Conjugation Mechanism for Pneumococcal Glycoconjugate Vaccines: Classic and Emerging Methods
Abstract
1. Introduction
2. Classic Conjugation Methods
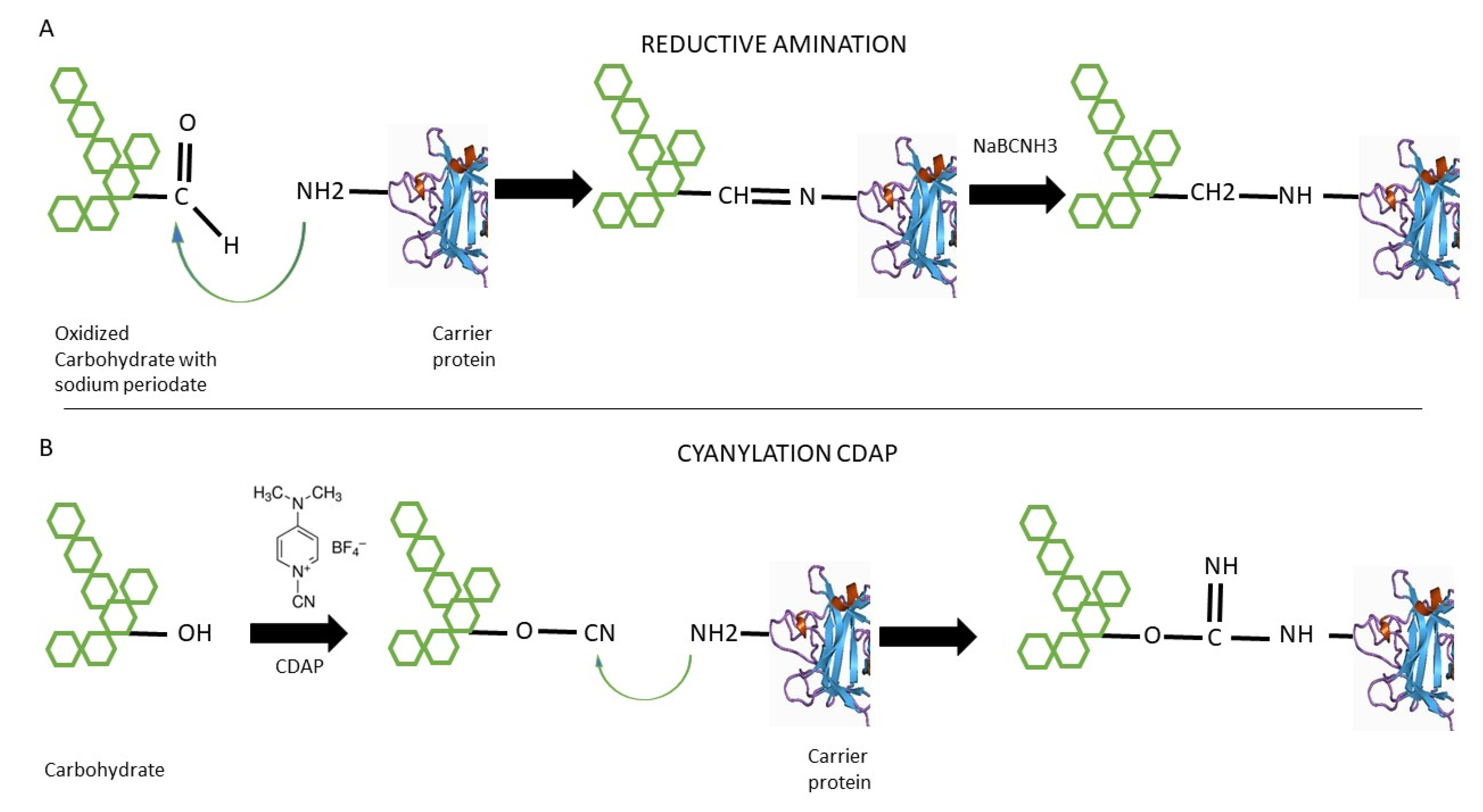
2.1. Reductive Amination
2.2. 1-Cyano-4-dimethylaminopyridine Tetrafluoroborate (CDAP) Cyanilation
3. Emerging Methods
3.1. The Galactose Oxidase (GO) Method for Reductive Amination
3.2. Multiple Antigen Presenting System (MAPS) Platform
3.3. Protein Glycan Coupling Technology (PGCT)

4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grabenstein, J.D.; Klugman, K.P. A Century of Pneumococcal Vaccination Research in Humans. Clin. Microbiol. Infect. 2012, 18, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Auzat, I.; Chapuy-Regaud, S.; le Bras, G.; dos Santos, D.; Ogunniyi, A.D.; le Thomas, I.; Garel, J.-R.; Paton, J.C.; Trombe, M.-C. The NADH Oxidase of Streptococcus Pneumoniae: Its Involvement in Competence and Virulence. Mol. Microbiol. 1999, 34, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Mantero, M.; Santus, P.; Tarsia, P. Understanding the Burden of Pneumococcal Disease in Adults. Clin. Microbiol. Infect. 2012, 18, 7–14. [Google Scholar] [CrossRef]
- Kim, G.L.; Seon, S.H.; Rhee, D.K. Pneumonia and Streptococcus Pneumoniae Vaccine. Arch. Pharm. Res. 2017, 40, 885–893. [Google Scholar] [CrossRef]
- Alonso, D.E.; Verheul, A.F.; Verhoef, J.; Snippe, H. Streptococcus Pneumoniae: Virulence Factors, Pathogenesis, and Vaccines. Microbiol. Rev. 1995, 59, 591–603. [Google Scholar]
- Morais, V.; Dee, V.; Suárez, N. Purification of Capsular Polysaccharides of Streptococcus Pneumoniae: Traditional and New Methods. Front Bioeng. Biotechnol. 2018, 6, 145. [Google Scholar] [CrossRef]
- Lund, E. Polyvalent, diagnostic pneumococcus sera. Acta Pathol. Microbiol. Scand. 1963, 59, 187–190. [Google Scholar] [CrossRef]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef]
- Ganaie, F.; Saad, J.S.; McGee, L.; van Tonder, A.J.; Bentley, S.D.; Lo, S.W.; Gladstone, R.A.; Turner, P.; Keenan, J.D.; Breiman, R.F.; et al. A New Pneumococcal Capsule Type, 10D, Is the 100th Serotype and Has a Large Cps Fragment from an Oral Streptococcus. mBio 2020, 11, e00937-20. [Google Scholar] [CrossRef]
- Kim, L.; McGee, L.; Tomczyk, S.; Beall, B. Biological and Epidemiological Features of Antibiotic-Resistant Streptococcus Pneumoniae in Pre- and Post-Conjugate Vaccine Eras: A United States Perspective. Clin. Microbiol. Rev. 2016, 29, 525–552. [Google Scholar] [CrossRef]
- AUSTRIAM, R.; GOLD, J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann. Int. Med. 1964, 60, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Tarahomjoo, S. Recent Approaches in Vaccine Development against Streptococcus Pneumoniae. J. Mol. Microbiol. Biotechnol. 2014, 24, 215–227. [Google Scholar] [CrossRef]
- Cecchini, P.; Entwisle, C.; Joachim, M.; Pang, Y.; Dalton, K.A.; Hill, S.; McIlgorm, A.; Chan, W.-Y.; Brown, J.S.; Colaco, C.A.; et al. Next Generation Vaccines: Development of a Novel Streptococcus Pneumoniae Multivalent Protein Vaccine. Bio. Processing J. 2015, 14, 18–33. [Google Scholar] [CrossRef]
- Morais, V.; Texeira, E.; Suarez, N. Next-Generation Whole-Cell Pneumococcal Vaccine. Vaccines 2019, 7, 151. [Google Scholar] [CrossRef]
- Dochez, A.R.; Avery, O.T. The elaboration of specific soluble substance by pneumococcus during growth. J. Exp. Med. 1917, 26, 477–493. [Google Scholar] [CrossRef]
- Dochez, A.R.; Avery, O.T. Soluble Substance of Pneumococcus Origin in the Blood and Urine during Lobar Pneumonia. Exp. Biol. Med. 1917, 14, 126–127. [Google Scholar] [CrossRef]
- Heidelberger, M.; Avery, O.T. The soluble specific substance of pneumococcus. J. Exp. Med. 1923, 38, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Dubos, R.; Avery, O.T. Decomposition of the capsular polysaccharide of pneumococcus type III by a bacterial enzyme. J. Exp. Med. 1931, 54, 51–71. [Google Scholar] [CrossRef]
- Goebel, W.F.; Avery, O.T. Chemo-Immunological studies on conjugated carbohydrate-proteins: I. The synthesis of p-aminophenol beta-glucoside, p-aminophenol beta-galactoside and their coupling with serum globulin. J. Exp. Med. 1929, 50, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Avery, O.T.; Goebel, W.F. Chemo-Immunological studies on conjugated carbohydrate-proteins: II. Immunological specificity of synthetic sugar-protein antigens. J. Exp. Med. 1929, 50, 533–550. [Google Scholar] [CrossRef]
- Daniels, C.C.; Rogers, P.D.; Shelton, C.M. A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. JPPT J. Pediatr. Pharm. 2016, 2721, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Abbas Abul, K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 9th ed.; Elsevier: Philadelphia, PA, USA, 2018; ISBN 978-0-323-47978-3. [Google Scholar]
- Musher, D.M.; Anderson, R.; Feldman, C. The Remarkable History of Pneumococcal Vaccination: An Ongoing Challenge. Pneumonia (Nathan) 2022, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schneerson, R.; Barrera, O.; Sutton, A.; Robbins, J.B. Preparation, Characterization, and Immunogenicity of Haemophilus Influenzae Type b Polysaccharide-Protein Conjugates. J. Exp. Med. 1980, 152, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Shinefield, H.; Fireman, B.; Lewis, E.; Ray, P.; Hansen, J.R.; Elvin, L.; Ensor, K.M.; Hackell, J.; Siber, G.; et al. Efficacy, Safety and Immunogenicity of Heptavalent Pneumococcal Conjugate Vaccine in Children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect Dis. J. 2000, 19, 187–195. [Google Scholar] [CrossRef]
- Del Bino, L.; Østerlid, K.E.; Wu, D.-Y.; Nonne, F.; Romano, M.R.; Codée, J.; Adamo, R. Synthetic Glycans to Improve Current Glycoconjugate Vaccines and Fight Antimicrobial Resistance. Chem. Rev. 2022, 122, 15672–15716. [Google Scholar] [CrossRef]
- Berti, F.; Adamo, R. Antimicrobial Glycoconjugate Vaccines: An Overview of Classic and Modern Approaches for Protein Modification. Chem. Soc. Rev. 2018, 47, 9015–9025. [Google Scholar] [CrossRef]
- Poolman, J.T.; Peeters, C.C.; van den Dobbelsteen, G.P. The History of Pneumococcal Conjugate Vaccine Development: Dose Selection. Expert Rev. Vaccines 2013, 12, 1379–1394. [Google Scholar] [CrossRef]
- Weinbergera, M.D.; Malley, R.; Lipsitch, M. Serotype Replacement in Disease Following Pneumococcal Vaccination: A Discussion of the Evidence. Lancet 2012, 378, 1962–1973. [Google Scholar] [CrossRef]
- WHO Pneumococcal Conjugate Vaccines in Infants and Children under 5 Years of Age: WHO Position Paper—February 2019. Wkly. Epidemiol. Rec. 2019, 94, 85–104.
- Jones, C.; Lemercinier, X. Full NMR Assignment and Revised Structure for the Capsular Polysaccharide from Streptococcus Pneumoniae Type 15B. Carbohydr. Res. 2005, 340, 403–409. [Google Scholar] [CrossRef]
- Jennings, H.J.; Young, N.M.; Lugowski, C. Structure of the Complex Polysaccharide C-Substance from Streptococcus Pneumoniae Type 1. Biochemistry 1980, 19, 4712–4719. [Google Scholar] [CrossRef] [PubMed]
- Laferrière, C.A.; Sood, R.K.; De Muys, J.M.; Michon, F.; Jennings, H.J. The Synthesis of Streptococcus Pneumoniae Polysaccharide-Tetanus Toxoid Conjugates and the Effect of Chain Length on Immunogenicity. Vaccine 1997, 15, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kossaczka, Z.; Shiloach, J.; Johnson, V.; Taylor, D.N.; Finkelstein, R.A.; Robbins, J.B.; Szu, S.C. Vibrio Cholerae O139 Conjugate Vaccines: Synthesis and Immunogenicity of V. Cholerae O139 Capsular Polysaccharide Conjugates with Recombinant Diphtheria Toxin Mutant in Mice. Infect Immun. 2000, 68, 5037–5043. [Google Scholar] [CrossRef] [PubMed]
- Kohn, J.; Wilchek, M. The Use of Cyanogen Bromide and Other Novel Cyanylating Agents for the Activation of Polysaccharide Resins. Appl. Biochem. Biotechnol. 1984, 9, 285–305. [Google Scholar] [CrossRef]
- Gray, G.R. The Direct Coupling of Oligosaccharides to Proteins and Derivatized Gels. Arch. Biochem. Biophys 1974, 163, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Jayaraman, N. Glycoconjugations of Biomolecules by Chemical Methods. Front Chem. 2020, 8, 570185. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.; Frasch, C.; Nurkka, A.; Käyhty, H.; Biemans, R.; Schuerman, L. Impact of the Conjugation Method on the Immunogenicity of Streptococcus Pneumoniae Serotype 19F Polysaccharide in Conjugate Vaccines. Clin. Vaccine Immunol. 2011, 18, 327–336. [Google Scholar] [CrossRef]
- Jennings, H.J.; Lugowski, C. Immunochemistry of Groups A, B, and C Meningococcal Polysaccharide-Tetanus Toxoid Conjugates. J. Immunol. 1981, 127, 1011–1018. [Google Scholar]
- Zou, W.; Williams, D.; Cox, A. Mitigating Base-Catalysed Degradation of Periodate-Oxidized Capsular Polysaccharides: Conjugation by Reductive Amination in Acidic Media. Vaccine 2019, 37, 1087–1093. [Google Scholar] [CrossRef]
- Lees, A.; Barr, J.F.; Gebretnsae, S. Activation of Soluble Polysaccharides with 1-Cyano-4-Dimethylaminopyridine Tetrafluoroborate (CDAP) for Use in Protein–Polysaccharide Conjugate Vaccines and Immunological Reagents. Iii Optimization of CDAP Activation. Vaccines 2020, 8, 777. [Google Scholar] [CrossRef]
- Kohn, J.; Wilchek, M. 1-Cyano-4-Dimethylamino Pyridinium Tetrafluoroborate as a Cyanylating Agent for the Covalent Attachment of Ligand to Polysaccharide Resins. FEBS Lett. 1983, 154, 209–210. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.; Nelson, B.L.; Mond, J.J. Activation of Soluble Polysaccharides with 1-Cyano-4-Dimethylaminopyridinium Tetrafluoroborate for Use in Protein-Polysaccharide Conjugate Vaccines and Immunological Reagents. Vaccine 1996, 14, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Suárez, N.; Massaldi, H.; Franco Fraguas, L.; Ferreira, F. Improved Conjugation and Purification Strategies for the Preparation of Protein-Polysaccharide Conjugates. J. Chromatogr. A 2008, 1213, 169–175. [Google Scholar] [CrossRef]
- Lees, A.; Zhou, J. Activation and Conjugation of Soluble Polysaccharides Using 1-Cyano-4-Dimethylaminopyridine Tetrafluoroborate (CDAP). JoVE 2021, 172, e62597. [Google Scholar] [CrossRef] [PubMed]
- Berti, F. Expanding Polysaccharide–Protein Coupling of Glycoconjugate Vaccines. J. Biol. Chem. 2022, 298, 101755. [Google Scholar] [CrossRef]
- Frasch, C.E. Preparation of Bacterial Polysaccharide-Protein Conjugates: Analytical and Manufacturing Challenges. Vaccine 2009, 27, 6468–6470. [Google Scholar] [CrossRef]
- Avci, F.; Berti, F.; Dull, P.; Hennessey, J.; Pavliak, V.; Prasad, A.K.; Vann, W.; Wacker, M.; Marcq, O. Glycoconjugates: What It Would Take To Master These Well-Known yet Little-Understood Immunogens for Vaccine Development. mSphere 2019, 4, e00520-19. [Google Scholar] [CrossRef]
- Duke, J.A.; Paschall, A.V.; Glushka, J.; Lees, A.; Moremen, K.W.; Avci, F.Y. Harnessing Galactose Oxidase in the Development of a Chemoenzymatic Platform for Glycoconjugate Vaccine Design. J. Biol. Chem. 2022, 298, 101453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lu, Y.J.; Malley, R. Multiple Antigen-Presenting System (MAPS) to Induce Comprehensive B-and T-Cell Immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 13564–13569. [Google Scholar] [CrossRef]
- Chichili, G.R.; Smulders, R.; Santos, V.; Cywin, B.; Kovanda, L.; van Sant, C.; Malinoski, F.; Sebastian, S.; Siber, G.; Malley, R. Phase 1/2 Study of a Novel 24-Valent Pneumococcal Vaccine in Healthy Adults Aged 18 to 64 Years and in Older Adults Aged 65 to 85 Years. Vaccine 2022, 40, 4190–4198. [Google Scholar] [CrossRef]
- Zhang, F.; Thompson, C.; Ma, N.; Lu, Y.-J.; Malley, R. Carrier Proteins Facilitate the Generation of Antipolysaccharide Immunity via Multiple Mechanisms. mBio 2022, 13, e0379021. [Google Scholar] [CrossRef]
- Oliver, E.; Pope, C.; Clarke, E.; Langton Hewer, C.; Ogunniyi, A.D.; Paton, J.C.; Mitchell, T.; Malley, R.; Finn, A.; Oliver, E. Th17 Responses to Pneumococcus in Blood and Adenoidal Cells in Children. Clin. Exp. Immunol. 2018, 195, 213–225. [Google Scholar] [CrossRef]
- Malley, R.; Trzcinski, K.; Srivastava, A.; Thompson, C.M.; Anderson, P.W.; Lipsitch, M. CD4 T Cells Mediate Antibody-Independent Acquired Immunity to Pneumococcal Colonization. Proc. Natl. Acad. Sci. USA 2005, 102, 4848–4853. [Google Scholar] [CrossRef]
- Basset, A.; Thompson, C.M.; Hollingshead, S.K.; Briles, D.E.; Ades, E.W.; Lipsitch, M.; Malley, R. Antibody-Independent, CD4+ T-Cell-Dependent Protection against Pneumococcal Colonization Elicited by Intranasal Immunization with Purified Pneumococcal Proteins. Infect Immun. 2007, 75, 5460–5464. [Google Scholar] [CrossRef]
- Moffitt, K.L.; Gierahn, T.M.; Lu, Y.J.; Gouveia, P.; Alderson, M.; Flechtner, J.B.; Higgins, D.E.; Malley, R. TH17-Based Vaccine Design for Prevention of Streptococcus Pneumoniae Colonization. Cell Host Microbe. 2011, 9, 158–165. [Google Scholar] [CrossRef]
- Malley, R.; Srivastava, A.; Lipsitch, M.; Thompson, C.M.; Watkins, C.; Tzianabos, A.; Anderson, P.W. Antibody-Independent, Interleukin-17A-Mediated, Cross-Serotype Immunity to Pneumococci in Mice Immunized Intranasally with the Cell Wall Polysaccharide. Infect Immun. 2006, 74, 2187–2195. [Google Scholar] [CrossRef]
- Szymanski, C.M.; Burr, D.H.; Guerry, P. Campylobacter Protein Glycosylation Affects Host Cell Interactions. Infect Immun. 2002, 70, 2242–2244. [Google Scholar] [CrossRef]
- Kowarik, M.; Young, N.M.; Numao, S.; Schulz, B.L.; Hug, I.; Callewaert, N.; Mills, D.C.; Watson, D.C.; Hernandez, M.; Kelly, J.F.; et al. Definition of the Bacterial N-Glycosylation Site Consensus Sequence. EMBO J. 2006, 25, 1957–1966. [Google Scholar] [CrossRef]
- Feldman, M.F.; Wacker, M.; Hernandez, M.; Hitchen, P.G.; Marolda, C.L.; Kowarik, M.; Morris, H.R.; Dell, A.; Valvano, M.A.; Aebi, M. Engineering N-Linked Protein Glycosylation with Diverse O Antigen Lipopolysaccharide Structures in Escherichia Coli. Proc. Natl. Acad. Sci. USA 2005, 102, 3016–3021. [Google Scholar] [CrossRef]
- Kay, E.; Cuccui, J.; Wren, B.W. Recent Advances in the Production of Recombinant Glycoconjugate Vaccines. NPJ Vaccines 2019, 4, 16. [Google Scholar] [CrossRef]
- Samaras, J.J.; Mauri, M.; Kay, E.J.; Wren, B.W.; Micheletti, M. Development of an Automated Platform for the Optimal Production of Glycoconjugate Vaccines Expressed in Escherichia Coli. Microb. Cell Fact. 2021, 20, 1–15. [Google Scholar] [CrossRef]
- Ielmini, M.V.; Feldman, M.F. Desulfovibrio Desulfuricans PglB Homolog Possesses Oligosaccharyltransferase Activity with Relaxed Glycan Specificity and Distinct Protein Acceptor Sequence Requirements. Glycobiology 2011, 21, 734–742. [Google Scholar] [CrossRef]
- Reglinski, M.; Ercoli, G.; Plumptre, C.; Kay, E.; Petersen, F.C.; Paton, J.C.; Wren, B.W.; Brown, J.S. A Recombinant Conjugated Pneumococcal Vaccine That Protects against Murine Infections with a Similar Efficacy to Prevnar-13. NPJ Vaccines 2018, 3, 1–11. [Google Scholar] [CrossRef]
| Vaccine | Producer | CPS Type | Protein | Conjugation Method |
|---|---|---|---|---|
| Prevnar PCV7 * | Pfizer | 4, 6B, 9V, 14,18C, 19F, 23F | CRM197 | Reductive amination |
| Prevnar PCV13 | Pfizer | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F | CRM197 | Reductive amination |
| Prevnar PCV20 | Pfizer | 1, 3, 4, 5, 6A, 6B, 7F, 8, 9 V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F | CRM197 | Reductive amination |
| Synflorix PCV10 | GSK | 1, 4, 5, 6B, 7F, 9 V, 14, 18C, 19F, 23F | NTHi PD, DT, TT | CDAP |
| Vaxneuvance PCV15 | Merck | 1, 3, 4, 5, 6A, 6B, 7F, 9 V, 14, 18C, 19F, 19A, 22F, 23F, 33F | CRM197 | Reductive amination |
| Method | Main Advantages | Main Disadvantages |
|---|---|---|
| Reductive amination | Proven efficacy in human use in commercial vaccines | Low yield Component degradation |
| CDAP | Proven effectivity in human use in commercial vaccines | Low yield |
| Goase | Improved yield and reduced component degradation | Not proven in humans. Still in preclinical phase |
| MAPS | Improved immunogenicity | Recently started in clinical phase |
| PGCT | Improves and facilitates vaccine production | Not proven in humans. Still in preclinical phase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, V.; Suarez, N. Conjugation Mechanism for Pneumococcal Glycoconjugate Vaccines: Classic and Emerging Methods. Bioengineering 2022, 9, 774. https://doi.org/10.3390/bioengineering9120774
Morais V, Suarez N. Conjugation Mechanism for Pneumococcal Glycoconjugate Vaccines: Classic and Emerging Methods. Bioengineering. 2022; 9(12):774. https://doi.org/10.3390/bioengineering9120774
Chicago/Turabian StyleMorais, Victor, and Norma Suarez. 2022. "Conjugation Mechanism for Pneumococcal Glycoconjugate Vaccines: Classic and Emerging Methods" Bioengineering 9, no. 12: 774. https://doi.org/10.3390/bioengineering9120774
APA StyleMorais, V., & Suarez, N. (2022). Conjugation Mechanism for Pneumococcal Glycoconjugate Vaccines: Classic and Emerging Methods. Bioengineering, 9(12), 774. https://doi.org/10.3390/bioengineering9120774






