Bioengineering to Accelerate Biodiesel Production for a Sustainable Biorefinery
Abstract
1. Introduction
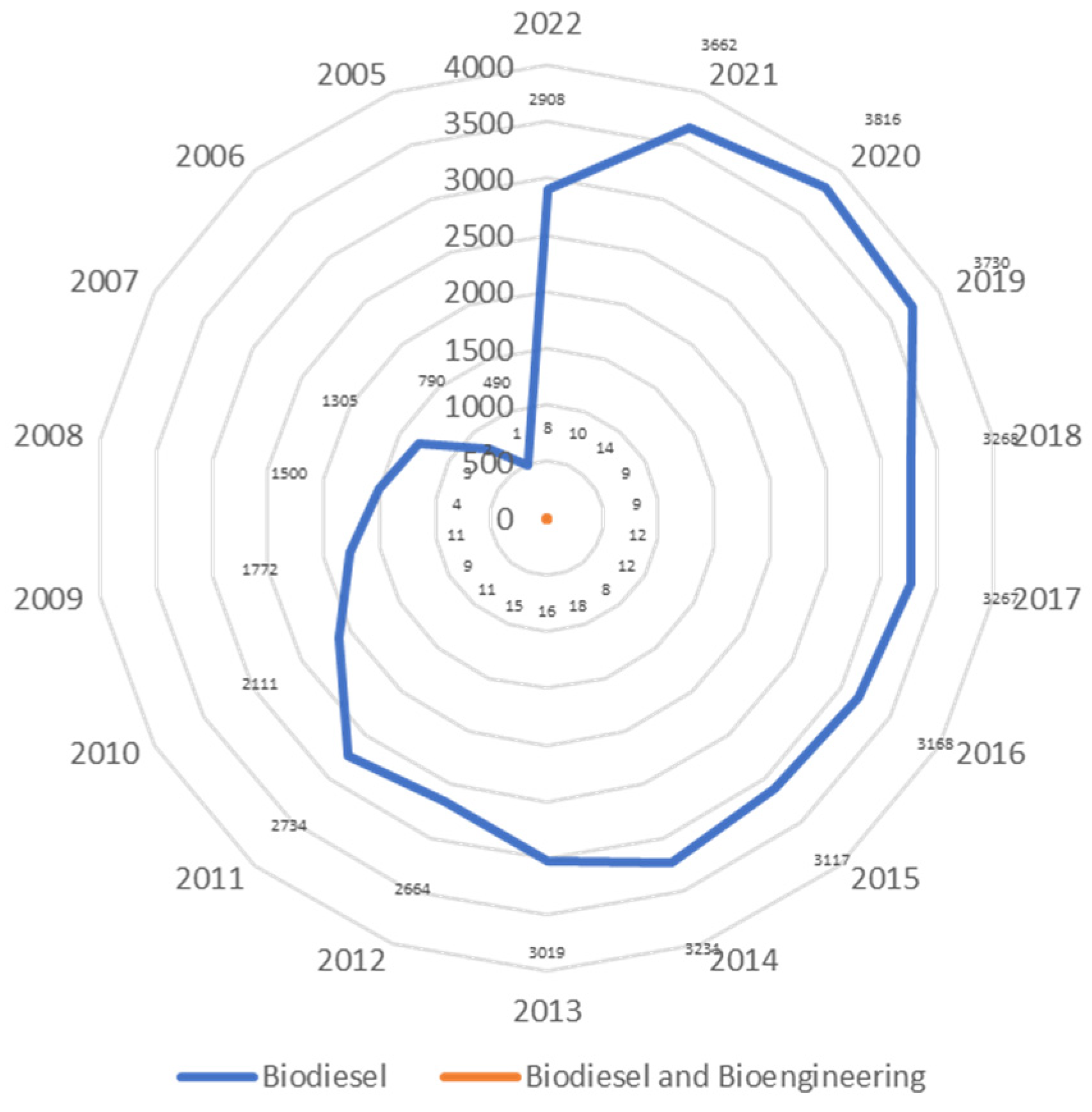
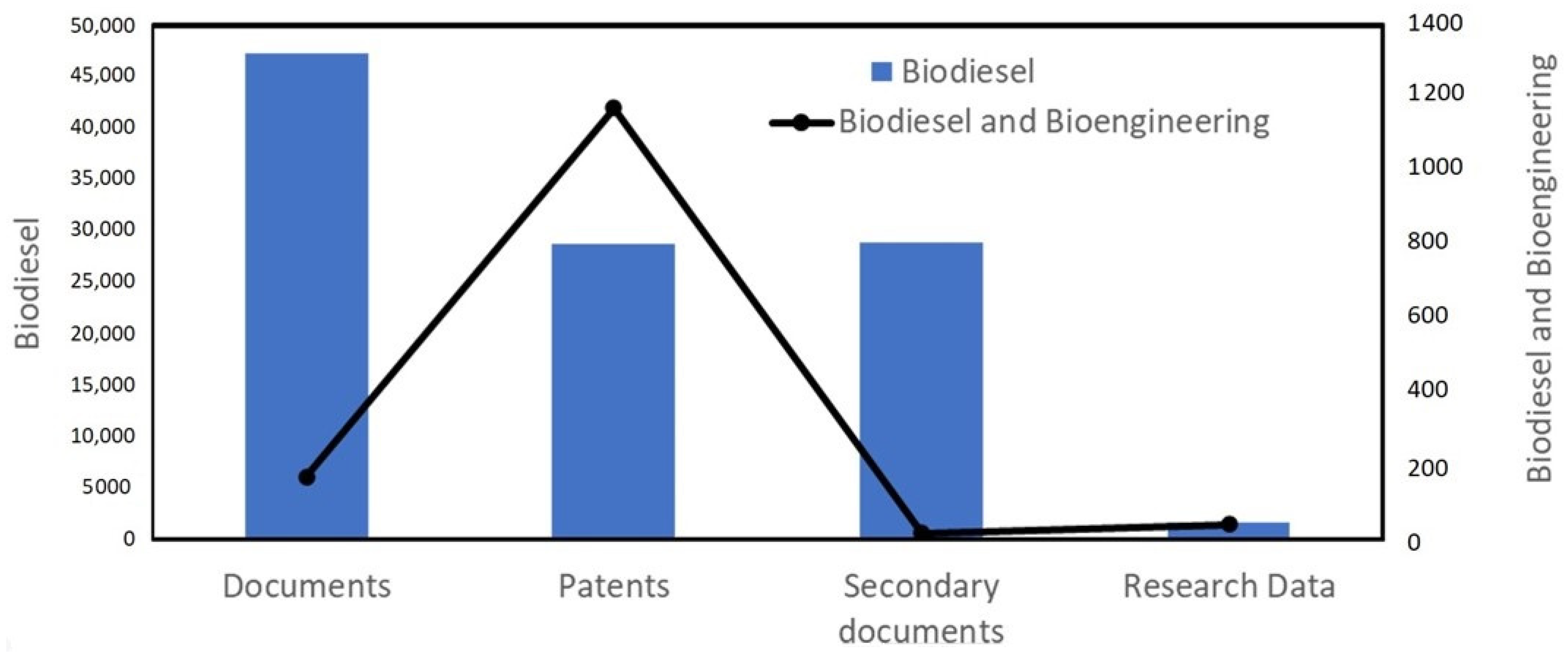
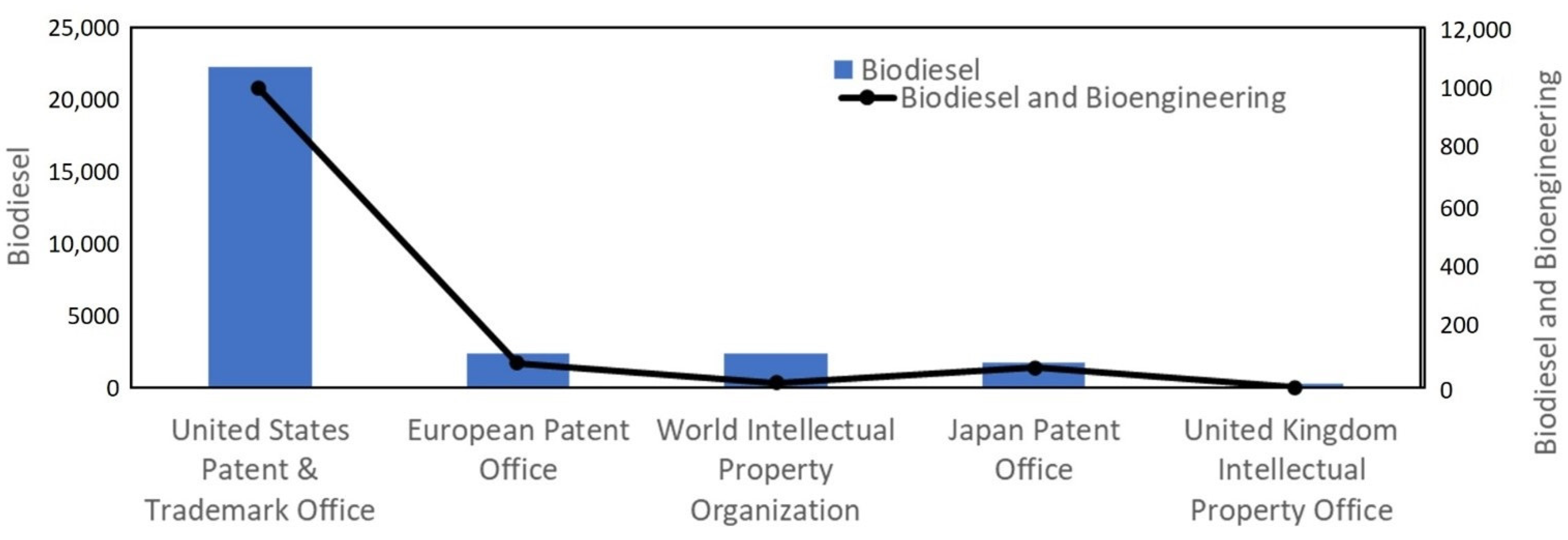
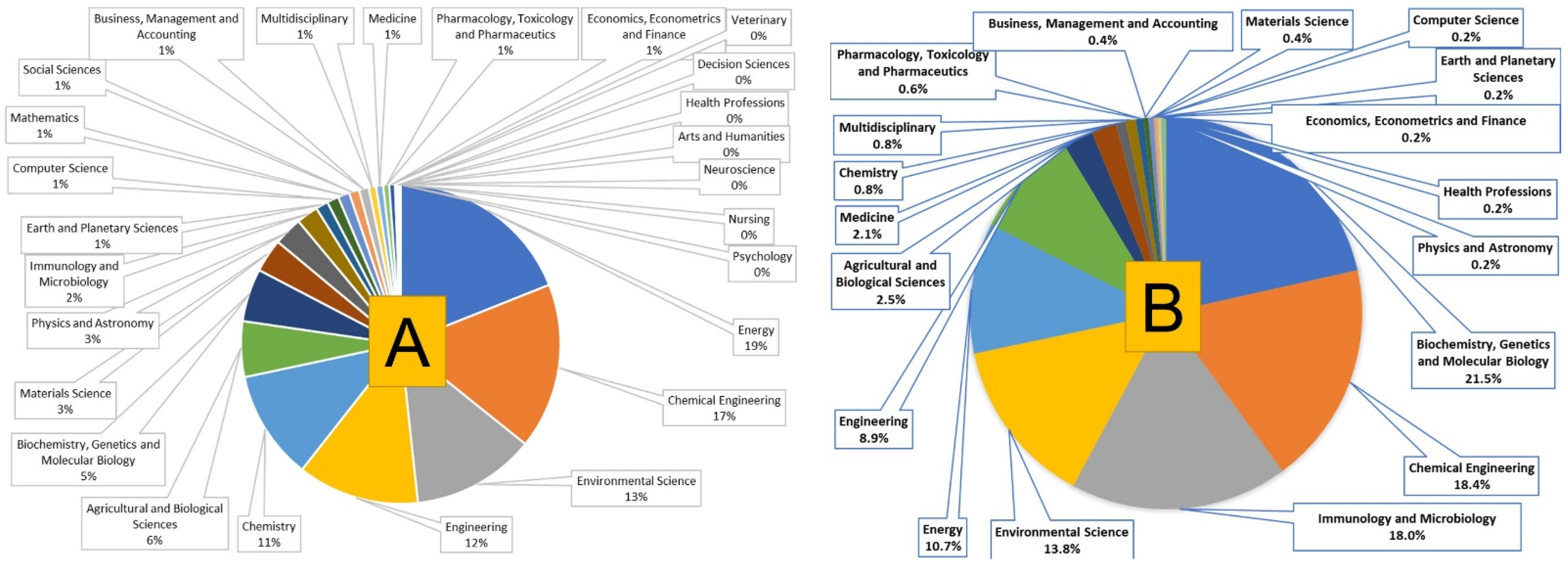
2. Scientometric Analysis
3. Approaches to Accelerate Biodiesel Production
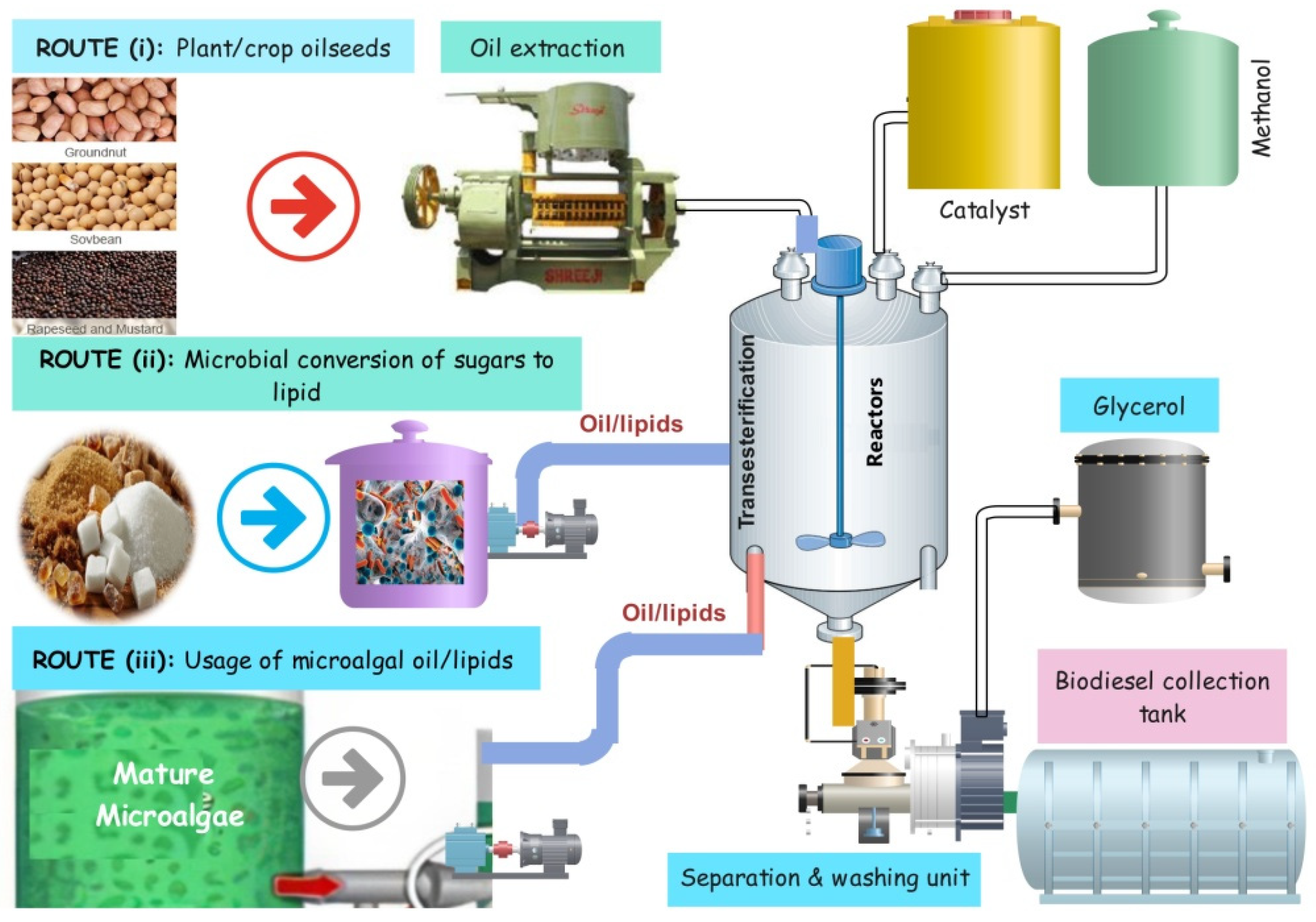
3.1. Biomass Selection in a Carbon-Neutral Manner
3.2. Constraints
3.3. Bioengineering
4. Waste Valorization
4.1. Current Status
| Waste from Biodiesel | Type of Feedstock | Applications | Outcomes | Reference |
|---|---|---|---|---|
| Jatropha curcas Deoiled Cake | Non-edible | Adsorption of Cr(VI) and Cu (II) from wastewater |
| Rawat et al. [83] |
| Oil Palm decanter cake (OPDC) | Edible | Adsorption of heavy metals such as Cu (II), Pb (II) and Zn (II) from waste streams |
| Yusoff et al. [84] |
| Mustard Oil Cake | Edible | Adsorption of Ni (II) from aqueous solution |
| Khan et al. [87] |
| Carbon derived from mustard oil cake (CMOC) | Edible | Adsorption of Zn (II) and Ni (II) from aqueous solution |
| Rao et al. [88] |
| Neem Oil Cake | Edible | Removal and recovery of Cu (II), Cd (II) and Pb (II) from wastewater |
| Rao and Khan [85] |
| Olive Oil Cake | Edible | Biogas Production |
| Sarkar [90] |
| Cotton Oil Cake | Non-edible | Biogas Production |
| Isci and Demirer [91] |
| Flaxseed Oil Cake | Edible | Preparation of Spray-dried functional powders for food applications as emulsion stabilizers |
| Drozlowska et al. [92] |
| Neem Oil Cake | Edible | Evaluation of the effect on plant growth, yield, and management of Alternaria tenuissima leaf spot disease, and rhizosphere microorganisms in chilli crop |
| VasudhaUdupa et al. [93] |
| Madhuca Oil Cake | Non-edible | |||
| Simarouba | Edible | |||
| Coconut kernel cake | Edible | Used as substrate for lipase production |
| Venkatesagowda et al. [94] |
| Neem Cake | Edible | Used for soil amendment against root knot nematode (Meloidogyne incognita) infecting Black gram (Vigna mungo) |
| Rehman et al. [95] |
| Mustard Cake | Edible | |||
| Castor Cake | Non-edible | |||
| Linseed Cake | Edible |
4.2. Opportunities
4.3. Challenges
5. Lifecycle Assessment and Sustainability
6. Conclusions and Future Prospects
- ➢
- The second and third generation feedstocks are latent resources that can overcome the feedstock restriction.
- ➢
- Advancements in bioengineering approaches used in biodiesel production can enhance production capacity and reduce production costs.
- ➢
- Adopting biorefinery approach provides additional benefit for commercial biodiesel production.
- ➢
- The LCA could be employed as the best tool to identify the best combination of various processes involved in the biodiesel refinery by comparing various pathways/techniques available for the sustainable biodiesel production system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Primary Energy Consumption. 2019. Available online: https://ourworldindata.org/grapher/primary-energy-cons?tab=chart&time=2000..latest&country=~OWID_WRL (accessed on 15 August 2022).
- USEIA. Today in Energy; U.S. Energy Information Administration: Washington, DC, USA, 2021.
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mußgnug, J.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenerg. Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- REN21. Renewables Global Status; Report: 2009 Update; Renewable Energy Global Status Report 2021; REN21: Paris, France, 2009. [Google Scholar]
- Awogbemi, O.; Kallon, D.V.V. Application of Tubular Reactor Technologies for the Acceleration of Biodiesel Production. Bioengineering 2022, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.S.; Sadik, W.A.; Sadek, O.M.; Kasaby, M.A. A review on biodiesel feedstocks and production technologies. J. Chil. Chem. Soc. 2021, 66, 5098–5109. [Google Scholar] [CrossRef]
- Linganiso, E.C.; Tlhaole, B.; Magagula, L.P.; Dziike, S.; Linganiso, L.Z.; Motaung, T.E.; Moloto, N.; Tetana, Z.N. Biodiesel Production from Waste Oils: A South African Outlook. Sustainability 2022, 14, 1983. [Google Scholar] [CrossRef]
- Rathore, D.; Nizami, A.S.; Singh, A.; Pant, D. Key issues in estimating energy and greenhouse gas savings of biofuels: Challenges and perspectives. Biofuel Res. J. 2016, 3, 380–393. [Google Scholar] [CrossRef]
- OECD; FAO. OECD-FAO Agricultural Outlook 2015–2024; OECD: Paris, France; FAO: Rome, Italy, 2015. [Google Scholar]
- IEA. Biofuel Production by Country/Region and Fuel Type, 2016–2022; IEA: Paris, France, 2021. [Google Scholar]
- Mukherjee, P.; Varshney, A.; Johnson, S.; Jha, T.B. Jatropha curcas: A review on biotechnological status and challenges. Plant Biotechnol. Rep. 2011, 5, 197–215. [Google Scholar] [CrossRef]
- Sharma, V.; Ramawat, K.G.; Choudhary, B.L. Biodiesel Production for Sustainable Agriculture. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Berlin/Heidelburg, Germany, 2012; pp. 133–160. [Google Scholar]
- Nehmeh, M.; Rodriguez-Donis, I.; Cavaco-Soares, A.; Evon, P.; Gerbaud, V.; Thiebaud-Roux, S. Bio-Refinery of Oilseeds: Oil Extraction, Secondary Metabolites Separation towards Protein Meal Valorisation—A Review. Processes 2022, 10, 841. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of biodiesel production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Phan, A.N.; Phan, T.M. Biodiesel Production from Waste Cooking Oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Navarro-Pineda, F.S.; Ponce-Marbán, D.V.; Sacramento-Rivero, J.C.; Barahona-Pérez, L.F. An economic model for estimating the viability of biodiesel production from Jatropha curcas L. J. Chem. Technol. Biotechnol. 2017, 92, 971–980. [Google Scholar] [CrossRef]
- Sun, J.; Xiong, X.; Wang, M.; Du, H.; Li, J.; Zhou, D.; Zuo, J. Microalgae biodiesel production in China: A preliminary economic analysis. Renew. Sustain. Energy Rev. 2019, 104, 296–306. [Google Scholar] [CrossRef]
- Deniz, I.; Aslanbay, B.; Imamoglu, E. State of Art Strategies for Biodiesel Production: Bioengineering Approaches. In Liquid Biofuel Production; Sigh, L.K., Chaudhary, G., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2019; pp. 319–350. [Google Scholar]
- Hegde, K.; Chandra, N.; Sarma, S.J.; Brar, S.K.; Veeranki, V.D. Genetic Engineering Strategies for Enhanced Biodiesel Production. Mol. Biotechnol. 2015, 57, 606–624. [Google Scholar] [CrossRef]
- Xie, D. Integrating Cellular and Bioprocess Engineering in the Non-Conventional Yeast Yarrowia lipolytica for Biodiesel Production: A Review. Front. Bioeng. Biotechnol. 2017, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Castro, N.M.; Bennett, G.N.; San, K.Y. Acetyl-CoA synthetase overexpression in Escherichia coli demonstrates more efficient acetate assimilation and lower acetate accumulation: A potential tool in metabolic engineering. Appl. Microbiol. Biotechnol. 2006, 71, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pan, A.; Spofford, C.; Zhou, N.; Alper, H.S. An evolutionary metabolic engineering approach for enhancing lipogenesis in Yarrowia lipolytica. Metab. Eng. 2015, 29, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Imam Abidi, S.H.; Liu, H.; Zhang, H.; Chakraborty, S.; Watson, N.; Ajikumar, P.K.; Stephanopoulos, G. Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica. Metab. Eng. 2015, 29, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Wasylenko, T.M.; Zhou, K.; Xu, P.; Stephanopoulos, G. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat. Biotechnol. 2017, 35, 173–179. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Dulermo, T.; Nicaud, J.-M. Engineering Yarrowia lipolytica to produce biodiesel from raw starch. Biotechnol. Biofuels 2015, 8, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Lazar, Z.; Rakicka, M.; Guo, Z.; Fouchard, F.; Coq, A.M.C.L.; Nicaud, J.-M. Metabolic engineering of Yarrowia lipolytica to produce chemicals and fuels from xylose. Metab. Eng. 2016, 38, 115–124. [Google Scholar] [CrossRef]
- Black, P.N.; DiRusso, C.C. Yeast acyl-CoA synthetases at the crossroads of fatty acid metabolism and regulation. Biochim. Biophys. Acta 2007, 1771, 286–298. [Google Scholar] [CrossRef]
- Davis, M.S.; Solbiati, J.; Cronan, J.E. Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J. Biol. Chem. 2000, 275, 28593–28598. [Google Scholar] [CrossRef]
- Klaus, D.; Ohlrogge, J.B.; Neuhaus, H.E.; Dörmann, P. Increased fatty acid production in potato by engineering of acetyl-CoA carboxylase. Planta 2004, 219, 389–396. [Google Scholar] [CrossRef]
- Ruenwai, R.; Cheevadhanarak, S.; Laoteng, K. Overexpression of acetyl-CoA carboxylase gene of Mucor rouxii enhanced fatty acid content in Hansenula polymorpha. Mol. Biotechnol. 2009, 42, 327–332. [Google Scholar] [CrossRef]
- Reik, A.; Zhou, Y.; Collingwood, T.N.; Warfe, L.; Bartsevich, V.; Kong, Y.; Henning, K.A.; Fallentine, B.K.; Zhang, L.; Zhong, X.; et al. Enhanced protein production by engineered zinc finger proteins. Biotechnol. Bioeng. 2007, 97, 1180–1189. [Google Scholar] [CrossRef]
- Liu, H.-H.; Ji, X.-J.; Huang, H. Biotechnological applications of Yarrowia lipolytica: Past, present and future. Biotechnol. Adv. 2015, 33, 1522–1546. [Google Scholar] [CrossRef]
- Doornbosch, R.; Steenblik, R. Biofuels: Is the Cure Worse than the Disease? OECD: Paris, France, 2007. [Google Scholar]
- Jayasree, A.V.; Baby, M.D. Scientometrics: Tools, techniques and software for analysis. Indian J. Inf. Sources Serv. 2019, 9, 116–121. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Korres, N.E.; Rathore, D.; Sevda, S.; Pant, D. Sustainable utilization of crop residues for energy generation: A Life Cycle Assessment (LCA) perspective. Bioresour. Technol. 2020, 303, 122964. [Google Scholar] [CrossRef]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef]
- Dickinson, S.; Mientus, M.; Frey, D.; Amini-Hajibashi, A.; Ozturk, S.; Shaikh, F.; Sengupta, D.; El-Halwagi, M.M. A review of biodiesel production from microalgae. Clean Technol. Environ. Policy 2017, 19, 637–668. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.L.; Molina-Jouve, C.; Nicaud, J.M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Gude, V.; Grant, G.; Patil, P.; Deng, S. Biodiesel production from low cost and renewable feedstock. Open Eng. 2013, 3, 595–605. [Google Scholar] [CrossRef]
- Chandel, A.K.; Garlapati, V.K.; Kumar, S.P.J.; Hans, M.; Singh, A.K.; Kumar, S. The role of renewable chemicals and biofuels in building a bioeconomy. Biofuels Bioprod. Biorefin. 2020, 14, 830–844. [Google Scholar] [CrossRef]
- Heo, H.Y.; Heo, S.; Lee, J.H. Comparative techno-economic analysis of transesterification technologies for microalgal biodiesel production. Ind. Eng. Chem. Res. 2019, 58, 18772–18779. [Google Scholar] [CrossRef]
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Bergmann, J.C.; Tupinambá, D.D.; Costa, O.Y.A.; Almeida, J.R.M.; Barreto, C.C.; Quirino, B.F. Biodiesel production in Brazil and alternative biomass feedstocks. Renew. Sustain. Energy Rev. 2013, 21, 411–420. [Google Scholar] [CrossRef]
- Fargione, J.; Hill, J.; Tilman, D.; Polasky, S.; Hawthorne, P. Land Clearing and the Biofuel Carbon Debt. Science 2008, 319, 1235–1238. [Google Scholar] [CrossRef]
- Hossain, A.K.; Davies, P.A. Plant oils as fuels for com-pression ignition engines: A technical review and life-cycle analysis. Renew. Energy 2010, 35, 1–13. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; Sadik, W.A.; Sadek, O.M.; Kasaby, M.A. Glycerolysis treatment to enhance biodiesel production from low-quality feedstocks. Fuel 2021, 284, 118970. [Google Scholar] [CrossRef]
- Bhatia, L.; Bachheti, R.K.; Garlapati, V.K.; Chandel, A.K. Third-generation biorefineries: A sustainable platform for food, clean energy, and nutraceuticals production. Biomass Conv. Bioref. 2022, 12, 4215–4230. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Zuccaro, G.; Kumar, M.; Kumar, S.P.J.; Garlapati, V.K.; Postemsky, P.D.; Kumar, N.S.S.; Chandel, A.K.; Simal-Gandara, J. Biodiesel production from lignocellulosic biomass using oleaginous microbes: Prospects for integrated biofuel production. Front. Microbiol. 2021, 12, 658284. [Google Scholar] [CrossRef]
- Shah, S.; Venkatramanan, V. Advances in microbial technology for upscaling sustainable biofuel production. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Secondary Metabolites Biochemistry and Applications; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 69–76. [Google Scholar] [CrossRef]
- Lu, H.; Yadav, V.; Zhong, M.; Bilal, M.; Taherzadeh, M.J.; Iqbal, H.M.N. Bioengineered microbial platforms for biomass-derived biofuel production—A review. Chemosphere 2022, 288, 132528. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Adegboye, M.F.; Ojuederie, O.B.; Talia, P.M.; Babalola, O.O. Bioprospecting of microbial strains for biofuel production: Metabolic engineering, applications, and challenges. Biotechnol. Biofuels 2021, 14, 5. [Google Scholar] [CrossRef]
- Xu, P.; Koffas, M.A. Metabolic engineering of Escherichia coli for biofuel production. Eng. Life Sci. 2010, 1, 493–504. [Google Scholar]
- Saha, R.; Mukhopadhyay, M. Prospect of metabolic engineering in enhanced microbial lipid production: A review. Biomass Convers. Biorefin. 2021, 1–22. [Google Scholar] [CrossRef]
- Ranjbar, S.; Malcata, F.X. Challenges and prospects for sustainable microalga-based oil: A comprehensive review, with a focus on metabolic and genetic engineering. Fuel 2022, 324, 124567. [Google Scholar] [CrossRef]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.M.; Ling, T.C.; Nagarajan, D.; Lee, D.J.; Chang, J.S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Iniyakumar, M.; Venkatramanan, V.; Ramalakshmi, A.; Bobita, R.; Tharunkumar, J.; Jothibasu, K.; Rakesh, S. Overview on Advanced Microalgae-Based Sustainable Biofuel Generation and Its Life Cycle Assessment. In Micro-Algae: Next-Generation Feedstock for Biorefineries: Contemporary Technologies and Future Outlook; Verma, P., Ed.; Springer Nature: Singapore, 2022; pp. 53–71. [Google Scholar] [CrossRef]
- Zhu, L.; Nugroho, Y.K.; Shakeel, S.R.; Li, Z.; Martinkauppi, B.; Hiltunen, E. Using microalgae to produce liquid transportation biodiesel: What is next? Renew. Sustain. Energy Rev. 2017, 78, 391–400. [Google Scholar] [CrossRef]
- Koyande, A.K.; Show, P.L.; Guo, R.; Tang, B.; Ogino, C.; Chang, J.S. Bio-processing of algal bio-refinery: A review on current advances and future perspectives. Bioengineered 2019, 10, 574–592. [Google Scholar] [CrossRef]
- Khan, T.A.; Khan, T.A.; Kumar Yadav, A. A Hydrodynamic Cavitation-Assisted System for Optimization of Biodiesel Production from Green Microalgae Oil Using a Genetic Algorithm and Response Surface Methodology Approach. Environ. Sci. Pollut. Res. 2022, 29, 49465–49477. [Google Scholar] [CrossRef]
- Singh, R.; Liu, H.; Shanklin, J.; Singh, V. Hydrothermal Pretreatment for Valorization of Genetically Engineered Bioenergy Crop for Lipid and Cellulosic Sugar Recovery. Bioresour. Technol. 2021, 341, 125817. [Google Scholar] [CrossRef]
- Vanhercke, T.; Petrie, J.R.; Singh, S.P. Energy Densification in Vegetative Biomass through Metabolic Engineering. Biocatal. Agric. Biotechnol. 2014, 3, 75–80. [Google Scholar] [CrossRef]
- Vanhercke, T.; Tahchy, A.E.; Liu, Q.; Zhou, X.-R.; Shrestha, P.; Divi, U.K.; Ral, J.-P.; Mansour, M.P.; Nichols, P.D.; James, C.N.; et al. Metabolic Engineering of Biomass for High Energy Density: Oilseed-like Triacylglycerol Yields from Plant Leaves. Plant Biotechnol. J. 2014, 12, 231–239. [Google Scholar] [CrossRef]
- Heater, B.S.; Chan, W.S.; Lee, M.M.; Chan, M.K. Directed Evolution of a Genetically Encoded Immobilized Lipase for the Efficient Production of Biodiesel from Waste Cooking Oil. Biotechnol. Biofuels 2019, 12, 165. [Google Scholar] [CrossRef]
- Takeshita, T.; Ivanov, I.N.; Oshima, K.; Ishii, K.; Kawamoto, H.; Ota, S.; Yamazaki, T.; Hirata, A.; Kazama, Y.; Abe, T.; et al. Comparison of Lipid Productivity of Parachlorella Kessleri Heavy-Ion Beam Irradiation Mutant PK4 in Laboratory and 150-L Mass Bioreactor, Identification and Characterization of Its Genetic Variation. Algal Res. 2018, 35, 416–426. [Google Scholar] [CrossRef]
- Santos Mendoza, M.; Dubreucq, B.; Miquel, M.; Caboche, M.; Lepiniec, L. LEAFY COTYLEDON 2 Activation Is Sufficient to Trigger the Accumulation of Oil and Seed Specific MRNAs in Arabidopsis Leaves. FEBS Lett. 2005, 579, 4666–4670. [Google Scholar] [CrossRef]
- Fan, J.; Yan, C.; Zhang, X.; Xu, C. Dual Role for Phospholipid:Diacylglycerol Acyltransferase: Enhancing Fatty Acid Synthesis and Diverting Fatty Acids from Membrane Lipids to Triacylglycerol in Arabidopsis Leaves. Plant Cell 2013, 25, 3506–3518. [Google Scholar] [CrossRef]
- Xu, C.; Fan, J.; Froehlich, J.E.; Awai, K.; Benning, C. Mutation of the TGD1 Chloroplast Envelope Protein Affects Phosphatidate Metabolism in Arabidopsis. Plant Cell 2005, 17, 3094–3110. [Google Scholar] [CrossRef]
- Sanjaya; Durrett, T.P.; Weise, S.E.; Benning, C. Increasing the Energy Density of Vegetative Tissues by Diverting Carbon from Starch to Oil Biosynthesis in Transgenic Arabidopsis. Plant Biotechnol. J. 2011, 9, 874–883. [Google Scholar] [CrossRef]
- Petrie, J.R.; Vanhercke, T.; Shrestha, P.; El Tahchy, A.; White, A.; Zhou, X.-R.; Liu, Q.; Mansour, M.P.; Nichols, P.D.; Singh, S.P. Recruiting a New Substrate for Triacylglycerol Synthesis in Plants: The Monoacylglycerol Acyltransferase Pathway. PLoS ONE 2012, 7, e35214. [Google Scholar] [CrossRef]
- Kalscheuer, R.; Stoelting, T.; Steinbuechel, A. Microdiesel: Escherichia coli engineered for fuel production. Microbiology 2006, 152, 2529–2536. [Google Scholar] [CrossRef]
- Steen, E.J.; Kang, Y.; Bokinsky, G.; Hu, Z.; Schirmer, A.; Mcclure, A.; del Cardayre, S.B.; Keasling, J.D. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 2010, 463, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Elbahloul, Y.; Steinbüchel, A. Pilot-scale production of fatty acid ethyl esters by an engineered Escherichia coli strain harboring the p (Microdiesel) plasmid. Appl. Environ. Microbiol. 2010, 76, 4560–4565. [Google Scholar] [CrossRef]
- de Jong, B.W.; Shi, S.; Siewers, V.; Nielsen, J. Improved production of fatty acid ethyl esters in Saccharomyces cerevisiae through up-regulation of the ethanol degradation pathway and expression of the heterologous phosphokinase pathway. Microb. Cell Fact. 2014, 13, 39. [Google Scholar] [CrossRef]
- Dulermo, R.; Gamboa-Meléndez, H.; Ledesma-Amaro, R.; Thevenieau, F.; Nicaud, J.-M. Yarrowia lipolytica AAL genes are involved in peroxisomal fatty acid activation. Biochim. Biophys. Acta 2016, 1861, 555–565. [Google Scholar] [CrossRef]
- Das, S.; Reshad, A.S.; Bhuyan, N.; Sut, D.; Tiwari, P.; Goud, V.V.; Kataki, R. Utilization of nonedible oilseeds in a biorefinery approach with special emphasis on rubber seeds. In Waste Biorefinery: Integrating Biorefineries for Waste Valorisation; Bhaskar, T., Pandey, A., Rene, E.R., Tsang, D.C.W., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2020; pp. 311–336. [Google Scholar]
- Bilgili, F.; Koçak, E.; Bulut, U.; Kuşkaya, S. Can biomass energy be an efficient policy tool for sustainable development? Renew. Sustain. Energy Rev. 2017, 71, 830–845. [Google Scholar] [CrossRef]
- FAO. Food Outlook; Food and Agricultural Organization (FAO): Rome, Italy, 2020. [Google Scholar]
- Mohanty, A.; Rout, P.R.; Dubey, B.; Meena, S.S.; Pal, P.; Goel, M. A critical review on biogas production from edible and non-edible oil cakes. Biomass Convers. Biorefin. 2022, 12, 949–966. [Google Scholar] [CrossRef]
- Das, A.; Busetty, S.; Goel, M.; Ram Kiran, B.; Sudharshan, K.; Kanapuram, R. Alternative usage of edible deoiled cake for decolonisation of Reactive Red Dye. Desalin. Water Treat. 2015, 53, 2720–2726. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, G.; Martin-Lara, M.A.; Blazquez, G.; Tenorio, G.; Calero, M. Hydrolyzed olive cake as novel adsorbent for copper removal from fertilizer industry wastewater. J. Clean. Prod. 2020, 268, 121935. [Google Scholar] [CrossRef]
- Rawat, A.P.; Rawat, M.; Rai, J.P.N. Toxic Metals Biosorption by Jatropha curcas Deoiled Cake: Equilibrium and Kinetic Studies. Water Environ. Res. 2013, 85, 733–742. [Google Scholar] [CrossRef]
- Yusoff, M.; Mohamed, E.; Idris, J.; Zainal, N.H.; Ibrahim, M.F.; Abd-Aziz, S. Adsorption of heavy metal ions by oil palm decanter cake activated carbon. Makara J. Technol. 2019, 23, 2. [Google Scholar]
- Rao, R.A.K.; Khan, M.A. Removal and recovery of Cu(II), Cd(II) and Pb(II) ions from single and multimetal systems by batch and column operation on neem oil cake (NOC). Sep. Purif. Technol. 2007, 57, 394–402. [Google Scholar] [CrossRef]
- Mahajan, G.; Garg, U.; Sud, D.; Garg, V. Utilization Properties of Jatropha De-Oiled Cake for Removal of Nickel (II) from Aqueous Solutions. Bioresources 2013, 8, 5596–5611. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngabura, M.; Choong, T.S.Y.; Masood, H.; Chuah, L.A. Biosorption and desorption of Nickel on oil cake: Batch and column studies. Bioresour. Technol. 2012, 103, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.A.K.; Khan, M.A.; Jeon, B.H. Utilization of carbon derived from mustard oil cake (CMOC) for the removal of bivalent metal ions: Effect of anionic surfactant on the removal and recovery. J. Hazard. Mater. 2010, 173, 273–282. [Google Scholar] [CrossRef]
- Upendar, K.; Sagar, T.V.; Raveendra, G.; Lingaiah, N.; Rao, B.V.S.K.; Prasad, R.B.N.; Sai Prasad, P.S. Development of a low temperature adsorbent from karanja seed cake for CO2 capture. RSC Adv. 2014, 4, 7142. [Google Scholar] [CrossRef]
- Sarkar, S. Exploring biogas potential data of cattle manure and olive cake to gain insight into farm and commercial scale production. Data Br. 2020, 32, 106045. [Google Scholar] [CrossRef]
- Isci, A.; Demirer, G.N. Biogas production potential from cotton wastes. Renew. Energy 2007, 32, 750–757. [Google Scholar] [CrossRef]
- Drozłowska, E.; Łopusiewicz, L.; Mezynska, M.; Bartkowiak, A. Valorization of Flaxseed Oil Cake Residual from Cold-Press Oil Production as a Material for Preparation of Spray-Dried Functional Powders for Food Applications as Emulsion Stabilizers. Biomolecules 2020, 10, 153. [Google Scholar] [CrossRef]
- VasudhaUdupa, A.; Balakrishna, G.; Shivanna, M.B. Influence of non-edible oil-cakes and their composts on growth, yield and Alternaria leaf spot disease in chilli. Int. J. Recycl. Org. Waste Agric. 2022, 11, 301–318. [Google Scholar]
- Venkatesagowda, B.; Ponugupaty, E.; Barbosa, A.M.; Dekker, R.F.H. Solid-state fermentation of coconut kernel-cake as substrate for the production of lipases by the coconut kernel-associated fungus Lasiodiplodia theobromae VBE-1. Ann. Microbiol. 2015, 65, 129–142. [Google Scholar] [CrossRef]
- Rehman, B.; Ganai, M.A.; Parihar, K.; Asif, M.; Siddiqui, M. A Biopotency of Oilcakes Against Meloidogyne incognita Affecting Vigna mungo. Eur. J. Appl. Sci. 2014, 6, 57–63. [Google Scholar] [CrossRef][Green Version]
- Singh, R.; Kumar, A.; Tomer, A. De-oiled Cakes of Neem, Jatropha, Mahua and Karanja: A New Substrate for Mass Multiplication of T. harzianum. J. Plant Pathol. Microbiol. 2015, 6, 1000288. [Google Scholar] [CrossRef]
- Radwan, M.A.; El-Maadawy, E.K.; Kassem, S.I.; Abu-Elamayem, M.M. Oil cakes soil amendment effects on Meloidogyne incognita, root-knot nematode infecting tomato. Arch. Phytopathol. Plant Prot. 2009, 42, 58–64. [Google Scholar] [CrossRef]
- Olowoake, A.A.; Osunlola, O.S.; Ojo, J.A. Influence of compost supplemented with jatropha cake on soil fertility, growth, and yield of maize (zea mays L.) in a degraded soil of Ilorin, Nigeria. Int. J. Recycl. Org. Waste Agric. 2018, 7, 67–73. [Google Scholar] [CrossRef]
- Hirota, M.; Suttajit, M.; Suguri, H.; Endo, Y.; Shudo, K.; Wongchai, V.; Hecker, E.; Fujiki, H. A new tumor promoter from the seed oil of Jatropha curcas L., an intramolecular diester of 12-deoxy-16-hydroxyphorbol. Cancer Res. 1988, 48, 5800–5804. [Google Scholar] [PubMed]
- Sharma, S.; Verma, M.; Sharma, A. Utilization of Non Edible Oil Seed Cakes as Substrate for Growth of Paecilomyces lilacinus and as Biopesticide Against Termites. Waste Biomass Valoriz. 2013, 4, 325–330. [Google Scholar] [CrossRef]
- Monlau, F.; Latrille, E.; Da Costa, A.C.; Steyer, J.P.; Carrere, H. Enhancement of methane production from sunflower oil cakes by dilute acid pretreatment. Appl. Energy 2013, 102, 1105–1113. [Google Scholar] [CrossRef]
- Fernandez-Cegri, V.; De la Rubia, M.A.; Raposo, F.; Borja, R. Effect of hydrothermal pre-treatment of sunflower oil cake on biomethane potential focusing on fibre composition. Bioresour. Technol. 2012, 123, 424–429. [Google Scholar] [CrossRef]
- Kumar, G.; Lin, C.Y. Biogenic Hydrogen Conversion of De-Oiled Jatropha Waste via Anaerobic Sequencing Batch Reactor Operation: Process Performance, Microbial Insights, and CO2 Reduction Efficiency. Sci. World J. 2014, 2014, 946503. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Vaštag, Ž.; Popović, L.; Popović, S.; Krimer, V.; Peričin, D. Production of enzymatic hydrolysates with antioxidant and angiotensin-I converting enzyme inhibitory activity from pumpkin oil cake protein isolate. Food Chem. 2011, 124, 1316–1321. [Google Scholar] [CrossRef]
- Jose, S.; Roy, R.; Phukan, A.R.; Shakyawar, D.B.; Sankaran, A. Biochar from oil cakes: An efficient and economical adsorbent for the removal of acid dyes from wool dye house effluent. Clean Technol. Environ. Policy 2022, 24, 1599–1608. [Google Scholar] [CrossRef]
- Kalinke, C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar prepared from castor oil cake at different temperatures: A voltammetric study applied for Pb2+, Cd2+ and Cu2+ ions preconcentration. J. Hazard. Mater. 2016, 318, 526–532. [Google Scholar] [CrossRef]
- Ancuța, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches: A review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Nevara, G.A.; Giwa Ibrahim, S.A.; Syed Muhammad, S.K.; Zawawi, N.; Mustapha, N.A.; Karim, R. Oilseed meals into foods: An approach for the valorization of oilseed by-products. Crit. Rev. Food Sci. Nutr. 2022, 28, 1–14. [Google Scholar] [CrossRef]
- Colletti, A.; Attrovio, A.; Boffa, L.; Mantegna, S.; Cravotto, G. Valorisation of by-products from soybean (Glycine max (L.) Merr.) processing. Molecules 2020, 25, 2129. [Google Scholar] [CrossRef]
- Martín-Cabrejas, M.A.; Aguilera, Y.; Pedrosa, M.M.; Cuadrado, C.; Hernández, T.; Díaz, S.; Esteban, R.M. The impact of dehydration process on antinutrients and protein digestibility of some legume flours. Food Chem. 2009, 114, 1063–1068. [Google Scholar] [CrossRef]
- Rabadán, A.; Álvarez-Ortí, M.; Martínez, E.; Pardo-Giménez, A.; Zied, D.C.; Pardo, J.E. Effect of replacing traditional ingredients for oils and flours from nuts and seeds on the characteristics and consumer preferences of lamb meat burgers. LWT 2021, 136, 110307. [Google Scholar] [CrossRef]
- Ilmi, M.; Hidayat, C.; Hastuti, P.; Heeres, H.J.; Van Der Maarel, M.J.E.C. Utilisation of Jatropha press cake as substrate in biomass and lipase production from Aspergillus niger 65I6 and Rhizomucor miehei CBS 360.62. Biocatal. Agric. Biotechnol. 2017, 9, 103–107. [Google Scholar] [CrossRef]
- Rathore, D.; Pant, D.; Singh, A. A comparison of life cycle assessment studies of different biofuels. In Life Cycle Assessment of Renewable Energy Sources, Series: Green Energy and Technology; Singh, A., Olsen, S.I., Pant, D., Eds.; Springer: London, UK, 2013. [Google Scholar] [CrossRef]
- Arguelles-Arguelles, A.; Amezcua-Allieri, M.A.; Ramírez-Verduzco, L.F. Life cycle assessment of green diesel production by hydrodeoxygenation of palm oil. Front. Energy Res. 2021, 9, 690725. [Google Scholar] [CrossRef]
- Foteinis, S.; Chatzisymeon, E.; Litinas, A.; Tsoutsos, T. Used-cooking-oil biodiesel: Life cycle assessment and comparison with first- and third-generation biofuel. Renew. Energy 2020, 153, 588–600. [Google Scholar] [CrossRef]
- Singh, A.; Olsen, S.I. Key Issues in Life Cycle Assessment of Biofuels. In Sustainable Bioenergy and Bioproducts: Value Added Engineering Applications, Green Energy and Technology; Gopalakrishnan, K., Leeuwen, J.H.v., Brown, R.C., Eds.; Springer: London, UK, 2012; pp. 213–228. [Google Scholar]
- Singh, A.; Pant, D.; Olsen, S.I.; Nigam, P.S. Key issues to consider in microalgae based biodiesel. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2012, 29, 687–700. [Google Scholar]
- Larson, E.D. A review of life-cycle analysis studies on liquid biofuel systems for the transport sector. Energy Sustain. Dev. 2006, 10, 109–126. [Google Scholar] [CrossRef]
- Karka, P.; Papadokonstantakis, S.; Hungerbühler, K.; Kokossis, A. Life Cycle Assessment of Biorefinery Products Based on Different Allocation Approaches. Comput. Aided Chem. Eng. 2015, 37, 2573–2578. [Google Scholar] [CrossRef]
- Gnansounou, E.; Dauriat, A.; Villegas, J.; Panichelli, L. Life cycle assessment of biofuels: Energy and greenhouse gas balances. Bioresour. Technol. 2009, 100, 4919–4930. [Google Scholar] [CrossRef]
- Cherubini, F.; Bird, N.D.; Cowie, A.; Jungmeier, G.; Schlamadinger, B.; Woess-Gallasch, S. Energy- and greenhouse gas-based LCA of biofuel and bioenergy systems: Key issues, ranges and recommendations. Resour. Conserv. Recycl. 2009, 53, 434–447. [Google Scholar]
- SAIC. Life Cycle Assessment: Principles and Practice; EPA/600/R-06/060; National Risk Management Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Cincinnati, OH, USA, 2006.
- Singh, A.; Pant, D.; Korres, N.E.; Nizami, A.S.; Prasad, S.; Murphy, J.D. Key issues in life cycle assessment of ethanol production from lignocellulosic biomass: Challenges and perspectives. Bioresour. Technol. 2010, 101, 5003–5012. [Google Scholar]
- Luo, L.; van der Voet, E.; Huppes, G.; Udo de Haes, H.A. Allocation issues in LCA methodology: A case study of corn stover-based fuel ethanol. Int. J. Life Cycle Assess. 2009, 14, 529–539. [Google Scholar] [CrossRef]
- Sheehan, J.; Camobreco, V.; Duffield, J.; Graboski, M.; Shapouri, H. An Overview of Biodiesel and Petroleum Diesel Life Cycles; National Renewable Energy Laboratory: Golden, CO, USA, 1998.
- Sieira, P.; Galante, E.B.F.; Mendes, A.J.B.; Haddad, A. Life Cycle Assessment of a Biodiesel Production Unit. Am. J. Chem. Eng. 2015, 3, 25–29. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Field, J.L.; Kwon, H.; Hawkins, T.R.; Paustian, K.; Wang, M.Q. A multi-product landscape life-cycle assessment approach for evaluating local climate mitigation potential. J. Clean. Prod. 2022, 354, 131691. [Google Scholar] [CrossRef]
- Malik, S.; Shahid, A.; Betenbaugh, M.J.; Liu, C.-G.; Mehmood, M.A. A novel wastewater-derived cascading algal biorefinery route for complete valorization of the biomass to biodiesel and value-added bioproducts. Energy Convers. Manag. 2022, 256, 115360. [Google Scholar] [CrossRef]
- Singh, A.; Olsen, S.I. Comparison of algal biodiesel production pathways using life cycle assessment tool. In Life Cycle Assessment of Renewable Energy Sources, Green Energy and Technology Series; Singh, A., Pant, D., Olsen, S.I., Eds.; Springer: London, UK, 2013; pp. 145–168. [Google Scholar]
- Gupta, R.; McRoberts, R.; Yu, Z.; Smith, C.; Sloan, W.; You, S. Life cycle assessment of biodiesel production from rapeseed oil: Influence of process parameters and scale. Bioresour. Technol. 2022, 360, 127532. [Google Scholar] [CrossRef]
- Vairaprakash, P.; Arumugam, A. Sustainability, Commercialization, and Future Prospects of Biodiesel Production. In Biodiesel Production: Feedstocks, Catalysts, and Technologies; Rokhum, S.L., Halder, G., Assabumrungrat, S., Ngaosuwan, K., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 355–376. [Google Scholar] [CrossRef]
- REN21. Renewables 2020 Global Status Report; REN21 Secretariat: Paris, France, 2020. [Google Scholar]
- Pavan, M.; Reinmets, K.; Garg, S.; Mueller, A.P.; Marcellin, E.; Kopke, M.; Valgepea, K. Advances in systems metabolic engineering of autotrophic carbon oxide-fixing biocatalysts towards a circular economy. Metab. Eng. 2022, 71, 117–141. [Google Scholar] [CrossRef]
- Hossain, S.M.Z.; Razzak, S.A.; Al-Shater, A.F.; Moniruzzaman, M.; Hossain, M.M. Recent Advances in Enzymatic Conversion of Microalgal Lipids into Biodiesel. Energy Fuels 2020, 34, 6735–6750. [Google Scholar] [CrossRef]
- Wang, H.; Peng, X.; Zhang, H.; Yang, S.; Li, H. Microorganisms-promoted biodiesel production from biomass: A review. Energy Convers. Manag. X 2021, 12, 100137. [Google Scholar] [CrossRef]
- Fan, J.; Andre, C.; Xu, C. A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii. FEBS Lett. 2011, 585, 1985–1991. [Google Scholar] [CrossRef]
- Cagliari, A.; Margis, R.; Maraschin, F.S.; Turchetto-Zolet, A.C.; Loss, G.; Margis-Pinheiro, M. Biosynthesis of triacylglycerols (TAGs) in plants and algae. Int. J. Plant Biol. 2011, 2, 40–52. [Google Scholar] [CrossRef]
- Severo, I.A.; Siqueira, S.F.; Deprá, M.C.; Maroneze, M.M.; Zepka, L.Q.; Jacob-Lopes, E. Biodiesel facilities: What can we address to make biorefineries commercially competitive? Renew. Sustain. Energy Rev. 2019, 112, 686–705. [Google Scholar] [CrossRef]



| Species | Tissue | Target Gene (s) | Total Fatty Acid (TFA) and Triacylglycerol (TGA) Level | Reference |
|---|---|---|---|---|
| Arabidopsis thaliana | Leaf | LEC2 | TAG not quantified | Santos Mendoza et al. [67] |
| Nicotiana tabacum | Leaf | WRI1, DGAT1, L-OLEOSIN | 17.7% TFA (DW), 15.8% TAG (DW) | Vanhercke et al. [64] |
| A. thaliana | Leaf | PDAT1 | 2.6% TAG (DW) | Fan et al. [68] |
| A. thaliana | Leaf | tgd1 | Not quantified | Xu et al. [69] |
| Solanum tuberosum | Tuber | ACCase | 0.03% TAG (DW) | Klaus et al. [29] |
| A. thaliana | Seedling | WRI1, AGPase RNAi | 5.8-fold increase | Sanjaya et al. [70] |
| Nicotiana benthamiana | Seedling | MGAT2 | 6.2-fold TAG increase | Petrie et al. [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathore, D.; Sevda, S.; Prasad, S.; Venkatramanan, V.; Chandel, A.K.; Kataki, R.; Bhadra, S.; Channashettar, V.; Bora, N.; Singh, A. Bioengineering to Accelerate Biodiesel Production for a Sustainable Biorefinery. Bioengineering 2022, 9, 618. https://doi.org/10.3390/bioengineering9110618
Rathore D, Sevda S, Prasad S, Venkatramanan V, Chandel AK, Kataki R, Bhadra S, Channashettar V, Bora N, Singh A. Bioengineering to Accelerate Biodiesel Production for a Sustainable Biorefinery. Bioengineering. 2022; 9(11):618. https://doi.org/10.3390/bioengineering9110618
Chicago/Turabian StyleRathore, Dheeraj, Surajbhan Sevda, Shiv Prasad, Veluswamy Venkatramanan, Anuj Kumar Chandel, Rupam Kataki, Sudipa Bhadra, Veeranna Channashettar, Neelam Bora, and Anoop Singh. 2022. "Bioengineering to Accelerate Biodiesel Production for a Sustainable Biorefinery" Bioengineering 9, no. 11: 618. https://doi.org/10.3390/bioengineering9110618
APA StyleRathore, D., Sevda, S., Prasad, S., Venkatramanan, V., Chandel, A. K., Kataki, R., Bhadra, S., Channashettar, V., Bora, N., & Singh, A. (2022). Bioengineering to Accelerate Biodiesel Production for a Sustainable Biorefinery. Bioengineering, 9(11), 618. https://doi.org/10.3390/bioengineering9110618










