Effect of Rho–Associated Kinase Inhibitor on Growth Behaviors of Human Induced Pluripotent Stem Cells in Suspension Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions of hiPSCs
2.2. hiPSC Aggregate Culture
2.3. Kinetic Analysis of Growth Behavior of hiPSCs
2.4. Time-Lapse Observation
2.5. Preparation and Staining of Frozen Sections
2.6. Scanning Electron Microscopy (SEM)
2.7. Statistical Analysis
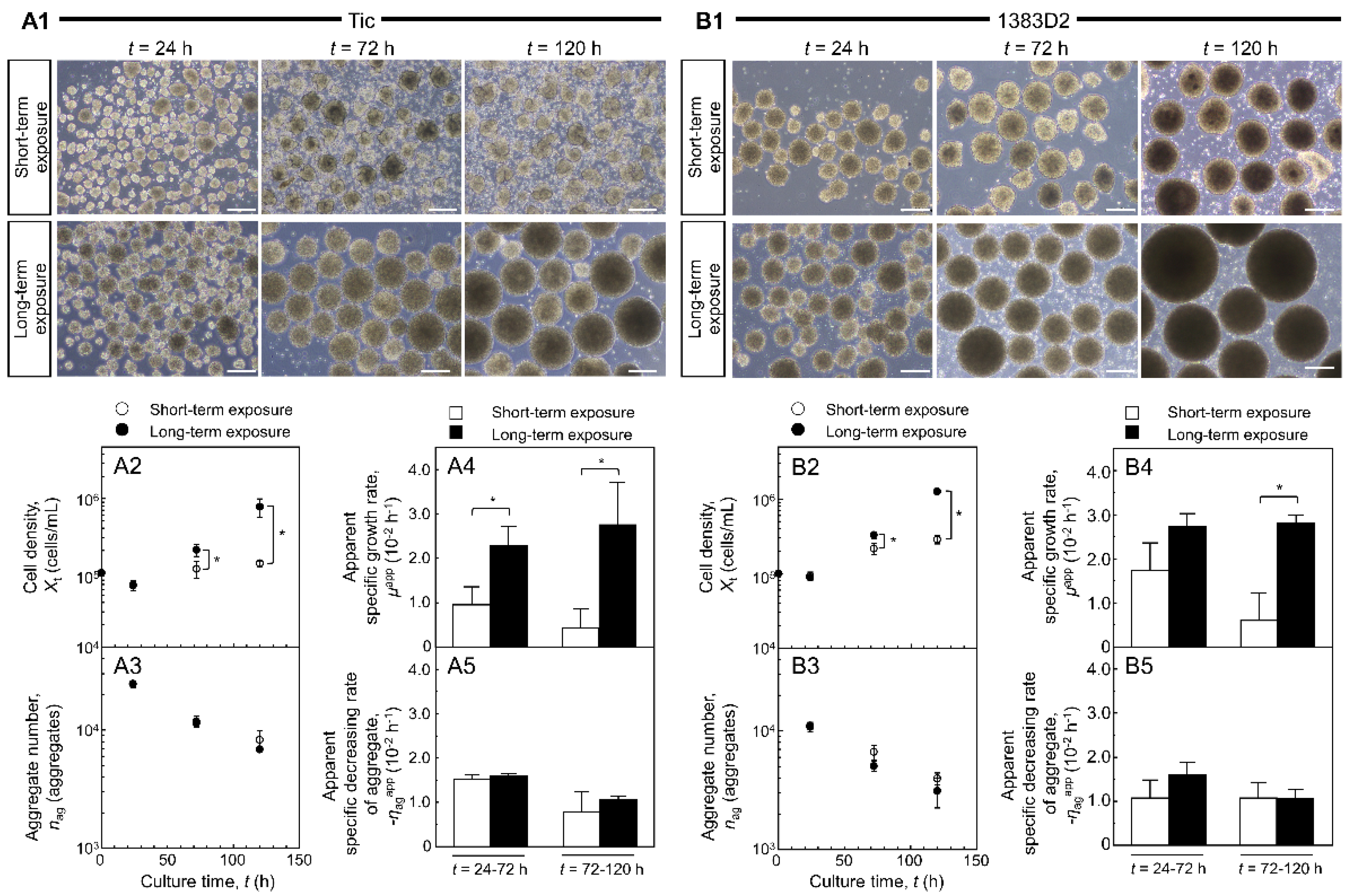
3. Results
3.1. Assessment of hiPSC Response in Suspension Culture following Short- and Long-Term Exposure to ROCK Inhibitor
3.2. The Effect of Long-Term Exposure to ROCK Inhibitor on Growth of hiPSCs
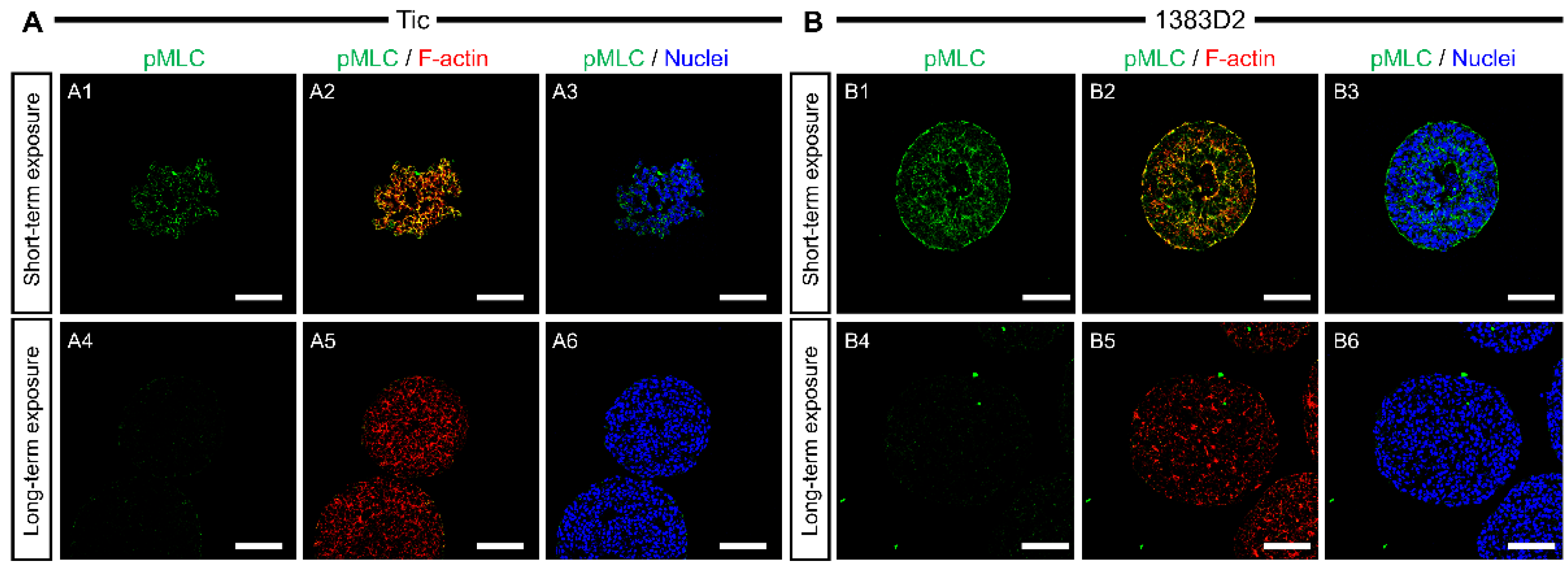
3.3. Localization Pattern of Collagen Type I and pMLC in Cell Aggregates by Prolonged Exposure to ROCK Inhibitor
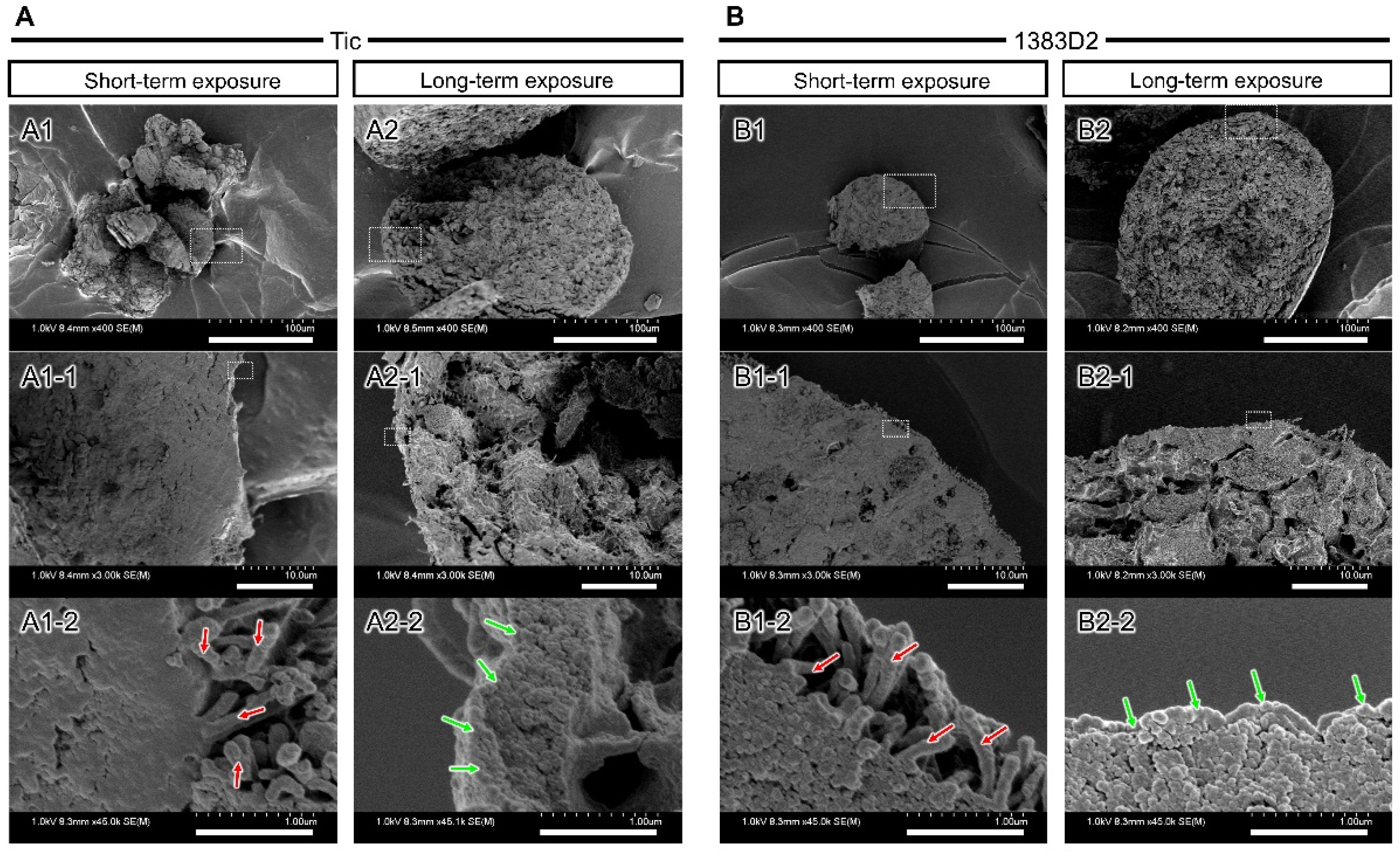
3.4. Time-Dependent Change of Microstructure on the Surface of Cell Aggregate by Prolonged Exposure to ROCK Inhibitor
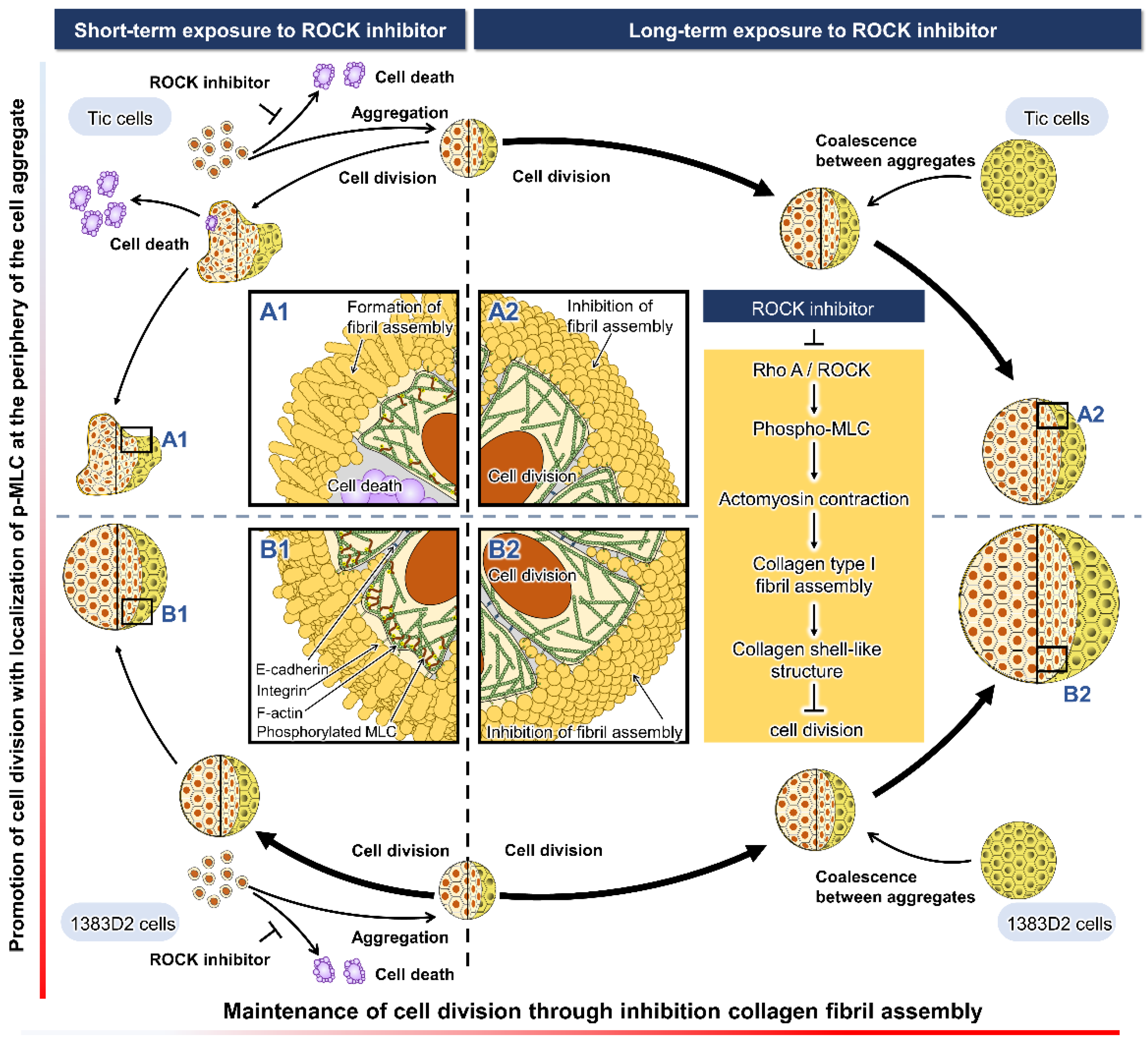
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Kimbrel, E.A.; Lanza, R. Current status of pluripotent stem cells: Moving the first therapies to the clinic. Nat. Rev. Drug Discov. 2015, 14, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Shafa, M.; Sjonnesen, K.; Yamashita, A.; Liu, S.Y.; Michalak, M.; Kallos, M.S.; Rancourt, D.E. Expansion and long–term maintenance of induced pluripotent stem cells in stirred suspension bioreactors. J. Tissue Eng. Regen. Med. 2012, 6, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chou, B.K.; Dowey, S.; He, C.X.; Gerecht, S.; Cheng, L.Z. Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res. 2013, 11, 1103–1116. [Google Scholar] [CrossRef]
- Kim, M.H.; Takeuchi, K.; Kino-oka, M. Role of cell-secreted extracellular matrix formation in aggregate formation and stability of human induced pluripotent stem cells in suspension culture. J. Biosci. Bioeng. 2019, 127, 372–380. [Google Scholar] [CrossRef]
- Nath, S.C.; Horie, M.; Nagamori, E.; Kino-oka, M. Size- and time dependent growth properties of human induced pluripotent stem cells in the culture of single aggregate. J. Biosci. Bioeng. 2017, 124, 469–475. [Google Scholar] [CrossRef]
- Kato, Y.; Kim, M.H.; Kino-oka, M. Comparison of growth kinetics between static and dynamic cultures of human induced pluripotent stem cells. J. Biosci. Bioeng. 2018, 125, 736–740. [Google Scholar] [CrossRef]
- Hashida, A.; Uemura, T.; Kino-Oka, M. Kinetics on aggregate behaviors of human induced pluripotent stem cells in static suspension and rotating flow cultures. J. Biosci. Bioeng. 2020, 129, 494–501. [Google Scholar] [CrossRef]
- Sanders, J.P.M.; Clark, J.H.; Harmsen, G.J.; Heeres, H.J.; Heijnen, J.J.; Kersten, S.R.A.; Van Swaaij, W.P.M.; Moulijn, J.A. Process Intensification process intensification in the future production of base chemicals from biomass. Chem. Eng. Process. 2012, 51, 117–136. [Google Scholar] [CrossRef]
- Gao, L.; Nath, S.C.; Jiao, X.; Zhou, R.; Nishikawa, S.; Krawetz, R.; Li, X.; Rancourt, D.E. Post-Passage rock inhibition induces cytoskeletal aberrations and apoptosis in Human embryonic stem cells. Stem Cell Res. 2019, 41, 101641. [Google Scholar] [CrossRef]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Sahai, E.A.; Yeo, M.; Bosch, M.; Dewar, A.; Olson, M.F. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 2001, 3, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Fujiwara, S.; Mizuno, K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J. Biochem. 2017, 161, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Evers, E.E.; Zondag, G.C.; Malliri, A.; Price, L.S.; ten Klooster, J.P.; van der Kammen, R.A.; Collard, J.G. Rho family proteins in cell adhesion and cell migration. Eur. J. Cancer 2000, 36, 1269–1274. [Google Scholar] [CrossRef]
- Croze, R.H.; Thi, W.J.; Clegg, D.O. ROCK inhibition promotes attachment, proliferation, and wound closure in human embryonic stem cell-derived retinal pigmented epithelium. Transl. Vis. Sci. Technol. 2016, 5, 7. [Google Scholar] [CrossRef]
- Pakzad, M.; Totonchi, M.; Taei, A.; Seifinejad, A.; Hassani, S.N.; Baharvand, H. Presence of a ROCK Inhibitor in Extracellular Matrix Supports More Undifferentiated Growth of Feeder-Free Human Embryonic and Induced Pluripotent Stem Cells upon Passaging. Stem Cell Rep. 2010, 6, 96–107. [Google Scholar] [CrossRef]
- Sun, C.C.; Chiu, H.T.; Lin, Y.F.; Lee, K.Y.; Pang, J.H. Y-27632, a ROCK Inhibitor, Promoted Limbal Epithelial Cell Proliferation and Corneal Wound Healing. PLoS ONE 2015, 10, e0144571. [Google Scholar] [CrossRef]
- Wang, T.; Kang, W.; Du, L.; Ge, S. Rho-kinase inhibitor Y-27632 facilitates the proliferation, migration and pluripotency of human periodontal ligament stem cells. J. Cell Mol. Med. 2017, 21, 3100–3112. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, Y.; Mi, J.; Zhou, Q.; Bai, F.; Xu, X.; Fisher, D.E.; Sun, Q.; Wu, X. ROCK inhibitor enhances the growth and migration of BRAF-mutant skin melanoma cells. Cancer Sci. 2018, 109, 3428–3437. [Google Scholar] [CrossRef]
- Ohgushi, M.; Matsumura, M.; Eiraku, M.; Murakami, K.; Aramaki, T.; Nishiyama, A.; Muguruma, K.; Nakano, T.; Suga, H.; Ueno, M.; et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell 2010, 7, 225–239. [Google Scholar] [CrossRef]
- Nakagawa, M.; Taniguchi, Y.; Senda, S.; Takizawa, N.; Ichisaka, T.; Asano, K.; Morizane, A.; Doi, D.; Takahashi, J.; Nishizawa, M.; et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Stem Cell Rep. 2014, 4, 3594. [Google Scholar] [CrossRef] [PubMed]
- Thanuthanakhun, N.; Kino-oka, M.; Borwornpinyo, S.; Ito, Y.; Kim, M.H. The impact of culture dimensionality on behavioral epigenetic memory contributing to pluripotent state of iPS cells. J. Cell. Physiol. 2021, 236, 4985–4996. [Google Scholar] [CrossRef] [PubMed]
- Sasano, Y.; Fukumoto, K.; Tsukamoto, Y.; Akagi, T.; Akashi, M.J. Construction of 3D cardiac tissue with synchronous powerful beating using human cardiomyocytes from human iPS cells prepared by a convenient differentiation method. Biosci. Bioeng. 2020, 129, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Matsumoto, T.; Kino-oka, M. Effect of liquid flow by pipetting during medium change on deformation of hiPSC aggregates. Regen. Med. 2019, 12, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.C.; Liu, Y.; Yuan, X.; Ma, T. Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue Eng. Part A 2015, 21, 1705–1719. [Google Scholar] [CrossRef]
- Cai, S.; Pestic-Dragovich, L.; O’Donnell, M.E.; Wang, N.; Ingber, D.; Elson, E.; Lanerolle, P.E. Regulation of cytoskeletal mechanics and cell growth by myosin light chain phosphorylation. Am. J. Phys. 1998, 275, C1349–C1356. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Webster, K.D.; Ng, W.P.; Fletcher, D.A. Tensional homeostasis in single fibroblasts. Biophys. J. 2014, 107, 146–155. [Google Scholar] [CrossRef]
- Zeng, D.; Ou, D.B.; Wei, T.; Ding, L.; Liu, X.T.; Hu, X.L.; Li, X.; Zheng, Q.S. Collagen/β1 integrin interaction is required for embryoid body formation during cardiogenesis from murine induced pluripotent stem cells. BMC Cell Biol. 2013, 14, 5. [Google Scholar] [CrossRef]
- Sachlos, E.; Auguste, D.T. Embryoid body morphology influences diffusive transport of inductive biochemicals: A strategy for stem cell differentiation. Biomaterials 2008, 29, 4471–4480. [Google Scholar] [CrossRef]
- Okumura, N.; Fujii, K.; Kagami, T.; Makiko, N.; Kitahara, M.; Kinoshita, S.; Koizumi, N. Activation of the Rho/Rho kinase signaling pathway is involved in cell death of corneal endothelium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6843–6851. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, K.; TeSlaa, T.; Koehler, C.M.; Teitell, M.A. P53 regulates rapid apoptosis in human pluripotent stem cells. J. Mol. Biol. 2016, 428, 1465–1475. [Google Scholar] [CrossRef]
- Okumura, N.; Sakamoto, Y.; Fujii, K.; Kitano, J.; Nakano, S.; Tsujimoto, Y.; Nakamura, S.; Ueno, M.; Hagiya, M.; Hamuro, J.; et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci. Rep. 2016, 6, 26113. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Fan, Y.; Guo, Z.; Wang, Y.; Zheng, X.; Huang, C.; Liang, B.; Gao, L.; Cao, Y.; Chen, Y.; et al. Compression generated by a 3D supracellular actomyosin cortex promotes embryonic stem cell colony growth and expression of Nanog and Oct4. Cell Syst. 2019, 9, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Versaevel, M.; Grevesse, T.; Gabriele, S. Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nat. Commun. 2012, 3, 671. [Google Scholar] [CrossRef]
- Takaki, T.; Montagner, M.; Serres, M.P.; Le Berre, M.; Russell, M.; Collinson, L.; Szuhai, K.; Howell, M.; Boulton, S.J.; Sahai, E.; et al. Actomyosin drives cancer cell nuclear dysmorphia and threatens genome stability. Nat. Commun. 2017, 8, 16013. [Google Scholar] [CrossRef]
- Amano, M.; Ito, M.; Kimura, K.; Fukata, Y.; Chihara, K.; Nakano, T.; Matsuura, Y.; Kaibuchi, K. Phosphorylation and activation of myosin by Rho–associated kinase (Rho-kinase). J. Biol. Chem. 1996, 271, 20246–20249. [Google Scholar] [CrossRef]
- Kalson, N.S.; Starborg, T.; Lu, Y.; Mironov, A.; Humphries, S.M.; Holmes, D.F.; Kadler, K.E. Nonmuscle myosin II powered transport of newly formed collagen fibrils at the plasma membrane. Proc. Natl. Acad. Sci. USA 2013, 110, 4743–4752. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T.; Kim, M.-H.; Kino-oka, M. Effect of Rho–Associated Kinase Inhibitor on Growth Behaviors of Human Induced Pluripotent Stem Cells in Suspension Culture. Bioengineering 2022, 9, 613. https://doi.org/10.3390/bioengineering9110613
Matsumoto T, Kim M-H, Kino-oka M. Effect of Rho–Associated Kinase Inhibitor on Growth Behaviors of Human Induced Pluripotent Stem Cells in Suspension Culture. Bioengineering. 2022; 9(11):613. https://doi.org/10.3390/bioengineering9110613
Chicago/Turabian StyleMatsumoto, Takaki, Mee-Hae Kim, and Masahiro Kino-oka. 2022. "Effect of Rho–Associated Kinase Inhibitor on Growth Behaviors of Human Induced Pluripotent Stem Cells in Suspension Culture" Bioengineering 9, no. 11: 613. https://doi.org/10.3390/bioengineering9110613
APA StyleMatsumoto, T., Kim, M.-H., & Kino-oka, M. (2022). Effect of Rho–Associated Kinase Inhibitor on Growth Behaviors of Human Induced Pluripotent Stem Cells in Suspension Culture. Bioengineering, 9(11), 613. https://doi.org/10.3390/bioengineering9110613






