as, by 2045, the number of people with diabetes is estimated to reach 629 million, making diabetes management a critical target for social and economic stability [5,6]. Further, though the diagnosis of T1DM can take place using a number of measures, including common symptoms of frequent urination and fatigue, problems can arise with late or delayed diagnosis [2,7]. Therefore, due to regular complications, the anticipated rise in the number of diabetic patients, and the rising costs of insulin, there is a drastic need for a change in approach and alternative treatments for type 1 diabetes.
One potential option in the management of T1DM is the use of EVs. EVs are a heterogeneous population of membranous vesicles secreted from diverse cells that have assorted cargoes such as proteins, lipids, and RNAs that are representative of their parent cells. These small particles are shown to play essential roles in disease and offer an impactful insight into cellular communication during the challenge. This review covers current solutions in the treatment of T1DM, with a focus on pancreatic and islet transplantation and the potential benefits of EVs in T1DM diagnosis and management.
2. Extracellular Vesicles in T1DM
T1DM is a complicated disorder that results from an intricate interaction between environmental and genetic components, with the former involving many susceptibility genes. The majority of T1DM patients are dependent on lifelong insulin treatment, which can only alleviate symptoms rather than restore function. EVs have lately gained popularity as possible diagnostic tools that could lead to the discovery of new biomarkers and therapeutic targets. Additionally, EVs have a unique ability to deliver bioactive molecules to precise locations and so have significant therapeutic implications. The following sections summarize about existing knowledge of EVs in T1DM in terms of diagnostics and therapy, as well as emerging EV based therapies.
3. Extracellular Vesicles
EVs are endogenous lipid bilayer vesicles that are secreted by the majority of cell types and are detected in virtually all bodily fluids [8,9,10,11]. EVs were thought to be a result of cells secreting and eliminating garbage [12,13,14], but research has determined that EVs are released into the surrounding environment by parent cells to execute a range of biological activities, including cell signaling and genetic material exchange (Figure 1A) [15,16,17,18]. This review gives a brief introduction to EVs; for more specific detailed information about the biological roles of EVs, cell biology, or cell to cell communication, readers are encouraged to refer to the excellent review articles by Ramis et al., 2020, and Kalluri et al., 2020 [19,20].
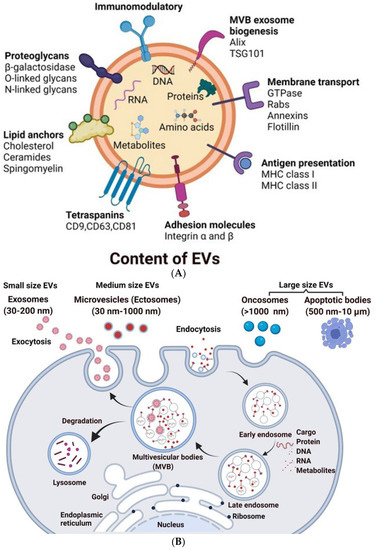
Figure 1.
Schematic representation of the biogenesis of EVs: (A) Composition of EVs: EVs consist of cargo made up of bioactive molecules such as proteins, nucleotides, secondary metabolites, and lipids. Heat shock proteins (Hsp90 and Hsp70), Tetraspanins (CD63, CD9, and CD81), cytoskeletal proteins (Fibronectin and Actin), viral proteins (Tsg101), and enzymes are examples of proteins. EVs also contains DNA and RNA. (B) During endosomal maturation, multivesicular bodies (MVBs) develop, and exosomes are released when the MVBs fuse with the plasma membrane. Microvesicles, on the other hand, are derived directly via cell membrane budding and fission. Apoptotic bodies are formed by the death of apoptotic cells.
3.1. Classification and Origin of EVs
EVs are heterogeneous, ranging from 30 nm to 10 µm in diameter and encompass several types of vesicles, including but not limited to apoptotic bodies, which are large vesicles released from cells undergoing apoptosis; microvesicles, which are released from an evagination of the plasma membrane; and exosomes, which are formed in multivesicular bodies (MVBs), that then fuse with the plasma membrane to release the vesicles into the extracellular fluid (Figure 1B, Table 1) [10,12,21,22].
The endosomal membrane pathway produces exosomes from a variety of cell types. Multiple endocytic bodies cluster together to create early endosomes after the plasma membrane invaginates to make endocytic vesicles. The invagination of early endosomes then progressed to the formation of multivesicular bodies. Finally, MVBs migrate and make contact with the plasma membrane in order to secrete vesicles into the extracellular environment, which are known as exosomes [23]. Exosome formation includes cargo sorting and releasing material, and the correct modulation of this process necessitates the coordination of many different proteins and can be modified by lysosomal status [24]. Exosome biogenesis requires subunits of endosomal sorting complex required for transport (ESCRT), which include ESCRT complexes 0 to III [25].
Exosome secretion is divided into two types: ESCRT-independent and ESCRT-dependent. The ESCRT-0 complex recruits proteins via ubiquitin or clathrin in the ESCRT-dependent pathway. ESCRT-I and ESCRT-III regulate the budding process, the production of ILVs, and the subsequent synthesis of exosomes. Syntenin, ALIX, and ESCRT-III are involved in the ESCRT-independent mechanism. Syntenin promotes proteins to form cargo clusters, and then ALIX and ESCRT-III govern the budding process, ILV development, and exosome formation. Protein engagement and cargo sorting can be accomplished by either ESCRT-independent mechanisms or ESCRT-dependent mechanisms [25,26].

Table 1.
Classification of EVs [27,28].
Table 1.
Classification of EVs [27,28].
| Characteristics of EVs | ||||
|---|---|---|---|---|
| Small-Sized EVs | Medium-Sized EVs | Large EVs | ||
| Exosomes (Nanovesicles) | Microvesicles (Ectosomes) | Oncosomes | Apoptotic Bodies | |
| Origin | Endocytic pathway | Cell plasma membrane | Cell plasma membrane | Cell plasma membrane |
| Size | 30–200 nm | 200–1000 nm | >1000 nm | 500–10 µm |
| Common markers | CD63, CD9, CD81, TSG101, flotillin, Alix, ESCRT-3 | Integrins, Selectins, CD40 ligand, ARF6, VCAMP3 | Annexin A1, Annexin A2, ARF2 | Annexin V, Phosphatidylserine, Thrombospondin |
| Content of EVs | Secondary metabolites, proteins and nucleic acids (mRNA, miRNA and other non-coding RNAs), lipids | Secondary metabolites, lipids, proteins and nucleic acids (mRNA, miRNA and other non-coding RNAs) | Nuclear fractions, cell organelles, proteins, DNA, coding and non-coding RNA, lipids | Nuclear fractions, cell organelles, proteins, DNA, coding and non-coding RNA, lipids |
| Pathways | Stimuli, ESCRT, and tetraspanin dependent | Stimuli, Ca2+, cell-dependent | Apoptosis related | Apoptosis related |
| Functions | Intercellular communication | Intercellular communication | Intercellular communication | Phagocytosis |
3.2. Composition of EVs
The contents of EVs represent the function and content of their parent cells, and as such, a variety of proteins, enzymes, transcription factors, lipids, extracellular matrix proteins, receptors, metabolites, and nucleic acids are present both inside and on the surface of an EV (Figure 1A) [9,12,18,21,22,29,30]. The cargo contents vary depending on the specific stimulus or disease conditions during EV formation. Cells consist of several thousands of lipid species, and using mass spectrometry (MS), it is now possible to quantify approximately 1000 lipids in a sample. EV membrane is made up entirely of lipids, and it is widely known that certain lipids are more abundant in EVs than in their parent cells. EVs derived from known cell types contain the composition of lipid classes, including cholesterol (CHOL), phosphatidylinositol (PI), glycosphingolipids, SM, phosphatidylcholine (PC), phosphatidylserine (PS), and phosphatidylethanolamine (PE). The lipid composition of EVs from body fluids includes sphingolipid and PC [31,32]. In EVs, a wide range of genetic material is detected. A large number of short RNAs are identified using various approaches, including next-generation sequencing. Y RNA, tRNA fragments, long and short non-coding RNA, vault RNA, and piwi-interacting RNA are all discovered in EVs, in addition to generally recognized RNA species such as mRNAs, miRNAs, and rRNAs. Specific miRNA and mRNA packaging into EVs may be aided by certain sequence motifs and posttranscriptional modifications in the 3′ end of miRNAs [33,34]. Recent investigations demonstrated that EXOs include both mitochondrial DNA (mtDNA) and fragmented chromosomal DNA [35,36]. EVs contain DNA ranging from 100 base pairs to 2.5 kilobase pairs [37]. Despite the strong evidence that EV-DNA is present in EVs, the DNA cargo’s functional importance is unknown. The protein content of EVs is related to the type of cell and the biogenesis mechanism in which they are produced. Tetraspanins CD81, CD37, CD63, CD53, and CD82 and major histocompatibility complex class II (MHC class II) are more abundant in EVs originating from the endolysosomal compartment. Alix and tumor susceptibility gene protein 101 (TSG101) are two auxiliary proteins required for the endosomal sorting complex required for the transport (ESCRT) pathway. EVs, regardless of cell type, contain ESCRT proteins, Alix, TSG101, and chaperones such as Hcs70 and Hsp90. Typical protein families present in EVs are given in Table 2 [38,39].

Table 2.
Typical protein families found within EVs [38,39].
3.3. Technologies for EV Isolation
Blood, urine, breast milk, cerebrospinal fluid, tears, saliva and nasal secretions, ascites, and sperm are all found to contain EVs. Many breakthroughs in EV detection and separation techniques were made in recent decades. As a result, EV recovery, purity, sensitivity, and specificity were all improved (Table 3). It is crucial to establish that the isolated vesicles are EVs with no contaminating components before doing any functional study. Despite this, challenges in isolation approaches exist due to overlapping size ranges, small diameters, and comparable morphologies to other EVs.

Table 3.
Characteristics of EV isolation methods (Adapted from [38]).
There is no unanimous agreement on the ideal EV isolation strategy. Despite the fact that MVs and EVs are formed by independent pathways, due to their size overlap, most separation methods do not separate a pure population. Density gradient centrifugation, ultrafiltration, ultracentrifugation/differential centrifugation, polymer-based precipitation, immunoprecipitation, microfluidic device isolation, and size exclusion chromatography (SEC) are just a few of the EV separation technologies that are developed thus far [40].
4. Background in T1DM
4.1. Healthy Blood Sugar Regulation
Diabetes is a disease in which metabolic processing and energy regulation malfunction. Energy in the body is provided through an intricate balance of food intake, breakdown, and uptake. In a healthy, non-diabetic system, the carbohydrates, lipids, and proteins from food are broken down into glucose, which is then delivered to cells via facilitated diffusion to be used for energy. Insulin is the vital gatekeeper in this process, regulating blood glucose levels (also known as blood sugar) by triggering cells to take up glucose from the blood (Figure 2A) [41,42,43]. This increase in membrane permeability is thought to be a result of the translocation of glucose receptors to the cell membrane [42,43]. The pancreas plays a key role in this process, as it aids in the digestion, absorption, utilization, and storage of energy substrates [41,44]. The pancreas is located close to other important organs in metabolism, lying below the stomach and extending from the spleen to the duodenum [45]. The pancreas is structurally distinct, containing cells that perform exocrine (nutrient digestion) and endocrine functions (glucose homeostasis) [44,45]. Though endocrine cells, which make up clusters called the islets of Langerhans (or pancreatic islets), are vital in the regulation of blood sugar, they make up only 1–2% of the adult pancreas [7,44,45,46]. Each islet has a number of distinct cell types, which produce hormones that play different roles in blood sugar maintenance. This includes α-cells and δ-cells, which produce glucagon (which has a role opposite to that of insulin, raising blood glucose levels) and somatostatin (which regulates the secretion of other hormones from the pancreas), respectively (Figure 2A) [44,45]. β-cells, which make up 65 to 80% of islets, produce insulin and amylin at a 100:1 ratio, which collaboratively works to prevent blood sugar spikes by triggering the uptake of glucose into cells and promoting satiety [44,45,46,47]. Each β-cell produces up to 1 million insulin molecules/minute (a number that is further elevated in response to eating) [7,46]. Insulin is considered a “key” in sugar regulation, allowing cells to uptake glucose by increasing the permeability of plasma membrane to glucose, inducing the conversion of glucose to glycogen in the liver, and promoting the production of fatty acids and proteins [46]. Together, the cells of the pancreas and the hormones secreted regulate blood sugar, with the average, optimal 24 h glucose levels of 99 ± 7 mg/dL [48]. In non-optimal, hypoglycemic conditions, blood sugar levels are below ideal levels, triggering the secretion of glucagon from α-cells, which promotes the breakdown of glycogen stored in the liver to glucose [46]. When the body has high blood glucose content, called a hyperglycemic state, β-cells release insulin and amylin to reduce the blood sugar levels and decrease food intake [46]. This system malfunctions in T1DM causing insulin levels drop, and blood sugar levels spike as cells are unable to take up glucose.
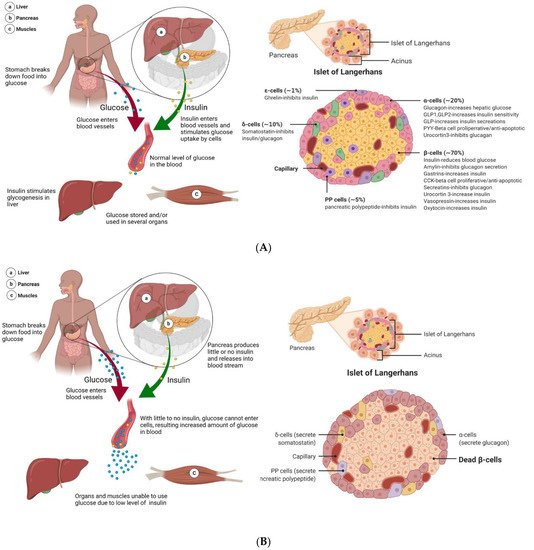
Figure 2.
Processing of glucose in healthy and T1DM patients. (A) As food is ingested in a healthy system, it gets broken down into glucose, which is then released into the bloodstream. In response, the pancreas secretes insulin. Glucose is transported across the membrane via facilitated diffusion. As such, as insulin increases the glucose permeability of cells, this allows the uptake and utilization of glucose. The pancreas is made up of many important cell structures, including the islets of Langerhans. The islets are a low percentage of the total pancreas mass but include cells that are vital in glucose regulation. This includes β-cells, the primary component of islets, making up 65–80% of the total islet cell count. β-cells are responsible for the production and secretion of insulin and amylin. (B) In diabetic patients, as food is digested and converted into glucose to be released into the bloodstream, the normal corresponding insulin response is lacking. This is because the pancreas is no longer producing enough insulin to enable the uptake and utilization of glucose, leading to a state of hyperglycemia. In T1DM, a large number of β-cells in the pancreas are killed due to autoimmune instigated T-cell attacks. Though some β-cells may remain, the pancreas is no longer capable of insulin independence.
4.2. Mechanisms in T1DM
Type 1 diabetes is a multifaceted disorder produced by a complex interplay of environmental and genetic variables, the former of which includes several susceptibility genes. The discovery of genes that confer vulnerability to T1DM would help to clarify pathogenetic processes in the establishment and progression of the disease, paving the way for the development of effective approaches for disease prevention and management. Genome-wide linkage analysis and genome-wide association studies (GWAS) and genome-wide linkage analysis have recently contributed significantly to our understanding of the role of genetics in the onset and progression of T1DM. Nearly 60 risk regions all over the human genome were found by GWAS, which is designated by single nucleotide polymorphisms that confer genetic susceptibility to T1DM [49,50]. Several studies claimed that 50% of the risk factors for T1DM are genetic [51]. When researchers first began investigating the causes of T1DM, genetic risk factors were focused on as the sole trigger, as susceptibility to developing T1DM is influenced by carrying specific high-risk haplotypes of class II human leukocyte antigens (HLA), such as HLA-DR (DR3/4) and HLA-DQ (DQ8) [4,52,53]. Over 50 non-human leukocyte antigen (HLA) regions and HLA genes were discovered in T1DM using genomic screening [51]. HLA-DQ and HLA-DR, in particular, are closely linked to T1DM. Haplotypes of class II molecules antigens can substantially increase or decrease the binding properties of associated autoantigens, resulting in T1DM development [54,55]. The impact of HLA on T1DM risk is supported by a twin concordance rate of up to 70% and 8–10% of risk shared between siblings [56]. HLA genetic susceptibility contributes to the loss of tolerance of β-cells antigens due to T-cell mediated islet cell death, insulin deficiency, and elevated blood glucose levels, suggesting that these genes operate at the β-cell level and play a crucial role in the pathogenesis of T1DM [3,57]. Despite initial thoughts, genetic predisposition alone cannot explain the incidence of T1DM, as recent work has identified that HLA loci account for 50% of familial T1DM [4,6,53,56,58]. Now, T1DM is considered an intricate interaction and balance between the genome, metabolism, and gut microbiome diversity [4,6,53,59,60,61]. There are also a number of environmental influences, including diet, vitamin D deficiency, infections, pollutants, and oxidative stress, associated with the increasing presentation of type 1 diabetes, with β-cell stress potentially providing a mechanistic underpinning [6,7,56,59,60]. In addition, comorbidities are often found in those diagnosed with T1DM, as the majority of T1DM patients (60%) are obese or overweight, while many others have hyperlipidemia and hypertension [62]. Comorbidities can occur as complications of T1DM, with individuals with poorly regulated T1DM having a 10 times higher chance of dying from cardiovascular disease than healthy individuals [4]. Though T1DM is slightly more common in males than females, females are more likely to experience complications of T1DM such as hypoglycemia, vascular events, and premature lethality in response to T1DM than males [6,62,63].
The mechanisms of T1DM begin well before full diabetes, as T-cells attack insulin-producing β-cells (Figure 2B) [6,7,53]. During this prediabetes phase, the pancreas can maintain normal glucose processing. However, the amount of insulin that can be secreted in the body is reliant both upon the absolute number of β-cells and β-cell function [46]. As the immune-mediated destruction of β-cells continues, this puts increasing demand and thus limits the function of the remaining β-cells. The body enters a state of hyperglycemia, with excessive levels of unused glucose in the bloodstream. In a healthy system, protective immune activated inflammation is terminated after a threat is eliminated, but the autoimmunity of T1DM leads to chronic inflammation, further promoting disease progression, β-cell apoptosis, and influencing insulin resistance [64,65]. Though some patients suffer from a complete loss of functional β-cells, measurable levels of C-peptide, which is the biologically inactive peptide formed when β-cells convert proinsulin to insulin, indicate that some T1DM patients can produce insulin; nevertheless, the functional β-cell mass is still decreased to the level of insulin-dependency [2,7]. In the 100 years since the discovery of insulin, knowledge of the T1DM pathology process, improved treatment options, and novel diagnostic measures were developed.
4.3. Role of EVs in Pancreas and Islet β-Cell Transplantation
The continuous glucose monitoring systems, unfortunately, are not capable of rapid responses to glycemic variability as might be produced by illness, stress, or a simple unannounced meal [2]. Moreover, though insulin administration offers some regulatory abilities, many patients develop chronic complications or become disabled by refractory hypoglycemia [66,67]. An alternative treatment option is the transplantation of the pancreas or islet β-cells to prevent or slow the complications and progression of T1DM, improving longevity and quality of life (Figure 3A). Though pancreas transplantations continued to evolve and develop over time (for the full record, see [66]), the number of pancreas transplants are dropping. This is due to inherent surgery risk and improvements in other treatment options, with a 20% decrease in transplants in the USA between 2005 and 2014, transplants peaking in 2004 [44,68]. Transplantations as treatments for T1DM are generally only considered in patients who exhibit a history of hard to control diabetes, defined as ≥1 episode of severe hypoglycemia per year, frequent and severe metabolic or microvascular complications, and problems with exogenous insulin therapy [69,70,71,72]. As such, pancreas transplantations are typically only performed 20 years after diabetes diagnosis [69]. Transplanting a pancreas can fully replace the β-cell mass and as such, is considered the only “near-cure” for T1DM, while transplanting islet β-cells can only partially restore the population of insulin-secreting cells needed for insulin and glucose regulation [66]. The first documented pancreas transplants were performed in the 1960s–1970s on uremic diabetic patients in combination with a kidney transplant [44,48,66]. Early surgeries found that transplanting a pancreas alone led to a high rate of organ rejection, while cases in which a pancreas and a kidney were simultaneously transplanted had minimal organ failure (though were susceptible to numerous other issues accompanying surgery such as thrombosis or infection) [66]. Recent reports indicate that this graft survival trend has continued, with 70% graft survival in patients with simultaneous pancreas and kidney transplant, and only ~50% for pancreas in isolation [2,44,68].
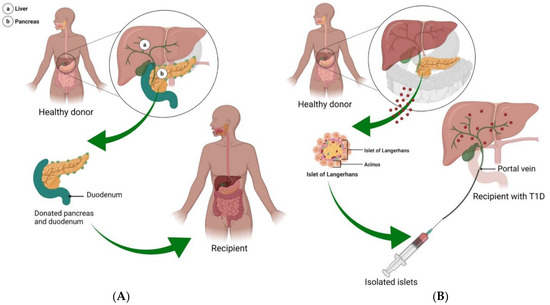
Figure 3.
Pancreas and islet cell transplantation. (A) In T1DM, many of the islet β-cells die, limiting insulin production and leading to a number of life-long complications. The only near-cure treatment strategy is the transplantation of a pancreas from a healthy donor. In this situation, a pancreas with a small portion of the duodenum attached is inserted into the T1DM patient. With this approach, recipients are able to achieve up to 5 years of insulin independence. (B) Considered an experimental procedure, delivery of islet cells to T1DM patients offers a less invasive and lower risk treatment option, in which islet cells from 1 to 3 healthy donors are isolated and delivered to the pancreas via the portal vein.
As an alternative to pancreas transplant, islet β-cell transplantation is a low-risk procedure, with donor islets infused into the liver via the portal vein (Figure 3B) [6,73]. Though islet transplants are practiced internationally, this procedure is still considered experimental in the USA [74]. Though islet transplantation does not replace the entire β-cell mass, it is less invasive than a pancreas transplant while still providing the potential to restore normal glycemic function, re-establish hypoglycemia awareness, and minimize severe hypoglycemic responses [2,67]. Islet transplants were first attempted in the 1970s, with very low success rates [71]. The first fruitful islet transplantations occurred in the late 1980s and 1990s, with the ability to consistently establish endogenous insulin secretion via transplant taking place in the 2000s [48]. The principal indications for β-cell replacement therapy are to treat a small subset of insulin-dependent T1DM and T2DM patients with severe or uncontrollable hypoglycemia [6,75]. An estimated 50% of transplant recipients maintain insulin independence one to two years post islet transplant [45,73,76]. A recent approach in islet delivery is to encapsulate islets to protect them from the immunological environment, allowing greater survival and function [2]. Islet cells may be transplanted in combination with hormones or growth factors such as glucagon-like peptide-1 to augment insulin secretion and inhibit glucagon release [56,77,78].
There are several pieces of evidence that indicate that EVs may increase the survival and efficacy of pancreatic islet transplantation [79]. The communication between β-cells and endothelial cells is imperative for islet transplantation because it helps the body re-grow new blood vessels. Figliolini et al. showed that EVs derived from human islets could be taken up by endothelial cells inside the islets and help with angiogenesis and engraftment, according to in vitro experiments [80]. Poor graft vascularization limits the success of islet transplantation. Cantaluppi et al. investigated that EVs secreted by endothelial progenitor cells promoted human islet vascularization. EVs stimulated migration, proliferation, cell survival, and organization in vessel-like structures in islet endothelial cells [81]. EVs derived from endothelial progenitor cells increased insulin secretion, survival, and angiogenesis of islets implanted in severe combined immunodeficiency (SCID) mice after incorporation into islet endothelium and β-cells [81]. EVs can facilitate islet transplantation through immunomodulatory effects in addition to enhancing revascularization. Human bone marrow MSCs and their EVs have been shown to be capable of suppressing immunological responses and delivering small RNAs. As a result, they may be able to improve islet transplantation by delivering small RNAs that enhance islet function while preventing immunological rejection. EVs produced from MSCs are shown to promote islet transplantation by promoting regulatory T cell function and suppressing peripheral blood mononuclear cell proliferation, according to a study conducted by Wen et al. [82]. The therapeutic cargo contained within EVs must be appropriately studied for health and safety issues. In conclusion, exosomes may portray an enthralling therapy in the context of islet transplantation, not only for enhancing revascularization but also for stimulating graft tolerance.
4.4. EVs and T1DM Pathogenesis
T1DM is a complex, multifactorial disease that is heavily influenced by cross-talk between metabolically active tissues. EVs are thought to be a major mediator of cellular communication and have been found to be increasingly important in disease pathogenesis, as exposure of materials from cells in crisis affects the survival of recipient cells [38,83,84]. For example, EVs from obese or diabetic mouse models can induce insulin resistance and glucose intolerance, while EVs from healthy lean mice can reverse these symptoms [85,86]. EVs are shown to contain hormones and autoantigens that have a key role in insulin sensitivity and the initiation of the strong, detrimental autoimmune response in T1DM, including insulin receptor substrate 1, GAD65, IA-2, and proinsulin [87,88,89,90,91,92]. The injection of EVs containing autoantigens was found to increase cytokine release, stimulate Toll-like receptors, and facilitate graft rejection [90,93]. EVs containing high levels of autoantigens are released as the pancreas undergoes excess stress, as increased levels of glucose or treatment with proinflammatory cytokines were found to drastically increase EV secretion [87,89,91]. Interestingly, macrophage-derived EVs are able to activate memory T-cells [94]. In a similar fashion, EVs isolated from insulinomas can induce the secretion of inflammatory cytokines in diabetes mouse models through the MyD88-dependent pathway, which is downstream of inflammatory signals of Toll-like receptors and interleukin-1. Activation of MyD88 can trigger T-cell proliferation and activation, further stimulating detrimental autoimmune response in T1DM [88,95]. EVs also participate in a negative feedback loop of cytokine production, which further drives macrophage activation [96].
Micro-RNAs (miRNAs) play key regulatory functions in various aspects of blood sugar regulation, such as embryonic development in pancreatic islets, maintenance of α- and β-cell phenotypes, insulin secretion, and exocytosis [84]. The miRNA content of EVs can play a major role in propagating T1DM pathology. EVs secreted from cells exposed to cytokines led to apoptosis when delivered to β-cells, an effect that can be blocked through the inactivation of miRNA activity through inhibition of Ago2 (a component of the RISC complex that is essential for miRNA action) [83]. Increased EV expression of miR-21, miR-34a, miR-146a, and miR-29 as triggered by exposure to cytokines early in disease contributes to the cytokine-induced β-cell dysfunction [53]. The release of miR-375 and miR-148a-3p in EVs are associated with β-cell death [97]. Similarly, EV-contained miR-142-3p/5p and miR-155 lead to the upregulation of cytokines in pancreatic islets β-cells, with in vitro inhibition of these miRNAs able to reduce apoptosis rate in primary rat islet cells [90]. When splenocytes isolated from diabetes-prone mice are exposed to β-cell EVs, there is a miR-29b-dependent increase in the production and secretion of TNF-α [90]. Further understanding of how the contents of EVs are modified in disease states can give insight into the spread of inflammation and pathology in T1DM.
4.5. EVs as Novel Biomarkers in T1DM
Several lines of evidence suggest that many factors induce T1DM, including genetic predisposing factors, non-infectious environment agents, endogenous antigens, exogenous infectious pathogens, and physiological stress [4,6,56,59,60]. As such, the underlying autoimmune process of T1DM precedes the onset of clinical symptoms. Though the early stage of T1DM, called prediabetes, is asymptomatic, it provides an excellent advantage for early prognosis and prevention of disease [98]. Currently, susceptibility genes (high-risk HLA genes; HLA-DR (DR3/4) and HLA-DQ (DQ8)) and hallmark islet autoantibodies (GAD65, IA-2, insulin, and ZnT8) are the gold-standard approaches for the detection of T1DM. Notably, a clinical study has revealed that HLA typing identified only 10% of study subjects with high T1DM risk while the remaining 90% displayed no autoimmunity, and less than 50% of cases were identified by a combination of genetic markers [52,53]. Further, a major limitation of diagnostic autoantibodies is that they appear relatively late during disease progression when almost 90% of β-cells are lost [99,100]. Additionally, a subset of individuals who showed seropositivity for autoantibodies will not develop clinical diabetes [101]. Therefore, current diagnostic tools do not meet the need for early diagnosis, creating a great demand for the identification of a suitable biomarker for early risk prediction.
In recent years, several studies documented the potential utility of EV-based biomarkers in ailments such as liver disease, neurodegenerative diseases, and cardiac disorders [102,103,104,105,106,107]. EVs have easy accessibility from bodily fluids and molecular and structural stabilities, making them an excellent candidate for harboring biomarkers in clinical settings [108]. In the context of T1DM, EVs drawn from blood can offer a new understanding of the status of sugar regulation (Table 4). Evidence indicates that EVs secreted by human pancreatic insulin-producing β-cells may trigger and accelerate disease progression via stimulating autoimmune responses [88,109,110]. Studies also implicated that the severity of T1DM and its pathophysiological conditions, such as inflammation, can alter the yield, content, and composition of EVs released from β-cells, information that can be utilized in the discovery of biomarkers [47,83,108,111,112,113,114,115,116]. A study by Garcia-Contreas and colleagues found that T1DM patients had reduced plasma EV size and concentration compared to healthy controls [108]. A recent report of EV RNA profiling of human islets from T1DM patients revealed modified EV RNA expression in response to proinflammatory cytokine treatment. Treating islets with proinflammatory cytokines mimics the chronic inflammation found in T1DM and results in the differential expression of RNAs associated with necrosis, insulin secretion, calcium signaling, and apoptosis, emphasizing the toxic effect of inflammation on islet survival [117]. Krishnan and colleagues identified miR-155-5p as the most upregulated miRNA, while miR-4485 was the most downregulated [117]. Significantly, islet-derived EVs act as antigen carriers, including autoantibodies GAD65, ZnT8, and β-cell glucose transporter 2, which are released in response to cellular stress, and serve as markers of T1DM progression [87,95,97,114,118,119,120]. Importantly, islet EVs were found to specifically activate memory T-cell responses in diabetic patients, but not healthy controls [119].
MiRNAs are crucial in blood sugar regulation, and a diverse population of miRNAs can be found within EVs [84]. As such, EV miRNAs are considered attractive candidates for biomarkers to diagnose T1DM or determine the success of a treatment plan [84,121,122]. A number of miRNAs are consistently upregulated in T1DM, including miR-21-5p, miR-100-5p, miR-150-5p, miR-181a-5p, miR-210-5p, and miR-375, many of which can be found in EVs [53,122]. Work studying young, newly diagnosed T1DM patients identified distinct EV miRNAs modifications compared to healthy controls, with downregulated levels of miR-195 and miR-455, while miR185 was upregulated [115]. A study employed miRNA microarray and qPCR analysis to characterize plasma-derived EVs and identified a distinct miRNA profile found in patients with long-duration T1DM compared with healthy controls with seven differentially expressed miRNAs [123]. Distinctions in EV miRNA content were also found in children with urinary EV miR-424 and miR-218, and serum EV miR-21-5p was found to be elevated in children with T1DM compared to healthy controls [114,124,125]. Of vital importance, research has suggested that miRNAs such as miR-21-5p have detrimental impacts in T1DM and can be linked with β-cell inflammation and apoptosis [126,127,128,129]. Work studying young, newly diagnosed T1DM patients identified distinct EV miRNA modifications compared to healthy controls, with downregulated levels of miR-195 and miR-455, while miR185 was upregulated [115]. Importantly, certain EV miRNAs are elevated in distinct timeframes and conditions; for example, work within a mouse model of diabetes found that miR-375 was elevated two weeks before the onset of diabetes and was thought to be a specific marker of deteriorating β-cell health and function [53,83,84]. Studies reported elevated levels of β-cell miR-21-5p in EVs in response to inflammatory cytokines treatments, both in animal models of type 1 diabetes and in vitro, while total levels are decreased [95,126,127,128,129].
There are a number of complications that can occur in diabetic patients, including diabetic nephropathy and retinopathy, a kidney disease that and complication damaging the blood vessels in the eye, respectively. Recent studies found that EVs and the miRNA content within EVs can aid in the early diagnoses of these complications [84,85,130,131,132,133,134,135]. Barutta and colleagues determined that miRNA-145 within urinary EVs is elevated in diabetic patients with diabetic nephropathy [130], while Kalani et al. demonstrated that increased levels of Wilm’s tumor-1 protein within urinary EVs could predict a decline in renal function [131]. Further, peroxisome proliferator-activated receptor-γ within EVs increases with disease severity of diabetic retinopathy, while miR-222 is decreased [132]. Furthermore, certain populations of EVs have significantly elevated levels of creatine in response to hyperglycemic events in T1DM patients [133].
In transplantation, the primary target time frame for both biomarkers and therapeutics is immediately after the graft to prevent surgical complications and graft rejection [136]. In the instance of transplantation, the organ or cells release donor-specific EVs that are involved in immune recognition and often influence rejection or graft tolerance; in the case of graft rejection, there is a significant decrease in transplant islet EV signal, as well as changes in EV miRNA and proteomic profiles, displaying distinct biomarker potential [137,138,139]. Notably, miRNAs in EVs can be beneficial in identifying successful T1DM treatments, as in many cases of malfunctioning transplantations, there are distinct upregulated and dysregulated miRNA and protein profiles that can be identified in EVs up to 5 years post-transplantation [138,140]. Additionally, studies identified that islet endothelial cells (IECs) and β-cells secrete a subset of miRNAs in EVs that take part in cross-talk between IECs and β-cells, thus promoting angiogenesis, a process limited in T1DM and inflammatory conditions, and often a limiting factor in transplantation efficacy [80,81,82,83,140]. As a marker of vascularization, these miRNAs can identify early problems in transplantation. Time course studies found that EVs from islet cells contain varying miRNA in response to inflammation and hypoxia, with miR-29b-3p and miR-216a-5p released in EVs 6 h after hypoxia and cytokine exposure, while miR-375 and miR-148a-3p were secreted 24 h after exposure [91]. Importantly, these stress conditions replicate negative conditions found in diabetic mice following islet transplantation [91]. Cumulatively, this evidence suggests that EVs and EV miRNAs are promising biomarkers for early T1DM prognosis and development, as well as conditions following pancreas or islet transplant.

Table 4.
Studies utilizing EVs as biomarkers in diabetes.
Table 4.
Studies utilizing EVs as biomarkers in diabetes.
| Study | Experimental Subject | Collected Specimen | EV Isolation | EV Size | MSC EV Characterization | Biomarkers | Important Finding from the Studies |
|---|---|---|---|---|---|---|---|
| [141] | Rat | Blood | UC | NA | WB, FC | eNOS and caveolin-1 | Decreased levels of eNOS and overexpression of caveolin-1 may serve as a biomarker for vascular injury |
| [117] | Human | Human islet cell | UC | <150 nm | EM, WB | piRNAs, snoRNAs, tRNAs, and lncRNAs | EV miRNAs may consider as potential circulating biomarkers for T1DM |
| [139] | Human | Plasma samples | SEC, UC | NA | WB | Islet endocrine hormone proteins and mRNAs | Circulating transplant islet-specific EVs has the potential to be a diagnostic tool for recurrent autoimmune T1DM after islet transplantation |
| [137] | Mice and Human | Human plasma and urine samples | SEC, UC | 30–200 nm | NTA, EM, WB | miRNA | EV miRNAs as biomarkers for monitoring immune rejection |
| [142] | Human | Urine samples | UC | NA | WB | Water channel aquaporins | Role of water channel aquaporins AQP5 and AQP2 as novel biomarkers to help in classifying the clinical stage of diabetic nephropathy |
| [133] | Human | Urine samples | UC | 40–100 nm | EM, NTA | Urinary podocyte | Analysis of urinary podocyte MPs may act as an early biomarker of glomerular injury in uncomplicated T1DM |
| [143] | Human | Plasma samples | UC | NA | NA | Cytokines and angiogenic factors | EVs isolated from plasma shows upregulated levels of cytokines and angiogenic agents in diabetic patients. |
| [134] | Human | Urine samples | UC, Filtration | NA | WB | Cystatin B and altered protease profiles | Enhanced cystatin B and altered protease profiles in EVs isolated from urine may act as biomarkers of kidney dysfunction in T1DM |
| [144] | Rat and Human | Kidney tissue and Urine samples | UC | NA | WB | Regucalcin | Lower levels of urinary exosomal regucalcin may act as a biomarker of diabetic kidney disease |
| [130] | Human | Urine samples | UC | <100 nm | EM, NTA, WB | miRNAs | EV miR-145 may act as a biomarker of T1DM |
| [131] | Human | Urine samples | UC | NA | WB | WT1 protein | Elevated expression of EV WT1 protein may act as a biomarker of T1DM |
4.6. Paracrine Effects of EVs on Islet Cells
As carriers of vital proteins, miRNAs, and other contents, EVs have natural therapeutic benefits in various diseases, including T1DM (Table 5) [145]. Research found that the administration of EVs can help preserve organs in donor transplantation and aid in minimizing autoimmunity and cytokine release, thus minimizing immune rejection, improving viability and long-term survival [63,82,145,146,147]. Specifically, EVs derived from mesenchymal stem cells (MSCs) were found to suppress the development of T-cells and slow disease development [63,114,145]. EVs naturally contain a number of valuable proteins and miRNAs but can also be used to deliver select cargoes, either through modification of the parent cells or through specific loading of therapeutic content [148,149]. Wen et al. found that cell modifications in which EVs delivered plasmids to silence Fas and miR-375 in human islets improved resilience against inflammatory cytokines and suppressed immune reaction [82]. Application of MSC EVs, through the delivery and upregulation of vascular endothelial growth factor (VEGF), promoted insulin secretion in islets, as well as downregulated pro-apoptotic genes, upregulated pro-survival factors, and increased angiogenesis, thus targeting primary causes of graft loss [80,150]. MSC-derived EVs were found to be protective in diseases, including T1DM, serving to block apoptosis, a major issue in graft survival, through the inhibition of the phosphorylation of p38 [151]. In a mouse model of diabetes induced using streptozotocin, the delivery of bone-marrow MSC-derived EVs aided in the regeneration of islet cells [147,152]. EVs from adipocyte-derived stem cells can promote angiogenesis and modulate the immune response, attenuating T1DM pathology [95]. Delivery of EVs derived from endothelial progenitor cells can enhance human islet vascularization, promoting insulin secretion and islet survival [81], and it is hypothesized that EV delivery early in T1DM may help restore a patient’s original islet cells [153]. Adipocyte-derived stem cell EVs modulate the immune response and promote proangiogenic properties, a vital aid in transplant survival [95]. EVs derived from bone-marrow-MSCs have shown the ability to ameliorate insulin resistance developed through aging and T2DM through the delivery of miR-29b [154].
In addition, EVs from a number of cell types, including MSCs, can have therapeutic impacts on the many complications that surround T1DM, including cognitive deficits, cardiac dysfunction, microvascular complications, and even transplantation rejection [155,156,157,158]. EVs loaded with miR-222 injected into rabbits with T1DM, and diabetic retinopathy showed attenuation of retinal degeneration [159]. Diabetic nephropathy was improved through the delivery of EVs derived from MSCs and adipose-derived stem cells by induction of autophagy [85,160,161] Though a number of studies regarding EV therapeutics use a heterogeneous population of EVs, a study by McGuinness and team (2016) found that microvesicles specifically stimulated long-term recovery and function in diabetic mice, improving glucose, insulin, and glucagon levels compared to the smaller EV population exosomes [162].
Finally, many pancreas and islet transplant recipients receive treatments to aid in successful transplantation that, if loaded into EVs, may be better protected and enhance impact. For example, IL-2 receptor antibodies to provide better early posttransplant protection of islets and coadministration of a TNF-α inhibitor can lead to a higher probability of insulin independence, two cargos that may benefit from protective EV delivery [67,78]. The overexpression and delivery of insulin-like growth factor 1 and protein 3 gamma not only enhance the proliferation of β-cells but demonstrate the ability to regenerate and restore β-cells [56]. The restoration and regeneration of pancreatic islet cells are regulated in part through EV interaction with the pancreatic and duodenal homeobox-1 pathway, which is vital in pancreatic cell differentiation [163,164]. Further, MSC-EVs at the time of islet transplantation are shown to improve islet functions through the delivery of necessary nutrients and thus transplant tolerance [147,165]. The delivery of EV-loaded cargos is not limited to T1DM. Glucagon-like peptide-1 is a hormone commonly used in T2DM to augment insulin secretion, aiding in glycemic index and insulin independence, can be loaded into EVs for better, more protected distribution [77].

Table 5.
EVs as therapeutics in T1DM.
Table 5.
EVs as therapeutics in T1DM.
| Study | MSC Source | MSC EV Isolation | EV Size | MSC EV Characterization | Model Species/Cells | Intervention(s), Route, and Dose | Important Finding from the Studies |
|---|---|---|---|---|---|---|---|
| [166] | Bone marrow-derived MSCs | NA | NA | NA | Rat model of T1DM | Tail vein injection | EV miR-145 secreted by bone morrow MSCs shows neurorestorative effects in diabetic rats with stroke |
| [167] | Human urine-derived stem cells | UC 30% sucrose/D2O cushion | 50–100 nm | Flow cytometry, WB, NTA | Adult male Sprague Dawley (SD) rats | Weekly with 100 μg of EV dissolved in PBS to a final volume of 200 μL via the tail vein | Reduction of the urine volume and urinary microalbumin excretion; prevention of podocyte and tubular epithelial cell apoptosis in diabetic rats |
| [152] | Bone marrow-derived MSCs | UC | 100 nm | FC, EM | Albino female rat (STZ-induced rat model of T1DM) | Intraperitoneal injection | EVs derived from MSCs showed therapeutic and regenerative effects upon the pancreatic islet cells |
| [168] | Human umbilical cord blood-derivedendothelial progenitor cells | UC | 50–60 nm | Tunable resistive pulse sensing analysis, EM, WB, NTA | Adult male SD rats | Subcutaneous injection with EVs 2 × 1010 or 1 × 1011 particles, dissolved in 200 μL of PBS | Increased angiogenesis through Erk1/2 signaling |
| [169] | Human umbilical cord blood-derivedendothelial progenitor cells | UC | 40 to 80 nm | EM, WB | Adult male SD rats | Subcutaneous injection at wound sites with 100 μL PBS or EPC-EVs (100 μg) around the wounds | Enhanced wound healing by regulating vascular endothelial cells function |
| [170] | Adipose derived-MSCs | ExoQuick | 200 nm | EM, WB | 10-week-old SD rats | Intraperitoneal injection | Adipose tissue-derived MSC EVs enhanced the erectile function in diabetic rats |
| [155] | Bone-marrow MSCs | UC | NA | EM, WB | Streptozotocin-Induced Diabetic Nude Mouse Model | Intracerebroventricular administration PKH-labeled EVs (5 µg in 10 µL aCSF) | Improvement of cognitive impairments by repairing damaged neurons and astrocytes |
| [171] | Cardiomyocytes overexpressing HSP70 MSCs | UC | 50–60 nm | EM, WB, NTA | Streptozotocin-Induced Diabetic Nude Mouse Model | Intraperitonial injections | Enhancement of Hsp20 in cardiomyocytes can give protection in diabetic hearts via the release of EVs |
| [172] | Human bone marrow-derived MSCs | ExoQuick, Precipitation | NA | NA | Male C57BL/6J mice | Intravenous injections | Attenuation of renal fibrosis |
| [173] | Human fibrocytes | UC | 50–100 nm | EM, WB, NTA, FC | Diabetic B6.Leprdb/db mice (11–12 weeks old) | 200 μL of PBS containing 0, 5 or 50 μg EV; 40 μL subcutaneously injected around the wounds sites; 40 μL were directly applied to the wound beds | Enhanced proliferation and migration of diabetic keratinocyte; increased wound closure |
| [174] | Mouse serum | ExoQuick | NA | NA | C57BL/6J mice, RIP-CreER mice, and Rosa26-GNZ mice | Intraperitoneal and intravenous injections | Increased pancreatic beta-cell proliferation |
| [82] | Human bone marrow MSCs | Total exosome isolation reagent (Invitrogen) | NA | NA | NSG Mice (NOD scid gamma) | Intraperitoneal infusion | Inhibition of immune rejection |
| [81] | Endothelial progenitor cells isolated from PBMCs | UC | NA | FC, EM | Immunodeficient (SCID) Mice | Subcutaneous implantation | Enhancement of neo-angiogenesis of human pancreatic islets |
| [175] | Adipose-derived MSCs | UC | 40–100 nm | FC, EM, DLS, SEM | 6- to 8-week old C57BL/6 male mice | Intraperitoneal injection | Enhanced regulatory T-cell population without change in the proliferation index of lymphocytes |
| [110] | Human MSCs | UC | NA | NTA | NA | NA | MSC-derived MVs suppress inflammatory T-cell responses in the islet antigens through the promotion of regulatory dendritic cells in T1DM |
| [80] | Human islets | UC | 54–256 nm | FC, EM, WB, SEM | NA | NA | Human islet-derived EVs participate in beta cell-endothelium cross-talk and the neoangiogenesis process |
| [163] | Menstrual blood-derived MSC | Exo-spin kit | 30–150 µm | AFM, FE-SEM, WB | Male Wistar Rats (STZ-induced rat model of T1DMM) | Intravenous injection | EVs isolated from stem cells may regenerate beta-cells of islets through the Pdx-1 pathway |
4.7. EV Based Clinical Trials in T1DM
One of the main reasons that EVs are a preferred option as therapeutic delivery vehicles is their ability to decrease the harmful effects that foreign compounds have on the body when introduced. Because of their biological origin, EVs are unlikely to elicit a strong immunological response. EVs are also safe because they are fully non-replicative and non-mutagenic; therefore, there are no regulatory considerations about side effects or the development of neoplasia [176]. The low toxicity of EVs in EV-based in vivo therapies has corroborated these benefits. However, there are a number of possible safety concerns. Further studies are warranted to assure that any individual application for EVs is safe. To date, there are now seven T1DM related clinical studies (Table 6) available by searching the clinicaltrials.gov database with keywords such as “Type 1 diabetes”, “extracellular vesicle”, “exosome”, or “microvesicle.” The majority of these clinical trials utilize EVs as biomarkers for clinical diagnosis and tracking disease progression post-treatments since EVs carry lipids, proteins, DNA, mRNAs, miRNAs, lipids, and proteins representative of their parent cell. Due to the ubiquitous use of MSC therapies, MSC EVs are used in the majority of clinical and preclinical investigations. Since MSC-derived EVs contain MSC-released secretomes such as cytokines, chemokines, and anti-inflammatory substances, they may have similar therapeutic potential [177]. This research, both clinical and in cell or animal models, on the use of EVs as biomarkers and therapeutics points to the great value EVs can add to the field of T1DM.

Table 6.
EV-based clinical trials in T1DM.
5. Conclusions
T1DM and its related complications have increasingly become a worldwide health problem and are associated with an enormous healthcare and economic burden. Patients diagnosed with type 1 diabetes often suffer from hypo- or hyper-glycemic episodes due to the cost and biological limitations of current therapeutic options. Transplants, in particular islet transplants, are long-lasting and impactful therapeutic alternatives to continuous exogenous insulin administration. However, before islet transplants can be regularly used, there are a number of complications that must be addressed, including the fact that up to 70% of transplanted islet cells may be destroyed during cell culture or after transplantation, with a very slow rate of self-replication [48,178]. This may be due to insults such as enzymatic damage, oxidative stress, and detachment of islet cells [78]. As such, future strategies for increased islet survival during transplantation include the generation of functional islet β-cells from easily replicating human or porcine pluripotent stem cells to allow for greater transplant numbers [48,78]. Alternative delivery techniques for islet cells such as encapsulation, scaffolds, or transplant sites may lead to less immune activation and improved long-term cell survival and function [72,78].
More work needs to be carried out to enhance biomarkers of T1DM, transplant rejection, and combined treatment options; EVs are a novel, versatile tool allowing new approaches for both biomarkers and therapeutics (Figure 4). EVs are mediators of intercellular communication, and cell-specific EVs carry disease-specific molecular signatures that allow the identification of T1DM from healthy patients, as well as serve as biomarkers of diabetic complications, including nephropathy and retinopathy [130,131,132,133,134]. Next, the focus should be on the identification of status-specific EV biomarkers for T1DM disease and transplant prognostic signature. Further, EVs are a pivotal strategy in ameliorating many of the complications associated with pancreas and islet transplantation outcomes [150]. However, additional investigations are needed regarding EV production, isolation, characterization, the underlying mechanism of actions, best route of administration, timing, and clearance patterns for a better understanding of T1DM therapeutic strategies [114,135,179,180].
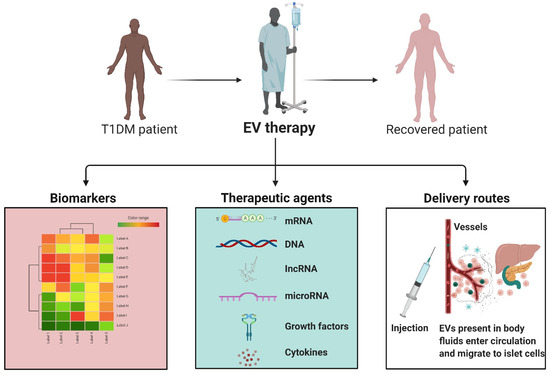
Figure 4.
EVs in T1DM. EVs can play a valuable role in T1DM. There are a number of RNAs and proteins that are modified in EVs in T1DM and rejected transplants. In addition, the natural components of EVs from a healthy system, such as anti-inflammatory cytokines or micRNAs, can aid in regulating and correcting a disease state. Moreover, EVs can be loaded with particular cargos and delivered alongside transplantation to aid in graft survival and long-term well-being. EVs can be are delivered into the bloodstream where they migrate to distant tissues such as pancreatic islets of Langerhans and are engulfed by target cells. As the EV payload is delivered into a target cell, the proteins and RNA species can have various impacts, triggering diverse cell signaling cascades and the regulation of gene expression.
Author Contributions
C.N.S. and M.D.H. contributed equally to planning, writing, editing, and finalizing the review article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Karen Toffler Charitable Trust as part of the Toffler Scholar Program, grant number F09048.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| UC | ultracentrifugation |
| EM | electron microscopy |
| WB | western blot |
| NTA | nanoparticle tracking analysis |
| FC | flow cytometry |
| PBS | phosphate buffered saline |
| DLS | dynamic light scattering |
| SEM | scanning electron microscopy |
References
- Wolkowicz, K.L.; Aiello, E.M.; Vargas, E.; Teymourian, H.; Tehrani, F.; Wang, J.; Pinsker, J.E.; Doyle, F.J., III; Patti, M.E.; Laffel, L.M.; et al. A review of biomarkers in the context of type 1 diabetes: Biological sensing for enhanced glucose control. Bioeng. Transl. Med. 2021, 6, e10201. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.A.B.D. The Management of Type 1 Diabetes, in Comprehensive Free Online Endocrinology Book; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ríos-Ríos, W.D.J.; Sosa-Luis, S.A.; Torres-Aguilar, H. Current advances in using tolerogenic dendritic cells as a therapeutic alternative in the treatment of type 1 diabetes. World J. Diabetes 2021, 12, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Snethlage, C.M.F.; Nieuwdorp, M.; van Raalte, D.H.; Rampanelli, E.; Verchere, B.C.; Hanssen, N.M. Auto-immunity and the gut microbiome in type 1 diabetes: Lessons from rodent and human studies. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101544. [Google Scholar] [CrossRef] [PubMed]
- Ewen, M.; Joosse, H.J.; Beran, D.; Laing, R. Insulin prices, availability and affordability in 13 low-income and middle-income countries. BMJ Glob. Health 2019, 4, e001410. [Google Scholar] [CrossRef]
- Dimeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Ikegami, H.; Babaya, N.; Noso, S. β-Cell failure in diabetes: Common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J. Diabetes Investig. 2021, 12, 1526–1539. [Google Scholar] [CrossRef]
- Clayton, A.; Boilard, E.; Buzas, E.I.; Cheng, L.; Falcón-Perez, J.M.; Gardiner, C.; Gustafson, D.; Gualerzi, A.; Hendrix, A.; Hoffman, A.; et al. Considerations towards a roadmap for collection, handling and storage of blood extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1647027. [Google Scholar] [CrossRef]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteom. 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 8–10. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Pant, S.; Hilton, H.; Burczynski, M.E. The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem. Pharmacol. 2012, 83, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.V.; Grunewald, T.G.P. Tumour-derived exosomes: Tiny envelopes for big stories. Biol. Cell 2015, 107, 287–305. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.H.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2009, 38, 215–224. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Ramis, J.M. Extracellular Vesicles in Cell Biology and Medicine. Sci. Rep. 2020, 10, 8667. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.; Su, X.; He, J.; Bai, W.; He, X. MSCs-derived exosomes and neuroinflammation, neurogenesis and therapy of traumatic brain injury. Front. Cell. Neurosci. 2017, 11, 55. [Google Scholar] [CrossRef]
- Stremersch, S.; Vandenbroucke, R.E.; Van Wonterghem, E.; Hendrix, A.; De Smedt, S.C.; Raemdonck, K. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J. Control. Release 2016, 232, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Suire, C.; Zhang, S.; Mattson, M.P. Impact of lysosome status on extracellular vesicle content and release. Ageing Res. Rev. 2016, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Desai, N.N.; Qureshi, M.Z.; Nogueira-Librelotto, D.R.; Gasparri, M.L.; Bishayee, A.; Nabavi, S.M.; Curti, V.; Daglia, M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 2018, 36, 328–334. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Coffey, R.J. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- He, N.; Thippabhotla, S.; Zhong, C.; Greenberg, Z.; Xu, L.; Pessetto, Z.; Godwin, A.K.; Zeng, Y.; He, M. Nano Pom-poms Prepared Highly Specific Extracellular Vesicles Expand the Detectable Cancer Biomarkers. bioRxiv 2021. [Google Scholar]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Batagov, A.O.; Kuznetsov, V.A.; Kurochkin, I.V. Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles. BMC Genom. 2011, 12 (Suppl. 3), S18. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Gutierrez-Vazquez, C.; Sanchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Guidolin, D.; Vallorani, L.; Casadei, L.; Gioacchini, A.M.; Tibollo, P.; Battistelli, M.; Falcieri, E.; Battistin, L.; Agnati, L.F.; et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp. Cell Res. 2010, 316, 1977–1984. [Google Scholar] [CrossRef]
- Ronquist, K.G.; Ronquist, G.; Carlsson, L.; Larsson, A. Human prostasomes contain chromosomal DNA. Prostate 2009, 69, 737–743. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef]
- Yu, L.-L.; Zhu, J.; Liu, J.-X.; Jiang, F.; Ni, W.-K.; Qu, L.-S.; Ni, R.-Z.; Lu, C.-H.; Xiao, M.-B. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. Biomed. Res. Int. 2018, 2018, 3634563. [Google Scholar] [CrossRef]
- Nakrani, M.N.; Wineland, R.H.; Anjum, F. Physiology, Glucose Metabolism, in Physiology, Glucose Metabolism; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Leroux, J.-P.; Marchand, J.-C.; Ha, R.H.T.; Cartier, P. The influence of insulin on glucose permeability and metabolism of human granulocytes. Eur. J. Biochem. 1975, 58, 367–373. [Google Scholar] [CrossRef]
- McConell, G.K.; Sjøberg, K.A.; Ceutz, F.; Gliemann, L.; Nyberg, M.P.; Hellsten, Y.; Frøsig, C.; Kiens, B.; Wojtaszewski, J.F.P.; Richter, E.A. Insulin-induced membrane permeability to glucose in human muscles at rest and following exercise. J. Physiol. 2020, 598, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Bahar, S.G.; Devulapally, P. Pancreas Transplantation; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Chen, J.; Gunton, J.E. Beta-cell function and human islet transplantation: Can we improve? J. Endocrinol. 2021, 248, R99–R112. [Google Scholar] [CrossRef] [PubMed]
- Cong, G.Z.; Ghosh, K.K.; Mishra, S.; Gulyás, M.; Kovács, T.; Máthé, D.; Padmanabhan, P.; Gulyás, B. Targeted pancreatic beta cell imaging for early diagnosis. Eur. J. Cell Biol. 2020, 99, 151110. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, G.; Jensen, S.S.; Le Bihan, M.C.; Laine, J.; McGuire, J.N.; Pociot, F.; Larsen, M.R. Characterization of membrane-shed microvesicles from cytokine-stimulated beta-cells using proteomics strategies. Mol. Cell Proteom. 2012, 11, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Piemonti, L. Felix dies natalis, insulin… ceterum autem censeo “beta is better”. Acta Diabetol. 2021, 58, 1287–1306. [Google Scholar] [CrossRef]
- Pociot, F. Type 1 diabetes genome-wide association studies: Not to be lost in translation. Clin. Transl. Immunol. 2017, 6, e162. [Google Scholar] [CrossRef]
- Ram, R.; Mehta, M.; Nguyen, Q.T.; Larma, I.; Boehm, B.O.; Pociot, F.; Concannon, P.; Morahan, G. Systematic Evaluation of Genes and Genetic Variants Associated with Type 1 Diabetes Susceptibility. J. Immunol. 2016, 196, 3043–3053. [Google Scholar] [CrossRef]
- Cerolsaletti, K.; Hao, W.; Greenbaum, C.J. Genetics Coming of Age in Type 1 Diabetes. Diabetes Care 2019, 42, 189–191. [Google Scholar] [CrossRef]
- Bonifacio, E.; Beyerlein, A.; Hippich, M.; Winkler, C.; Vehik, K.; Weedon, M.; Laimighofer, M.; Hattersley, A.; Krumsiek, J.; Frohnert, B.I.; et al. Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: A prospective study in children. PLoS Med. 2018, 15, e1002548. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, Z.; Lu, Q.; Chang, C.; Zhou, Z. Beyond Genetics: What Causes Type 1 Diabetes. Clin. Rev. Allergy Immunol. 2017, 52, 273–286. [Google Scholar] [CrossRef]
- Nyaga, D.M.; Vickers, M.H.; Jefferies, C.; Perry, J.K.; O’Sullivan, J.M. The genetic architecture of type 1 diabetes mellitus. Mol. Cell Endocrinol. 2018, 477, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Stankov, K.; Benc, D.; Draskovic, D. Genetic and epigenetic factors in etiology of diabetes mellitus type 1. Pediatrics 2013, 132, 1112–1122. [Google Scholar] [CrossRef]
- Akil, A.A.-S.; Yassin, E.; Al-Maraghi, A.; Aliyev, E.; Al-Malki, K.; Fakhro, K.A. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J. Transl. Med. 2021, 19, 137. [Google Scholar] [CrossRef]
- Santin, I.; Eizirik, D.L. Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and beta-cell apoptosis. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 71–81. [Google Scholar] [CrossRef]
- Pociot, F.; Lernmark, A. Genetic risk factors for type 1 diabetes. Lancet 2016, 387, 2331–2339. [Google Scholar] [CrossRef]
- Pearson, J.A.; Wong, F.S.; Wen, L. Inflammasomes and Type 1 Diabetes. Front. Immunol. 2021, 12, 686956. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes:Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. 1), S13–S27. [Google Scholar] [CrossRef]
- Peng, J.; Narasimhan, S.; Marchesi, J.R.; Benson, A.; Wong, F.S.; Wen, L. Long term effect of gut microbiota transfer on diabetes development. J. Autoimmun. 2014, 53, 85–94. [Google Scholar] [CrossRef]
- Berkovic, M.C.; Bilic-Curcic, I.; Sabolic, L.L.G.; Mrzljak, A.; Cigrovski, V. Fear of hypoglycemia, a game changer during physical activity in type 1 diabetes mellitus patients. World J. Diabetes 2021, 12, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jia, S.; Xu, H. Potential therapeutic applications of exosomes in different autoimmune diseases. Clin. Immunol. 2019, 205, 116–124. [Google Scholar] [CrossRef]
- Clark, M.; Kroger, C.J.; Tisch, R.M. Type 1 Diabetes: A Chronic Anti-Self-Inflammatory Response. Front. Immunol. 2017, 8, 1898. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.E.R.; Gruessner, R.W.G.; Dunn, D.L.; Matas, A.J.; Humar, A.; Kandaswamy, R.; Mauer, S.M.; Kennedy, W.R.; Goetz, F.C.; Robertson, R.P.; et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann. Surg. 2001, 233, 463–501. [Google Scholar] [CrossRef]
- Bellin, M.D.; Barton, F.B.; Heitman, A.; Harmon, J.V.; Kandaswamy, R.; Balamurugan, A.N.; Sutherland, D.E.R.; Alejandro, R.; Hering, B.J. Potent Induction Immunotherapy Promotes Long-Term Insulin Independence After Islet Transplantation in Type 1 Diabetes. Am. J. Transplant. 2012, 12, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, A.C.; Gruessner, R.W. Pancreas Transplantation of US and Non-US Cases from 2005 to 2014 as Reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR). Rev. Diabet. Stud. 2016, 13, 35–58. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Pancreas Transplantation in Type 1 Diabetes. Diabetes Care 2004, 27 (Suppl. 1), S105. [Google Scholar]
- Robertson, R.P.; Davis, C.; Larsen, J.; Stratta, R.; Sutherland, D.E. Pancreas and islet transplantation for patients with diabetes. Diabetes Care 2000, 23, 112–116. [Google Scholar] [CrossRef]
- Bellin, M.D.; Dunn, T.B. Transplant strategies for type 1 diabetes: Whole pancreas, islet and porcine beta cell therapies. Diabetologia 2020, 63, 2049–2056. [Google Scholar] [CrossRef]
- Triolo, T.M.; Bellin, M.D. Lessons from Human Islet Transplantation Inform Stem Cell-Based Approaches in the Treatment of Diabetes. Front. Endocrinol. 2021, 12, 636824. [Google Scholar] [CrossRef]
- Lehmann, R.; Graziano, J.; Brockmann, J.; Pfammatter, T.; Kron, P.; de Rougemont, O.; Mueller, T.; Zuellig, R.A.; Spinas, G.A.; Gerber, P.A. Glycemic Control in Simultaneous Islet-Kidney Versus Pancreas-Kidney Transplantation in Type 1 Diabetes: A Prospective 13-Year Follow-up. Diabetes Care 2015, 38, 752–759. [Google Scholar] [CrossRef]
- Naftanel, M.A.; Harlan, D.M. Pancreatic Islet Transplantation. PLoS Med. 2018, 1, e58. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Stock, P.G.; de Koning, E.J.P.; Piemonti, L.; Pratschke, J.; Alejandro, R.; Bellin, M.D.; Berney, T.; Choudhary, P.; Johnson, P.R.; et al. Defining outcomes for β-cell replacement therapy in the treatment of diabetes: A consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transpl. Int. 2018, 31, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Schutta, M.H.; Markmann, J.F.; Barker, C.F.; Naji, A.; Teff, K.L. Cell Function Following Human Islet Transplantation for Type 1 Diabetes. Diabetes 2005, 54, 100–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al Ghofaili, K.; Fung, M.; Ao, Z.; Meloche, M.; Shapiro, R.J.; Warnock, G.L.; Elahi, D.; Meneilly, G.S.; Thompson, D.M. Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation 2007, 83, 24–28. [Google Scholar] [CrossRef]
- Khosravi-Maharlooei, M.; Hajizadeh-Saffar, E.; Tahamtani, Y.; Basiri, M.; Montazeri, L.; Khalooghi, K.; Ashtiani, M.K.; Farrokhi, A.; Aghdami, N.; Nejad, A.S.H.; et al. THERAPY OF ENDOCRINE DISEASE: Islet transplantation for type 1 diabetes: So close and yet so far away. Eur. J. Endocrinol. 2015, 173, R165–R183. [Google Scholar] [CrossRef]
- Guay, C.; Regazzi, R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes. Metab. 2017, 19 (Suppl. 1), 137–146. [Google Scholar] [CrossRef]
- Figliolini, F.; Cantaluppi, V.; De Lena, M.; Beltramo, S.; Romagnoli, R.; Salizzoni, M.; Melzi, R.; Nano, R.; Piemonti, L.; Tetta, C.; et al. Isolation, characterization and potential role in beta cell-endothelium cross-talk of extracellular vesicles released from human pancreatic islets. PLoS ONE 2014, 9, e102521. [Google Scholar] [CrossRef]
- Cantaluppi, V.; Biancone, L.; Figliolini, F.; Beltramo, S.; Medica, D.; Deregibus, M.C.; Galimi, F.; Romagnoli, R.; Salizzoni, M.; Tetta, C.; et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transpl. 2012, 21, 1305–1320. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Liu, D.; Weizmann, Y.; Mahato, R.I. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J. Control. Release 2016, 238, 166–175. [Google Scholar] [CrossRef]
- Guay, C.; Menoud, V.; Rome, S.; Regazzi, R. Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Commun. Signal. 2015, 13, 17. [Google Scholar] [CrossRef]
- He, X.; Kuang, G.; Wu, Y.; Ou, C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin. Transl. Med. 2021, 11, e468. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Q.; Liu, D.; Liu, Z. Exosomes: Advances, development and potential therapeutic strategies in diabetic nephropathy. Metabolism 2021, 122, 154834. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef]
- Cianciaruso, C.; Phelps, E.A.; Pasquier, M.; Hamelin, R.; Demurtas, D.; Ahmed, M.A.; Piemonti, L.; Hirosue, S.; Swartz, M.A.; De Palma, M.; et al. Primary Human and Rat β-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together with Cytokine-Induced Enhancers of Immunity. Diabetes 2017, 66, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Hassanali, S.; Nugent, C.; Wen, L.; Hamilton-Williams, E.; Dias, P.; Dai, Y.D. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in nonobese diabetic mice. J. Immunol. 2011, 187, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Vomund, A.N.; Zinselmeyer, B.; Hughes, J.; Calderon, B.; Valderrama, C.; Ferris, S.; Wan, X.; Kanekura, K.; Carrero, J.A.; Urano, F.; et al. Beta cells transfer vesicles containing insulin to phagocytes for presentation to T cells. Proc. Natl. Acad. Sci. USA 2015, 112, E5496–E5502. [Google Scholar] [CrossRef] [PubMed]
- Grieco, G.E.; Fignani, D.; Formichi, C.; Nigi, L.; Licata, G.; Maccora, C.; Brusco, N.; Sebastiani, G.; Dotta, F. Extracellular Vesicles in Immune System Regulation and Type 1 Diabetes: Cell-to-Cell Communication Mediators, Disease Biomarkers, and Promising Therapeutic Tools. Front. Immunol. 2021, 12, 682948. [Google Scholar] [CrossRef]
- Saravanan, P.B.; Vasu, S.; Yoshimatsu, G.; Darden, C.M.; Wang, X.; Gu, J.; Lawrence, M.C.; Naziruddin, B. Differential expression and release of exosomal miRNAs by human islets under inflammatory and hypoxic stress. Diabetologia 2019, 62, 1901–1914. [Google Scholar] [CrossRef]
- Eitan, E.; Tosti, V.; Suire, C.N.; Cava, E.; Berkowitz, S.; Bertozzi, B.; Raefsky, S.M.; Veronese, N.; Spangler, R.; Spelta, F.; et al. In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell 2017, 16, 1430–1433. [Google Scholar] [CrossRef]
- Rahman, M.J.; Regn, D.; Bashratyan, R.; Dai, Y.D. Exosomes Released by Islet-Derived Mesenchymal Stem Cells Trigger Autoimmune Responses in NOD Mice. Diabetes 2014, 63, 1008–1020. [Google Scholar] [CrossRef]
- Giri, P.K.; Schorey, J.S. Exosomes Derived from M. Bovis BCG Infected Macrophages Activate Antigen-Specific CD4+ and CD8+ T Cells In Vitro and In Vivo. PLoS ONE 2008, 3, e2461. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Wang, J. Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus. Cells 2019, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jing, N.; Shen, A.; Guo, F.; Song, Y.; Pan, M.; Ma, X.; Zhao, L.; Zhang, H.; Wu, L.; et al. MiR-21-5p in macrophage-derived extracellular vesicles affects podocyte pyroptosis in diabetic nephropathy by regulating A20. J. Endocrinol. Investig. 2021, 44, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Mattke, J.; Vasu, S.; Darden, C.M.; Kumano, K.; Lawrence, M.C.; Naziruddin, B. Role of Exosomes in Islet Transplantation. Front. Endocrinol. 2021, 12, 681600. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N. Biomarkers for diabetes prediction, pathogenesis or pharmacotherapy guidance? Past, present and future possibilities. Diabet Med. 2012, 29, 5–13. [Google Scholar] [CrossRef]
- Ziegler, A.G.; Rewers, M.; Simell, O.; Simell, T.; Lempainen, J.; Steck, A.; Winkler, C.; Ilonen, J.; Veijola, R.; Knip, M.; et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013, 309, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Buron, F.; Reffet, S.; Badet, L.; Morelon, E.; Thaunat, O. Immunological Monitoring in Beta Cell Replacement: Towards a Pathophysiology-Guided Implementation of Biomarkers. Curr. Diabetes Rep. 2021, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Swensen, A.C.; Qian, W.J. Serum biomarkers for diagnosis and prediction of type 1 diabetes. Transl. Res. 2018, 201, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Ekroos, K.; Kauhanen, D.; Simolin, H.; Seierstad, T.; Berge, V.; Sandvig, K.; Llorente, A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur. J. Cancer 2017, 70, 122–132. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Saha, B.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J. Transl. Med. 2015, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Yousif, G.; Qadri, S.; Haik, M.; Haik, Y.; Parray, A.S.; Shuaib, A. Circulating Exosomes of Neuronal Origin as Potential Early Biomarkers for Development of Stroke. Mol. Diagn. Ther. 2021, 25, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Gercel-Taylor, C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Zhu, S.; Chen, L.; Liu, X.; Wei, R.; Zhao, L.; Yang, Y.; Zhang, Z.; Kong, G.; Li, P.; et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: A comparison with plasma total miRNAs. J. Extracell. Vesicles 2019, 8, 1643670. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Brooks, R.W.; Boccuzzi, L.; Robbins, P.D.; Ricordi, C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2940–2956. [Google Scholar]
- Bashratyan, R.; Sheng, H.; Regn, D.; Rahman, M.J.; Dai, Y.D. Insulinoma-released exosomes activate autoreactive marginal zone-like B cells that expand endogenously in prediabetic NOD mice. Eur. J. Immunol. 2013, 43, 2588–2597. [Google Scholar] [CrossRef]
- Favaro, E.; Carpanetto, A.; Caorsi, C.; Giovarelli, M.; Angelini, C.; Cavallo-Perin, P.; Tetta, C.; Camussi, G.; Zanone, M.M. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 2016, 59, 325–333. [Google Scholar] [CrossRef]
- Kilpinen, L.; Impola, U.; Sankkila, L.; Ritamo, I.; Aatonen, M.; Kilpinen, S.; Tuimala, J.; Valmu, L.; Levijoki, J.; Finckenberg, P.; et al. Extracellular membrane vesicles from umbilical cord blood-derived MSC protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J. Extracell. Vesicles 2013, 2, 21927. [Google Scholar] [CrossRef]
- Raimondo, F.; Corbetta, S.; Morosi, L.; Chinello, C.; Gianazza, E.; Castoldi, G.; Di Gioia, C.; Bombardi, C.; Stella, A.; Battaglia, C.; et al. Urinary exosomes and diabetic nephropathy: A proteomic approach. Mol. BioSyst. 2013, 9, 1139. [Google Scholar] [CrossRef]
- Wu, S.F.; Noren Hooten, N.; Freeman, D.W.; Mode, N.A.; Zonderman, A.B.; Evans, M.K. Extracellular vesicles in diabetes mellitus induce alterations in endothelial cell morphology and migration. J. Transl. Med. 2020, 18, 230. [Google Scholar] [CrossRef]
- Pang, H.; Luo, S.; Xiao, Y.; Xia, Y.; Li, X.; Huang, G.; Xie, Z.; Zhou, Z. Emerging Roles of Exosomes in T1DM. Front. Immunol. 2020, 11, 593348. [Google Scholar] [CrossRef] [PubMed]
- Tesovnik, T.; Kovač, J.; Pohar, K.; Hudoklin, S.; Dovč, K.; Bratina, N.; Trebušak Podkrajšek, K.; Debeljak, M.; Veranič, P.; Bosi, E.; et al. Extracellular Vesicles Derived Human-miRNAs Modulate the Immune System in Type 1 Diabetes. Front. Cell Dev. Biol. 2020, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.W.; Hooten, N.N.; Eitan, E.; Green, J.; Mode, N.A.; Bodogai, M.; Zhang, Y.; Lehrmann, E.; Zonderman, A.B.; Biragyn, A.; et al. Altered Extracellular Vesicle Concentration, Cargo, and Function in Diabetes. Diabetes 2018, 67, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Syed, F.; Kang, N.J.; Mirmira, R.G.; Evans-Molina, C. Profiling of RNAs from Human Islet-Derived Exosomes in a Model of Type 1 Diabetes. Int. J. Mol. Sci. 2019, 20, 5903. [Google Scholar] [CrossRef] [PubMed]
- Hasilo, C.P.; Negi, S.; Allaeys, I.; Cloutier, N.; Rutman, A.K.; Gasparrini, M.; Bonneil, É.; Thibault, P.; Boilard, É.; Paraskevas, S. Presence of diabetes autoantigens in extracellular vesicles derived from human islets. Sci. Rep. 2017, 7, 5000. [Google Scholar] [CrossRef]
- Rutman, A.K.; Negi, S.; Gasparrini, M.; Hasilo, C.P.; Tchervenkov, J.; Paraskevas, S. Immune Response to Extracellular Vesicles From Human Islets of Langerhans in Patients With Type 1. Diabetes Endocrinol. 2018, 159, 3834–3847. [Google Scholar] [CrossRef]
- Wu, W.-C.; Song, S.-J.; Zhang, Y.; Li, X. Role of Extracellular Vesicles in Autoimmune Pathogenesis. Front. Immunol. 2020, 11, 579043. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Negi, S.; Rutman, A.K.; Paraskevas, S. Extracellular Vesicles in Type 1 Diabetes: Messengers and Regulators. Curr. Diabetes Rep. 2019, 19, 69. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Shah, S.H.; Tamayo, A.; Robbins, P.D.; Golberg, R.B.; Mendez, A.J.; Ricordi, C. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci. Rep. 2017, 7, 5998. [Google Scholar] [CrossRef]
- Kong, Q.; Guo, X.; Guo, Z.; Su, T. Urinary Exosome miR-424 and miR-218 as Biomarkers for Type 1 Diabetes in Children. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef] [PubMed]
- Lakhter, A.J.; Pratt, R.E.; Moore, R.E.; Doucette, K.K.; Maier, B.F.; DiMeglio, L.A.; Sims, E.K. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia 2018, 61, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Roggli, E.; Britan, A.; Gattesco, S.; Lin-Marq, N.; Abderrahmani, A.; Meda, P.; Regazzi, R. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes 2010, 59, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Roggli, E.; Gattesco, S.; Caille, D.; Briet, C.; Boitard, C.; Meda, P.; Regazzi, R. Changes in microRNA expression contribute to pancreatic beta-cell dysfunction in prediabetic NOD mice. Diabetes 2012, 61, 1742–1751. [Google Scholar] [CrossRef]
- Ruan, Q.; Wang, T.; Kameswaran, V.; Wei, Q.; Johnson, D.S.; Matschinsky, F.; Shi, W.; Chen, Y.H. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proc. Natl. Acad. Sci. USA 2011, 108, 12030–12035. [Google Scholar] [CrossRef]
- Sims, E.K.; Lakhter, A.J.; Anderson-Baucum, E.; Kono, T.; Tong, X.; Evans-Molina, C. MicroRNA 21 targets BCL2 mRNA to increase apoptosis in rat and human beta cells. Diabetologia 2017, 60, 1057–1065. [Google Scholar] [CrossRef]
- Barutta, F.; Tricarico, M.; Corbelli, A.; Annaratone, L.; Pinach, S.; Grimaldi, S.; Bruno, G.; Cimino, D.; Taverna, D.; Deregibus, M.C.; et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS ONE 2013, 8, e73798. [Google Scholar] [CrossRef]
- Kalani, A.; Mohan, A.; Godbole, M.M.; Bhatia, E.; Gupta, A.; Sharma, R.K.; Tiwari, S. Wilm’s tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS ONE 2013, 8, e60177. [Google Scholar] [CrossRef]
- Katome, T.; Namekata, K.; Mitamura, Y.; Semba, K.; Egawa, M.; Naito, T.; Harada, C.; Harada, T. Expression of intraocular peroxisome proliferator-activated receptor gamma in patients with proliferative diabetic retinopathy. J. Diabetes Complicat. 2015, 29, 275–281. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Xiao, F.; Kennedy, C.R.J.; Perkins, B.A.; Reich, H.N.; Scholey, J.W.; Cherney, D.Z.; Burger, D. Assessment of urinary microparticles in normotensive patients with type 1 diabetes. Diabetologia 2017, 60, 581–584. [Google Scholar] [CrossRef]
- Musante, L.; Tataruch, D.; Gu, D.; Liu, X.; Forsblom, C.; Groop, P.-H.; Holthofer, H. Proteases and protease inhibitors of urinary extracellular vesicles in diabetic nephropathy. J. Diabetes Res. 2015, 2015, 289734. [Google Scholar] [CrossRef]
- Barreiro, K.; Dwivedi, O.P.; Leparc, G.; Rolser, M.; Delic, D.; Forsblom, C.; Groop, P.; Groop, L.; Huber, T.B.; Puhka, M.; et al. Comparison of urinary extracellular vesicle isolation methods for transcriptomic biomarker research in diabetic kidney disease. J. Extracell. Vesicles 2020, 10, e12038. [Google Scholar] [CrossRef] [PubMed]
- Troppmann, C.; Gruessner, A.C.; Dunn, D.L.; Sutherland, D.E.; Gruessner, R.W. Surgical complications requiring early relaparotomy after pancreas transplantation: A multivariate risk factor and economic impact analysis of the cyclosporine era. Ann. Surg. 1998, 227, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, P.; Korutla, L.; Habertheuer, A.; Yu, M.; Rostami, S.; Yuan, C.-X.; Reddy, S.; Liu, C.; Korutla, V.; Koeberlein, B.; et al. Tissue-specific exosome biomarkers for noninvasively monitoring immunologic rejection of transplanted tissue. J. Clin. Investig. 2017, 127, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Benichou, G.; Wang, M.; Ahrens, K.; Madsen, J.C. Extracellular vesicles in allograft rejection and tolerance. Cell Immunol. 2020, 349, 104063. [Google Scholar] [CrossRef] [PubMed]
- Korutla, L.; Rickels, M.R.; Hu, R.; Freas, A.; Reddy, S.; Habertheuer, A.; Harmon, J.; Korutla, V.; Ram, C.; Naji, A.; et al. Noninvasive diagnosis of recurrent autoimmune type 1 diabetes after islet cell transplantation. Am. J. Transplant. 2019, 19, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Chidester, S.; Livinski, A.A.; Fish, A.F.; Joseph, P.V. The Role of Extracellular Vesicles in beta-Cell Function and Viability: A Scoping Review. Front. Endocrinol. 2020, 11, 375. [Google Scholar] [CrossRef]
- Ishida, K.; Taguchi, K.; Hida, M.; Watanabe, S.; Kawano, K.; Matsumoto, T.; Hattori, Y.; Kobayashi, T. Circulating microparticles from diabetic rats impair endothelial function and regulate endothelial protein expression. Acta Physiol. 2016, 216, 211–220. [Google Scholar] [CrossRef]
- Rossi, L.; Nicoletti, M.C.; Carmosino, M.; Mastrofrancesco, L.; Di Franco, A.; Indrio, F.; Lella, R.; Laviola, L.; Giorgino, F.; Svelto, M.; et al. Urinary Excretion of Kidney Aquaporins as Possible Diagnostic Biomarker of Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 4360357. [Google Scholar] [CrossRef]
- Tokarz, A.; Szuścik, I.; Kuśnierz-Cabala, B.; Kapusta, M.; Konkolewska, M.; Żurakowski, A.; Georgescu, A.; Stępień, E. Extracellular vesicles participate in the transport of cytokines and angiogenic factors in diabetic patients with ocular complications. Folia Med. Crac. 2015, 55, 35–48. [Google Scholar]
- Zubiri, I.; Posada-Ayala, M.; Benito-Martin, A.; Maroto, A.S.; Martin-Lorenzo, M.; Cannata-Ortiz, P.; de la Cuesta, F.; Gonzalez-Calero, L.; Barderas, M.G.; Fernandez-Fernandez, B.; et al. Kidney tissue proteomics reveals regucalcin downregulation in response to diabetic nephropathy with reflection in urinary exosomes. Transl. Res. 2015, 166, 474–484.e4. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto-Kuroda, T.; Oh, J.Y.; Kim, D.-K.; Jeong, H.J.; Park, S.Y.; Lee, H.J.; Park, J.W.; Kim, T.W.; An, S.Y.; Prockop, D.J.; et al. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017, 8, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Bellucci, L.; Bussolati, B.; Ranghino, A. Potential Applications of Extracellular Vesicles in Solid Organ Transplantation. Cells 2020, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.R.; Rodriguez, S.M.; Luong, J.C.; Li, S.; Cao, R.; Alshetaiwi, H.; Lau, H.; Davtyan, H.; Jones, M.B.; Jafari, M.; et al. Exosome loaded immunomodulatory biomaterials alleviate local immune response in immunocompetent diabetic mice post islet xenotransplantation. Commun. Biol. 2021, 4, 685. [Google Scholar] [CrossRef] [PubMed]
- Nasiri Kenari, A.; Cheng, L.; Hill, A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods 2020, 177, 103–113. [Google Scholar] [CrossRef]
- Constantin, A.; Filippi, A.; Alexandru, N.; Nemecz, M.; Georgescu, A. Extracellular Vesicles from Adipose Tissue Stem Cells in Diabetes and Associated Cardiovascular Disease; Pathobiological Impact and Therapeutic Potential. Int. J. Mol. Sci. 2020, 21, 9598. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Sarvestani, F.S.; Ghahremani, M.H.; Aghdaei, M.H.; Al-Abdullah, I.H.; Azarpira, N. Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. EXCLI J. 2020, 19, 1064–1080. [Google Scholar]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Sabry, D.; Marzouk, S.; Zakaria, R.; Ibrahim, H.A.; Samir, M. The effect of exosomes derived from mesenchymal stem cells in the treatment of induced type 1 diabetes mellitus in rats. Biotechnol. Lett. 2020, 42, 1597–1610. [Google Scholar] [CrossRef]
- Newton, W.C.; Kim, J.W.; Luo, J.Z.Q.; Luo, L. Stem cell-derived exosomes: A novel vector for tissue repair and diabetic therapy. J. Mol. Endocrinol. 2017, 59, R155–R165. [Google Scholar] [CrossRef]
- Su, T.; Xiao, Y.; Xiao, Y.; Guo, Q.; Li, C.; Huang, Y.; Deng, Q.; Wen, J.; Zhou, F.; Luo, X.-H. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MiR-29b-3p Regulates Aging-Associated Insulin Resistance. ACS Nano 2019, 13, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Nagaishi, K.; Konari, N.; Saito, Y.; Chikenji, T.S.; Mizue, Y.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci. Rep. 2016, 6, 24805. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Shi, J.; Yang, X.; Yang, L.; Hua, F. The Exosome: A New Player in Diabetic Cardiomyopathy. J. Cardiovasc. Transl. Res. 2019, 12, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rong, P.; Ma, X.; Nie, W.; Chen, C.; Yang, C.; Zhang, J.; Dong, Q.; Wang, W. Paracrine effect of mesenchymal stem cell as a novel therapeutic strategy for diabetic nephropathy. Life Sci. 2018, 215, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Prattichizzo, F.; Matacchione, G.; Giuliani, A.; Sabbatinelli, J.; Olivieri, F.; de Candia, P.; De Nigris, V.; Ceriello, A. Extracellular vesicle-shuttled miRNAs: A critical appraisal of their potential as nano-diagnostics and nano-therapeutics in type 2 diabetes mellitus and its cardiovascular complications. Theranostics 2021, 11, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.; Shamardan, R. Adipose mesenchymal stem cells–derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7, 184945441880782. [Google Scholar] [CrossRef]
- Ebrahim, N.; Ahmed, I.A.; Hussien, N.I.; Dessouky, A.A.; Farid, A.S.; Elshazly, A.M.; Mostafa, O.; El Gazzar, W.B.; Sorour, S.M.; Seleem, Y.; et al. Mesenchymal Stem Cell-Derived Exosomes Ameliorated Diabetic Nephropathy by Autophagy Induction through the mTOR Signaling Pathway. Cells 2018, 7, 226. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. Ther. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- McGuinness, D.; Anthony, D.F.; Moulisova, V.; MacDonald, A.I.; MacIntyre, A.; Thomson, J.; Nag, A.; Davies, R.W.; Shiels, P.G. Microvesicles but Not Exosomes from Pathfinder Cells Stimulate Functional Recovery of the Pancreas in a Mouse Streptozotocin-Induced Diabetes Model. Rejuvenation Res. 2016, 19, 223–232. [Google Scholar] [CrossRef]
- Mahdipour, E.; Salmasi, Z.; Sabeti, N. Potential of stem cell-derived exosomes to regenerate beta islets through Pdx-1 dependent mechanism in a rat model of type 1 diabetes. J. Cell Physiol. 2019, 234, 20310–20321. [Google Scholar] [CrossRef]
- Xiong, J.; Hu, H.; Guo, R.; Wang, H.; Jiang, H. Mesenchymal Stem Cell Exosomes as a New Strategy for the Treatment of Diabetes Complications. Front. Endocrinol. 2021, 12, 646233. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Zhu, X.-Y.; Puranik, A.S.; Tang, H.; McGurren, K.A.; van Wijnen, A.J.; Lerman, A.; Lerman, L.O. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017, 92, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Ye, X.; Chopp, M.; Venkat, P.; Zacharek, A.; Yan, T.; Ning, R.; Yu, P.; Cui, G.; Chen, J. miR-145 Regulates Diabetes-Bone Marrow Stromal Cell-Induced Neurorestorative Effects in Diabetes Stroke Rats. Stem Cells Transl. Med. 2016, 5, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Z.; Liu, Y.-M.; Niu, X.; Yin, J.-Y.; Hu, B.; Guo, S.-C.; Fan, Y.; Wang, Y.; Wang, N.-S. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res. Ther. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Hu, B.; Niu, X.; Liu, X.; Zhang, G.; Zhang, C.; Li, Q.; Wang, Y. Exosomes Derived from Human Endothelial Progenitor Cells Accelerate Cutaneous Wound Healing by Promoting Angiogenesis Through Erk1/2 Signaling. Int. J. Biol. Sci. 2016, 12, 1472–1487. [Google Scholar] [CrossRef]
- Li, X.; Jiang, C.; Zhao, J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J. Diabetes Complicat. 2016, 30, 986–992. [Google Scholar] [CrossRef]
- Zhu, L.L.; Huang, X.; Yu, W.; Chen, H.; Chen, Y.; Dai, Y.T. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia 2018, 50, e12871. [Google Scholar] [CrossRef]
- Wang, X.; Gu, H.; Huang, W.; Peng, J.; Li, Y.; Yang, L.; Qin, D.; Essandoh, K.; Wang, Y.; Peng, T.; et al. Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice. Diabetes 2016, 65, 3111–3128. [Google Scholar] [CrossRef]
- Wang, B.; Yao, K.; Huuskes, B.M.; Shen, H.H.; Zhuang, J.; Godson, C.; Brennan, E.P.; Wilkinson-Berka, J.L.; Wise, A.F.; Ricardo, S.D. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol. Ther. 2016, 24, 1290–1301. [Google Scholar] [CrossRef]
- Geiger, A.; Walker, A.; Nissen, E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem. Biophys. Res. Commun. 2015, 467, 303–309. [Google Scholar] [CrossRef]
- Tsukita, S.; Yamada, T.; Takahashi, K.; Munakata, Y.; Hosaka, S.; Takahashi, H.; Gao, J.; Shirai, Y.; Kodama, S.; Asai, Y.; et al. MicroRNAs 106b and 222 Improve Hyperglycemia in a Mouse Model of Insulin-Deficient Diabetes via Pancreatic beta-Cell Proliferation. EBioMedicine 2017, 15, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Nojehdehi, S.; Soudi, S.; Hesampour, A.; Rasouli, S.; Soleimani, M.; Hashemi, S.M. Immunomodulatory effects of mesenchymal stem cell-derived exosomes on experimental type-1 autoimmune diabetes. J. Cell Biochem. 2018, 119, 9433–9443. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.D.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Ma, X.; Yang, C.; Chen, Z.; Rong, P.; Wu, M.; Jiang, J.; Tan, M.; Yi, S.; Wang, W. Human mesenchymal-stem-cells-derived exosomes are important in enhancing porcine islet resistance to hypoxia. Xenotransplantation 2018, 25, e12405. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; James, V.; Rizvanov, A.A. Therapeutic Application of Mesenchymal Stem Cells Derived Extracellular Vesicles for Immunomodulation. Front. Immunol. 2019, 10, 2663. [Google Scholar] [CrossRef]
- Fujita, Y.; Murakami, T.; Nakamura, A. Recent Advances in Biomarkers and Regenerative Medicine for Diabetic Neuropathy. Int. J. Mol. Sci. 2021, 22, 2301. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).