Upgrading of Biobased Glycerol to Glycerol Carbonate as a Tool to Reduce the CO2 Emissions of the Biodiesel Fuel Life Cycle
Abstract
1. Introduction
- (i).
- processes using activated phosgene-sourced reagents, such as phosgene itself, chloroformates, or carbonyldiimidazole;
- (ii).
- processes using activated reagents not sourced from phosgene, such as dialkyl carbonates, diaryl carbonates, or CO + O2;
- (iii).
- processes using non-activated CO2-sourced reagents, such as CO2 itself or urea.
2. Materials and Methods
2.1. General Information
2.2. Procedure for Preliminary Batch Reactions
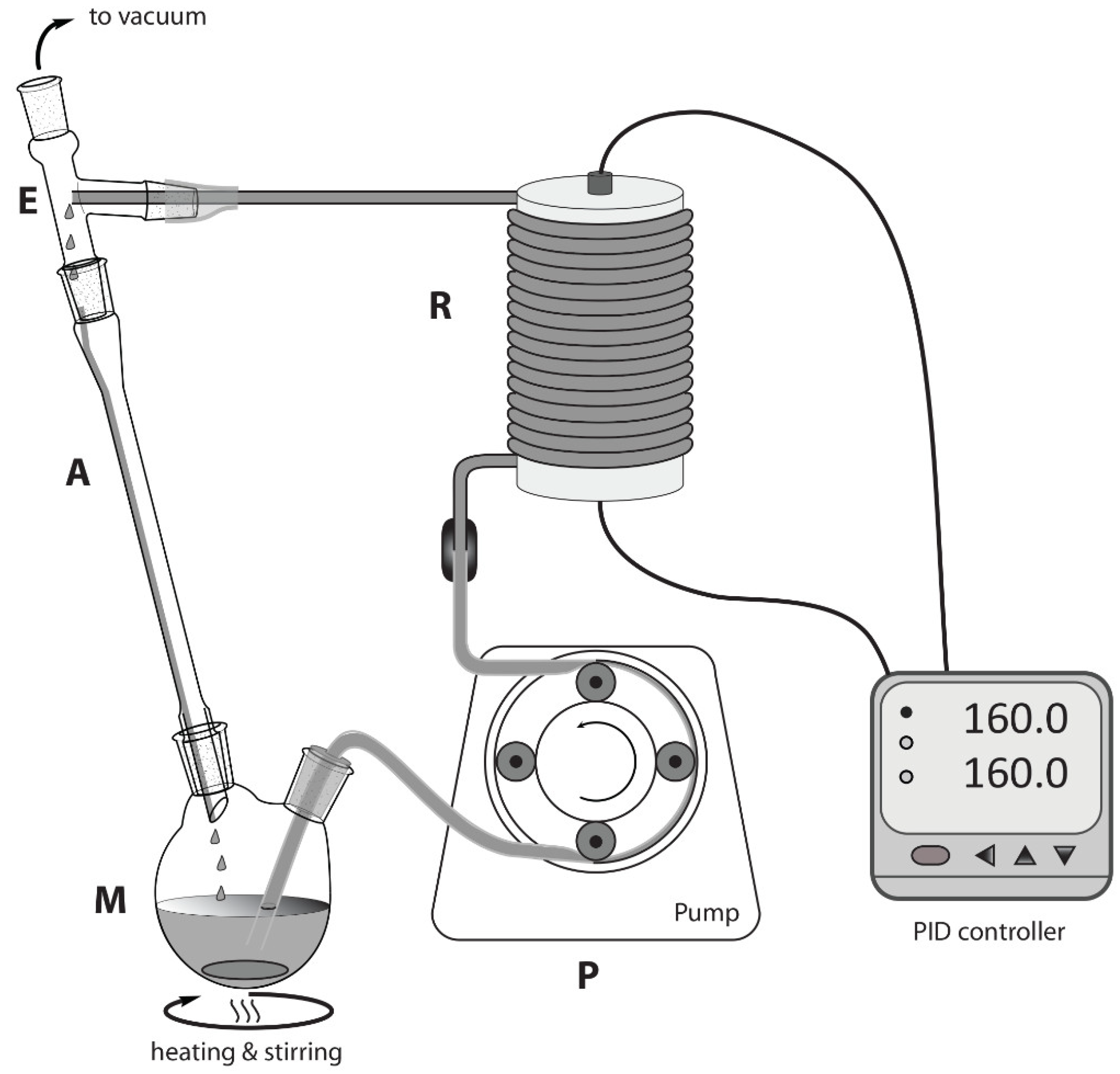
2.3. Procedure for Continuous Flow Reactions
2.4. Design of Experiments (DoE)
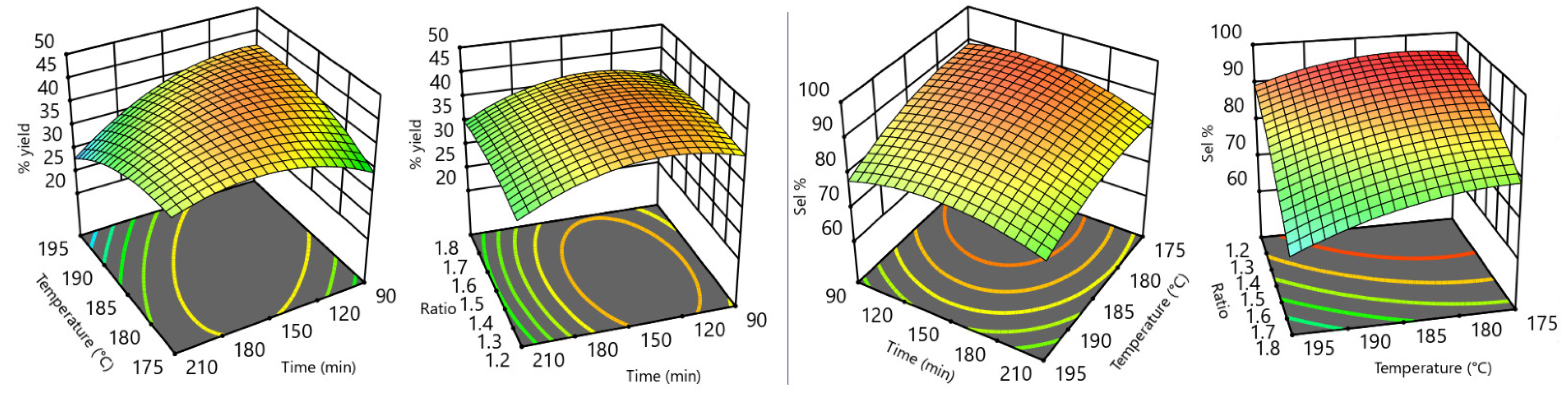
3. Results
- temperatures greater than 175 °C resulted in better GC yields;
- a minimum time of 90 min was required to obtain complete conversion of Gly;
- pressures lower than 400 mmHg resulted in unreliable flow due to peristaltic pump malfunction (tube squeezing) and minor urea losses due to sublimation;
- yields were unaffected by changes in flow rate, within the range from 0.5–5.0 mL/min;
- yields were unaffected by changes in catalyst amount, within the range from 0.03–0.05 molZnSO4·H2O/molGly;
- diglycerol tricarbonate (DGTC) was identified as the main by-product [23].
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. (Eds.) Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In IPCC 2022: Climate Change 2022: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; p. 3056. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Xu, H.; Ou, L.; Li, Y.; Hawkins, T.R.; Wang, M. Life Cycle Greenhouse Gas Emissions of Biodiesel and Renewable Diesel Production in the United States. Environ. Sci. Technol. 2022, 56, 7512–7521. [Google Scholar] [CrossRef] [PubMed]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2015, 56, 1022–1031. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Roncaglia, F.; Forti, L.; D’Anna, S.; Maletti, L. An Expedient Catalytic Process to Obtain Solketal from Biobased Glycerol. Processes 2021, 9, 141. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, H.; Zhu, X.; Lu, R.; Shi, L.; Lu, F. Effect of Tungsten Species on Selective Hydrogenolysis of Glycerol to 1,3-Propanediol. ChemSusChem 2020, 14, 569–581. [Google Scholar] [CrossRef]
- Sittijunda, S.; Reungsang, A. Valorization of crude glycerol into hydrogen, 1,3-propanediol, and ethanol in an up-flow anaerobic sludge blanket (UASB) reactor under thermophilic conditions. Renew. Energy 2020, 161, 361–372. [Google Scholar] [CrossRef]
- Zheng, Z.; Luo, M.; Yu, J.; Wang, J.; Ji, J. Novel Process for 1,3-Dihydroxyacetone Production from Glycerol. 1. Technological Feasibility Study and Process Design. Ind. Eng. Chem. Res. 2012, 51, 3715–3721. [Google Scholar] [CrossRef]
- Selva, M.; Fabris, M. The reaction of glycerol carbonate with primary aromatic amines in the presence of Y- and X-faujasites: The synthesis of N-(2,3-dihydroxy)propyl anilines and the reaction mechanism. Green Chem. 2009, 11, 1161–1172. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.; Raoul, Y.; Mouloungui, Z. Synthesis of five and six-membered cyclic glycerilic carbonates bearing exocyclic urethane functions. Eur. J. Lipid Sci. Technol. 2012, 115, 111–122. [Google Scholar] [CrossRef]
- Quienne, B.; Poli, R.; Pinaud, J.; Caillol, S. Enhanced aminolysis of cyclic carbonates by β-hydroxylamines for the production of fully biobased polyhydroxyurethanes. Green Chem. 2021, 23, 1678–1690. [Google Scholar] [CrossRef]
- Fricke, N.; Keul, H.; Möller, M. Carbonate Couplers and Functional Cyclic Carbonates from Amino Acids and Glucosamine. Macromol. Chem. Phys. 2009, 210, 242–255. [Google Scholar] [CrossRef]
- Bao, Y.-M.; Shen, G.-R.; He, J.; Li, Y.-S. Water-soluble hyperbranched poly(ester urethane)s based on d,l-alanine: Isocyanate-free synthesis, post-functionalization and application. Green Chem. 2012, 14, 2243–2250. [Google Scholar] [CrossRef]
- Galletti, G.; Prete, P.; Vanzini, S.; Cucciniello, R.; Fasolini, A.; De Maron, J.; Cavani, F.; Tabanelli, T. Glycerol Carbonate as a Versatile Alkylating Agent for the Synthesis of β-Aryloxy Alcohols. ACS Sustain. Chem. Eng. 2022, 10, 10922–10933. [Google Scholar] [CrossRef]
- Ghandi, M.; Mostashari, A.; Karegar, M.; Barzegar, M. Efficient Synthesis of α-Monoglycerides via Solventless Condensation of Fatty Acids with Glycerol Carbonate. J. Am. Oil Chem. Soc. 2007, 84, 681–685. [Google Scholar] [CrossRef]
- Simao, A.-C.; Lynikaite-Pukleviciene, B.; Rousseau, C.; Tatibouet, A.; Cassel, S.; Sackus, A.; Rauter, A.; Rollin, P. 1,2-Glycerol Carbonate: A Versatile Renewable Synthon. Lett. Org. Chem. 2006, 3, 744–748. [Google Scholar] [CrossRef]
- da Costa, P.L.F.; Melo, V.N.; Guimarães, B.M.; Schuler, M.; Pimenta, V.; Rollin, P.; Tatibouët, A.; de Oliveira, R.N. Glycerol carbonate in Ferrier reaction: Access to new enantiopure building blocks to develop glycoglycerolipid analogues. Carbohydr. Res. 2016, 436, 1–10. [Google Scholar] [CrossRef]
- Carré, C.; Zoccheddu, H.; Delalande, S.; Pichon, P.; Avérous, L. Synthesis and characterization of advanced biobased thermoplastic nonisocyanate polyurethanes, with controlled aromatic-aliphatic architectures. Eur. Polym. J. 2016, 84, 759–769. [Google Scholar] [CrossRef]
- Rousseau, J.; Rousseau, C.; Lynikaite, B.; Sackus, A.; de Leon, C.; Rollin, P.; Tatibouet, A. Tosylated glycerol carbonate, a versatile bis-electrophile to access new functionalized glycidol derivatives. Tetrahedron 2009, 65, 8571–8581. [Google Scholar] [CrossRef]
- Legros, V.; Taing, G.; Buisson, P.; Schuler, M.; Bostyn, S.; Rousseau, J.; Sinturel, C.; Tatibouët, A. Activated Glycerol Carbonates, Versatile Reagents with Aliphatic Amines: Formation and Reactivity of Glycidyl Carbamates and Trialkylamines. Eur. J. Org. Chem. 2017, 2017, 5032–5043. [Google Scholar] [CrossRef]
- Parzuchowski, P.G.; Świderska, A.; Roguszewska, M.; Frączkowski, T.; Tryznowski, M. Amine functionalized polyglycerols obtained by copolymerization of cyclic carbonate monomers. Polymer 2018, 151, 250–260. [Google Scholar] [CrossRef]
- Rokicki, G.; Rakoczy, P.; Parzuchowski, P.; Sobiecki, M. Hyperbranched aliphatic polyethers obtained from environmentally benign monomer: Glycerol carbonate. Green Chem. 2005, 7, 529–539. [Google Scholar] [CrossRef]
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Heal. Mater. 2021, 10, e2002026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Grinstaff, M.W. Recent Advances in Glycerol Polymers: Chemistry and Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 1906–1924. [Google Scholar] [CrossRef]
- Ekladious, I.; Liu, R.; Zhang, H.; Foil, D.H.; Todd, D.A.; Graf, T.N.; Padera, R.F.; Oberlies, N.H.; Colson, Y.L.; Grinstaff, M.W. Synthesis of poly(1,2-glycerol carbonate)–paclitaxel conjugates and their utility as a single high-dose replacement for multi-dose treatment regimens in peritoneal cancer. Chem. Sci. 2017, 8, 8443–8450. [Google Scholar] [CrossRef]
- Kundys, A.; Plichta, A.; Florjańczyk, Z.; Zychewicz, A.; Lisowska, P.; Parzuchowski, P.; Wawrzyńska, E. Multi-arm star polymers of lactide obtained in melt in the presence of hyperbranched oligoglycerols. Polym. Int. 2016, 65, 927–937. [Google Scholar] [CrossRef]
- Mamiński, M.; Czarzasta, M.; Parzuchowski, P. Wood adhesives derived from hyperbranched polyglycerol cross-linked with hexamethoxymethyl melamines. Int. J. Adhes. Adhes. 2011, 31, 704–707. [Google Scholar] [CrossRef]
- Jansen, J.F.G.A.; Dias, A.A.; Dorschu, M.; Coussens, B. Fast Monomers: Factors Affecting the Inherent Reactivity of Acrylate Monomers in Photoinitiated Acrylate Polymerization. Macromolecules 2003, 36, 3861–3873. [Google Scholar] [CrossRef]
- Mhanna, A.; Sadaka, F.; Boni, G.; Brachais, C.-H.; Brachais, L.; Couvercelle, J.-P.; Plasseraud, L.; Lecamp, L. Photopolymerizable Synthons from Glycerol Derivatives. J. Am. Oil Chem. Soc. 2013, 91, 337–348. [Google Scholar] [CrossRef]
- Sacripante, G.G.; Zhou, K.; Farooque, M. Sustainable Polyester Resins Derived from Rosins. Macromolecules 2015, 48, 6876–6881. [Google Scholar] [CrossRef]
- Ibn El Alami, M.S.; Suisse, I.; Fadlallah, S.; Sauthier, M.; Visseaux, M. Telomerization of 1,3-butadiene with glycerol carbonate and subsequent ring-opening lactone co-polymerization. Comptes Rendus. Chim. 2016, 19, 299–305. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, L.; Dai, J.; Qu, J. Synthesis and properties of aqueous cyclic carbonate dispersion and non-isocyanate polyurethanes under atmospheric pressure. Prog. Org. Coatings 2019, 136, 5209. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.; Jiang, S.; Liang, C.; Wang, J.; Kang, M.; Li, Q.; Zhao, Y. Promising approaches to improve the performances of hybrid non-isocyanate polyurethane. Polym. Int. 2018, 68, 651–660. [Google Scholar] [CrossRef]
- Annunziata, L.; Diallo, A.K.; Fouquay, S.; Michaud, G.; Simon, F.; Brusson, J.-M.; Carpentier, J.-F.; Guillaume, S.M. α,ω-Di(glycerol carbonate) telechelic polyesters and polyolefins as precursors to polyhydroxyurethanes: An isocyanate-free approach. Green Chem. 2013, 16, 1947–1956. [Google Scholar] [CrossRef]
- Duval, C.; Kébir, N.; Jauseau, R.; Burel, F. Organocatalytic synthesis of novel renewable non-isocyanate polyhydroxyurethanes. J. Polym. Sci. Part A: Polym. Chem. 2015, 54, 758–764. [Google Scholar] [CrossRef]
- Quienne, B.; Kasmi, N.; Dieden, R.; Caillol, S.; Habibi, Y. Isocyanate-Free Fully Biobased Star Polyester-Urethanes: Synthesis and Thermal Properties. Biomacromolecules 2020, 21, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Ekin, A.; Webster, D.C. Synthesis and Characterization of Novel Hydroxyalkyl Carbamate and Dihydroxyalkyl Carbamate Terminated Poly(dimethylsiloxane) Oligomers and Their Block Copolymers with Poly(ε-caprolactone). Macromolecules 2006, 39, 8659–8668. [Google Scholar] [CrossRef]
- Tachibana, Y.; Shi, X.; Graiver, D.; Narayan, R. The Use of Glycerol Carbonate in the Preparation of Highly Branched Siloxy Polymers. Silicon 2014, 7, 5–13. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.-L.; Lam, M.K.; Chen, W.-H. Organic Carbonate Production Utilizing Crude Glycerol Derived as By-Product of Biodiesel Production: A Review. Energies 2020, 13, 1483. [Google Scholar] [CrossRef]
- Magniont, C.; Escadeillas, G.; Oms-Multon, C.; De Caro, P. The benefits of incorporating glycerol carbonate into an innovative pozzolanic matrix. Cem. Concr. Res. 2010, 40, 1072–1080. [Google Scholar] [CrossRef]
- Hough, L.; Priddle, J.E.; Theobald, R.S. 363. Carbohydrate carbonates. Part II. Their preparation by ester-exchange methods. J. Chem. Soc. 1962, 9, 1934–1938. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, X.; Song, Y.; Tran, D.K.; Wang, H.; Wilson, J.; Dong, M.; Vazquez, M.; Sun, G.; Wooley, K.L. Complexities of Regioselective Ring-Opening vs. Transcarbonylation-Driven Structural Metamorphosis during Organocatalytic Polymerizations of Five-Membered Cyclic Carbonate Glucose Monomers. JACS Au 2022, 2, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Cao, Y.; Xu, S.; He, P.; Li, H.; Liu, H. Tris-imidazolinium-based porous poly(ionic liquid)s as an efficient catalyst for decarboxylation of cyclic carbonate to epoxide. RSC Adv. 2021, 11, 14193–14202. [Google Scholar] [CrossRef] [PubMed]
- Ochoa Gómez, J.R.; Gómez de Miranda Jiménez de Aberastul, O.; Blanco Pérez, N.; Maestro Madurga, B.; Prieto Fernández, S. Glycidol Synthesis Method. Patent WO2017017307A1, 30 July 2015. [Google Scholar]
- Teng, W.K.; Ngoh, G.C.; Yusoff, R.; Aroua, M.K. A review on the performance of glycerol carbonate production via catalytic transesterification: Effects of influencing parameters. Energy Convers. Manag. 2014, 88, 484–497. [Google Scholar] [CrossRef]
- Van Mileghem, S.; De Borggraeve, W.M.; Baxendale, I.R. A Robust and Scalable Continuous Flow Process for Glycerol Carbonate. Chem. Eng. Technol. 2018, 41, 12. [Google Scholar] [CrossRef]
- Singh, D.; Reddy, B.; Ganesh, A.; Mahajani, S. Zinc/Lanthanum Mixed-Oxide Catalyst for the Synthesis of Glycerol Carbonate by Transesterification of Glycerol. Ind. Eng. Chem. Res. 2014, 53, 18786–18795. [Google Scholar] [CrossRef]
- Okoye, P.; Abdullah, A.; Hameed, B. Glycerol carbonate synthesis from glycerol and dimethyl carbonate using trisodium phosphate. J. Taiwan Inst. Chem. Eng. 2016, 68, 51–58. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Cui, P.; Cheng, W.; Zhang, S. Glycerol carbonate synthesis from glycerol and dimethyl carbonate using guanidine ionic liquids. Chin. J. Chem. Eng. 2017, 25, 1182–1186. [Google Scholar] [CrossRef]
- Nascimento, M.A.D.; Gotardo, L.E.; Leão, R.A.C.; de Castro, A.M.; de Souza, R.O.M.A.; Itabaiana, J.I. Enhanced Productivity in Glycerol Carbonate Synthesis under Continuous Flow Conditions: Combination of Immobilized Lipases from Porcine Pancreas and Candida antarctica (CALB) on Epoxy Resins. ACS Omega 2019, 4, 860–869. [Google Scholar] [CrossRef]
- Gérardy, R.; Estager, J.; Luis, P.; Debecker, D.P.; Monbaliu, J.-C.M. Versatile and scalable synthesis of cyclic organic carbonates under organocatalytic continuous flow conditions. Catal. Sci. Technol. 2019, 9, 6841–6851. [Google Scholar] [CrossRef]
- Zheng, Q.; Nishimura, R.; Sato, Y.; Inomata, H.; Ota, M.; Watanabe, M.; Camy, S. Dimethyl carbonate (DMC) synthesis from methanol and carbon dioxide in the presence of ZrO2 solid solutions and yield improvement by applying a natural convection circulation system. Chem. Eng. J. 2021, 429, 132378. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, D.; Jia, B.; Huang, Y.; Cheng, Y.; Luo, X.; Liang, Z. Study on catalytic performance and kinetics of high efficiency CeO2 catalyst prepared by freeze drying for the synthesis of dimethyl carbonate from CO2 and methanol. Chem. Eng. Sci. 2022, 254, 117614. [Google Scholar] [CrossRef]
- Huang, S.; Yan, B.; Wang, S.; Ma, X. Recent advances in dialkyl carbonates synthesis and applications. Chem. Soc. Rev. 2015, 44, 3079–3116. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, D. Transformation of CO2 with glycerol to glycerol carbonate by a novel ZnWO4-ZnO catalyst. J. CO2 Util. 2018, 26, 370–379. [Google Scholar] [CrossRef]
- Park, C.-Y.; Nguyen-Phu, H.; Shin, E.W. Glycerol carbonation with CO2 and La2O2CO3/ZnO catalysts prepared by two different methods: Preferred reaction route depending on crystalline structure. Mol. Catal. 2017, 435, 99–109. [Google Scholar] [CrossRef]
- Chaugule, A.A.; Tamboli, A.H.; Kim, H. Ionic liquid as a catalyst for utilization of carbon dioxide to production of linear and cyclic carbonate. Fuel 2017, 200, 316–332. [Google Scholar] [CrossRef]
- He, Y.; Lu, H.; Li, X.; Wu, J.; Pu, T.; Du, W.; Li, H.; Ding, J.; Wan, H.; Guan, G. Insight into the reversible behavior of Lewis–Brønsted basic poly(ionic liquid)s in one-pot two-step chemical fixation of CO2 to linear carbonates. Green Chem. 2021, 23, 8571–8580. [Google Scholar] [CrossRef]
- Giannoccaro, P.; Casiello, M.; Milella, A.; Monopoli, A.; Cotugno, P.; Nacci, A. Synthesis of 5-membered cyclic carbonates by oxidative carbonylation of 1,2-diols promoted by copper halides. J. Mol. Catal. A Chem. 2012, 365, 162–171. [Google Scholar] [CrossRef]
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating Worldwide use of Urea—A Global Change Contributing to Coastal Eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]
- Estiu, G.; Merz, J.K.M. The Hydrolysis of Urea and the Proficiency of Urease. J. Am. Chem. Soc. 2004, 126, 6932–6944. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M. Deep eutectic solvents based on urea, polyols and sugars for starch treatment. Int. J. Biol. Macromol. 2021, 176, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.S.; Lala, L.K. Urea as a base in organic reactions. Tetrahedron Lett. 1967, 8, 3267–3269. [Google Scholar] [CrossRef]
- Rodríguez-Santiago, L.; Noguera, M.; Sodupe, M.; Salpin, J.Y.; Tortajada, J. Gas Phase Reactivity of Ni+ with Urea. Mass Spectrometry and Theoretical Studies. J. Phys. Chem. A 2003, 107, 9865–9874. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, N.; Wei, W.; Sun, Y. Catalytic performance of metal oxides for the synthesis of propylene carbonate from urea and 1,2-propanediol. J. Mol. Catal. A: Chem. 2007, 270, 44–49. [Google Scholar] [CrossRef]
- Nguyen-Phu, H.; Shin, E.W. Disordered structure of ZnAl2O4 phase and the formation of a Zn NCO complex in ZnAl mixed oxide catalysts for glycerol carbonylation with urea. J. Catal. 2019, 373, 147–160. [Google Scholar] [CrossRef]
- Elman, A.R.; Davydov, I.E.; Stepanov, A.A. Synthesis of Urea by Ammonolysis of Propylene Carbonate. J. Chem. Chem. Eng. 2018, 12, 26–30. [Google Scholar] [CrossRef]
- Calvino-Casilda, V.; Mul, G.; Fernández, J.; Rubio-Marcos, F.; Bañares, M. Monitoring the catalytic synthesis of glycerol carbonate by real-time attenuated total reflection FTIR spectroscopy. Appl. Catal. A Gen. 2011, 409, 106–112. [Google Scholar] [CrossRef]
- Park, J.-H.; Choi, J.S.; Woo, S.K.; Lee, S.D.; Cheong, M.; Kim, H.S.; Lee, H. Isolation and characterization of intermediate catalytic species in the Zn-catalyzed glycerolysis of urea. Appl. Catal. A Gen. 2012, 433, 35–40. [Google Scholar] [CrossRef]
- Wang, H.; Xin, Z.; Li, Y. Synthesis of Ureas from CO2. Top. Curr. Chem. 2017, 375, 49. [Google Scholar] [CrossRef]
- Ishaq, H.; Siddiqui, O.; Chehade, G.; Dincer, I. A solar and wind driven energy system for hydrogen and urea production with CO2 capturing. Int. J. Hydrogen Energy 2020, 46, 4749–4760. [Google Scholar] [CrossRef]
- Li, J.; Wang, T. Chemical equilibrium of glycerol carbonate synthesis from glycerol. J. Chem. Thermodyn. 2011, 43, 731–736. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Ramírez-López, C.; Belsué, M. A Brief Review on Industrial Alternatives for the Manufacturing of Glycerol Carbonate, a Green Chemical. Org. Process. Res. Dev. 2012, 16, 389–399. [Google Scholar] [CrossRef]
- Indran, V.P.; Saud, A.S.H.; Maniam, G.P.; Yusoff, M.M.; Taufiq-Yap, Y.H.; Rahim, M.H.A. Versatile boiler ash containing potassium silicate for the synthesis of organic carbonates. RSC Adv. 2016, 6, 34877–34884. [Google Scholar] [CrossRef]
- Fernandes, G.P.; Yadav, G.D. Selective glycerolysis of urea to glycerol carbonate using combustion synthesized magnesium oxide as catalyst. Catal. Today 2018, 309, 153–160. [Google Scholar] [CrossRef]
- Chaves, D.M.; Da Silva, M.J. A selective synthesis of glycerol carbonate from glycerol and urea over Sn(OH)2: A solid and recyclable in situ generated catalyst. New J. Chem. 2019, 43, 3698–3706. [Google Scholar] [CrossRef]
- Mallesham, B.; Rangaswamy, A.; Rao, B.G.; Rao, T.V.; Reddy, B.M. Solvent-Free Production of Glycerol Carbonate from Bioglycerol with Urea Over Nanostructured Promoted SnO2 Catalysts. Catal. Lett. 2020, 150, 3626–3641. [Google Scholar] [CrossRef]
- Turney, T.W.; Patti, A.; Gates, W.; Shaheen, U.; Kulasegaram, S. Formation of glycerol carbonate from glycerol and urea catalysed by metal monoglycerolates. Green Chem. 2013, 15, 1925–1931. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Yamanishi, Y.; Arai, M. Synthesis of glycerol carbonate from glycerol and urea using zinc-containing solid catalysts: A homogeneous reaction. J. Catal. 2013, 297, 137–141. [Google Scholar] [CrossRef]
- Kulasegaram, S.; Shaheen, U.; Turney, T.W.; Gates, W.P.; Patti, A.F. Zinc monoglycerolate as a catalyst for the conversion of 1,3- and higher diols to diurethanes. RSC Adv. 2015, 5, 47809–47812. [Google Scholar] [CrossRef]
- Nguyen-Phu, H.; Do, L.T.; Shin, E.W. Investigation of glycerolysis of urea over various ZnMeO (Me = Co, Cr, and Fe) mixed oxide catalysts. Catal. Today 2020, 352, 80–87. [Google Scholar] [CrossRef]
- Nguyen-Phu, H.; Park, C.-Y.; Shin, E.W. Dual catalysis over ZnAl mixed oxides in the glycerolysis of urea: Homogeneous and heterogeneous reaction routes. Appl. Catal. A Gen. 2018, 552, 1–10. [Google Scholar] [CrossRef]
- Endah, Y.K.; Kim, M.S.; Choi, J.; Jae, J.; Lee, S.D.; Lee, H. Consecutive carbonylation and decarboxylation of glycerol with urea for the synthesis of glycidol via glycerol carbonate. Catal. Today 2017, 293, 136–141. [Google Scholar] [CrossRef]
- Kaur, A.; Prakash, R.; Ali, A. 1H NMR assisted quantification of glycerol carbonate in the mixture of glycerol and glycerol carbonate. Talanta 2018, 178, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Isa, K. Thermal Decomposition of Urea and Urea Derivatives by Simultaneous TG/(DTA)/MS. J. Mass Spectrom. Soc. Jpn. 1998, 46, 299–303. [Google Scholar] [CrossRef]
- Lertlukkanasuk, N.; Phiyanalinmat, S.; Kiatkittipong, W.; Arpornwichanop, A.; Aiouache, F.; Assabumrungrat, S. Reactive distillation for synthesis of glycerol carbonate via glycerolysis of urea. Chem. Eng. Process. Process Intensif. 2013, 70, 103–109. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wu, C.; Qian, Q.; Ma, J.; Jiang, L.; Han, B. Microwave assisted synthesis of glycerol carbonate from glycerol and urea. Pure Appl. Chem. 2017, 90, 1–6. [Google Scholar] [CrossRef]
- Romano, G.; Paradisi, E.; Rosa, R.; Leonelli, C.; Roncaglia, F. Synthesis of Glycerol Carbonate from Glycerol and Urea Using a Microwave Reactor. AMPERE Newsl. 2022, 111, 1–8. Available online: www.ampereeurope.org/issue-111 (accessed on 22 November 2022).
- She, Q.M.; Liu, J.H.; Aymonier, C.; Zhou, C.H. In situ fabrication of layered double hydroxide film immobilizing gold nanoparticles in capillary microreactor for efficient catalytic carbonylation of glycerol. Mol. Catal. 2021, 513, 111825. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry II. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Tennant, A.J.; Krause, M.J.; Kujawski, M.P.; Sherren, B.T. Compositions de Polyuréthane. Patent WO2021222192A1, 29 April 2020. [Google Scholar]
- Aresta, M.; Dibenedetto, A.; di Bitonto, L. New efficient and recyclable catalysts for the synthesis of di- and tri-glycerol carbonates. RSC Adv. 2015, 5, 64433–64443. [Google Scholar] [CrossRef]
- Atlantic Richfield Company. Patent GB1 458 595 A, 15 December 1976.
- Arne Them Jensen. Subtilases. Patent EP0 611 243 A1, 21 March 2021. [Google Scholar]



| Entry | Catalyst | Isolated Yield (%) |
|---|---|---|
| 1 | ZnSO4·7H2O | 27 |
| 2 | ZnSO4·H2O | 30 |
| 3 b | ZnSO4·H2O | 27 |
| 4 | ZnCl2 | 30 |
| 5 | FeCl3 | 20 |
| 6 | MgO | 22 |
| Exp. n. | Temperature (°C) | Urea:Gly MR | Time (min) | GC Yield (%) | GC Selectivity (%) |
|---|---|---|---|---|---|
| 1 | 195 | 1.8 | 210 | 25.8 | 57 |
| 2 | 175 | 1.2 | 90 | 33.6 | 93 |
| 3 | 175 | 1.8 | 210 | 37.9 | 68 |
| 4 | 185 | 1.5 | 210 | 35.2 | 85 |
| 5 | 185 | 1.2 | 150 | 40.3 | 91 |
| 6 | 175 | 1.2 | 210 | 37.9 | 90 |
| 7 | 195 | 1.2 | 90 | 41.9 | 86 |
| 8 | 185 | 1.5 | 150 | 42.9 | 88 |
| 9 | 185 | 1.8 | 150 | 42.3 | 80 |
| 10 | 175 | 1.8 | 90 | 33.4 | 82 |
| 11 | 195 | 1.2 | 210 | 27.0 | 84 |
| 12 | 185 | 1.5 | 150 | 45.1 | 88 |
| 13 | 185 | 1.5 | 150 | 45.7 | 86 |
| 14 | 185 | 1.5 | 150 | 42.8 | 89 |
| 15 | 185 | 1.5 | 150 | 41.3 | 89 |
| 16 | 195 | 1.8 | 90 | 38.7 | 64 |
| 17 | 175 | 1.5 | 150 | 39.0 | 87 |
| 18 | 185 | 1.5 | 90 | 41.0 | 82 |
| 19 | 195 | 1.5 | 150 | 40.3 | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderlini, B.; Ughetti, A.; Cristoni, E.; Forti, L.; Rigamonti, L.; Roncaglia, F. Upgrading of Biobased Glycerol to Glycerol Carbonate as a Tool to Reduce the CO2 Emissions of the Biodiesel Fuel Life Cycle. Bioengineering 2022, 9, 778. https://doi.org/10.3390/bioengineering9120778
Anderlini B, Ughetti A, Cristoni E, Forti L, Rigamonti L, Roncaglia F. Upgrading of Biobased Glycerol to Glycerol Carbonate as a Tool to Reduce the CO2 Emissions of the Biodiesel Fuel Life Cycle. Bioengineering. 2022; 9(12):778. https://doi.org/10.3390/bioengineering9120778
Chicago/Turabian StyleAnderlini, Biagio, Alberto Ughetti, Emma Cristoni, Luca Forti, Luca Rigamonti, and Fabrizio Roncaglia. 2022. "Upgrading of Biobased Glycerol to Glycerol Carbonate as a Tool to Reduce the CO2 Emissions of the Biodiesel Fuel Life Cycle" Bioengineering 9, no. 12: 778. https://doi.org/10.3390/bioengineering9120778
APA StyleAnderlini, B., Ughetti, A., Cristoni, E., Forti, L., Rigamonti, L., & Roncaglia, F. (2022). Upgrading of Biobased Glycerol to Glycerol Carbonate as a Tool to Reduce the CO2 Emissions of the Biodiesel Fuel Life Cycle. Bioengineering, 9(12), 778. https://doi.org/10.3390/bioengineering9120778










